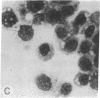
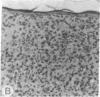
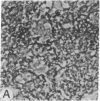
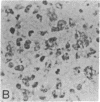
Abstract
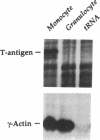
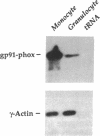
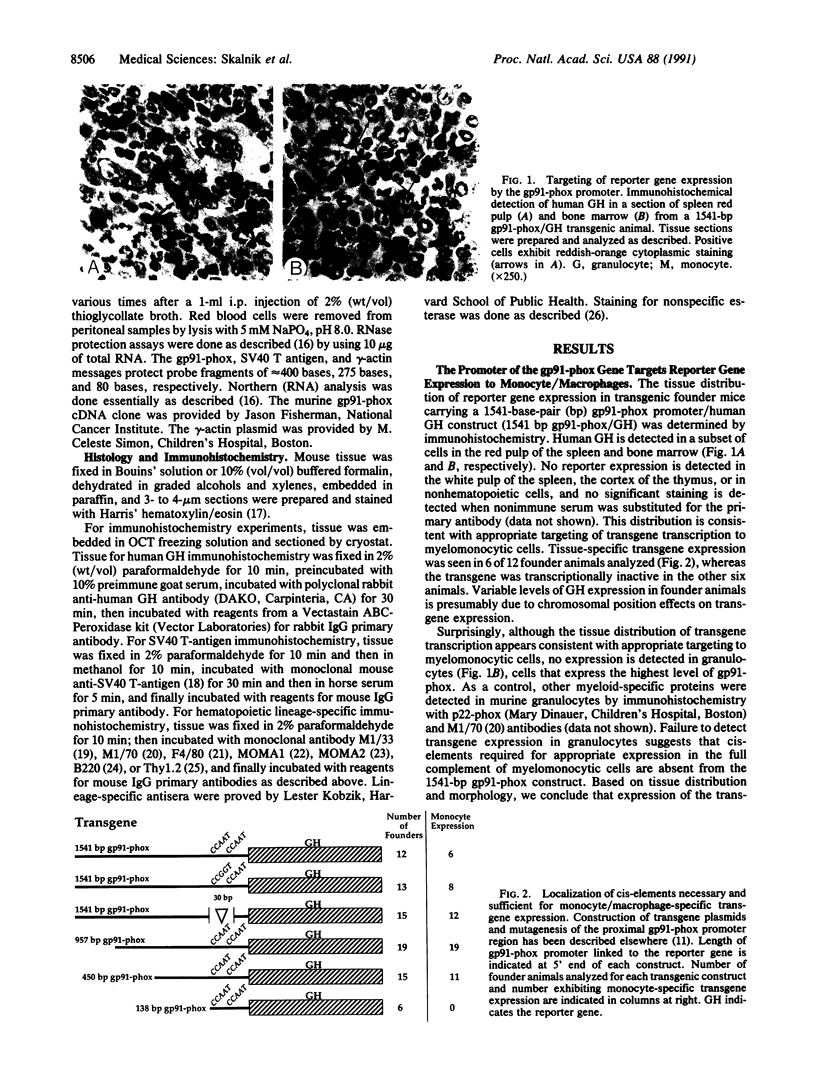
A component of a heterodimeric cytochrome b, designated gp91-phox, is required for the microbicidal activity of phagocytic cells and is expressed exclusively in differentiated myelomonocytic cells (granulocytes; monocyte/macrophages). In an attempt to identify cis-elements responsible for this restricted pattern of expression, we produced transgenic mice carrying reporter genes linked to the human gp91-phox promoter. Immunohistochemical and RNA analyses indicate that 450 base pairs of the proximal gp91-phox promoter is sufficient to target reporter expression to a subset of monocyte/macrophages. Mice expressing simian virus 40 large tumor antigen under control of the gp91-phox promoter develop monocyte/macrophage-derived malignancies with complete penetrance at 6-12 mo of age and provide an animal model of true histiocytic lymphoma. As these transgenes are inactive in most phagocytic cells that express the endogenous gp91-phox-encoding gene, we infer that additional genomic regulatory elements are necessary for appropriate targeting to the full complement of phagocytes in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Chen S., Botteri F., van der Putten H., Landel C. P., Evans G. A. A lymphoproliferative abnormality associated with inappropriate expression of the Thy-1 antigen in transgenic mice. Cell. 1987 Oct 9;51(1):7–19. doi: 10.1016/0092-8674(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990 Nov;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. J., Springer T. A., Thorbecke G. J. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Fritton H. P., Igo-Kemenes T., Nowock J., Strech-Jurk U., Theisen M., Sippel A. E. Alternative sets of DNase I-hypersensitive sites characterize the various functional states of the chicken lysozyme gene. Nature. 1984 Sep 13;311(5982):163–165. doi: 10.1038/311163a0. [DOI] [PubMed] [Google Scholar]
- Greer P., Maltby V., Rossant J., Bernstein A., Pawson T. Myeloid expression of the human c-fps/fes proto-oncogene in transgenic mice. Mol Cell Biol. 1990 Jun;10(6):2521–2527. doi: 10.1128/mcb.10.6.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson P., Wright D. H., Jones D. B. Malignant lymphoma of true histiocytic (monocyte/macrophage) origin. Cancer. 1983 Jan 1;51(1):80–91. doi: 10.1002/1097-0142(19830101)51:1<80::aid-cncr2820510118>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Hotchkiss J. A., Rorvik M. C., Allison D. P., Foote L. J. Rat monoclonal antibodies to mouse lung components for analysis of fibrosis. Exp Mol Pathol. 1987 Aug;47(1):110–124. doi: 10.1016/0014-4800(87)90012-8. [DOI] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Rep M., Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987 Dec;26(6):653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Molecular genetics of chronic granulomatous disease. Annu Rev Immunol. 1989;7:277–307. doi: 10.1146/annurev.iy.07.040189.001425. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Walker L. E., Allen R. A., Jesaitis A. J., Orkin S. H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988 May;85(10):3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parkos C. A., Walker L., Orkin S. H., Dinauer M. C., Jesaitis A. J. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989 Nov 9;342(6246):198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. C., Witte O. N. The role of multiple oncogenes in hematopoietic neoplasia. Mutat Res. 1988 May;195(3):245–253. doi: 10.1016/0165-1110(88)90003-6. [DOI] [PubMed] [Google Scholar]
- Skalnik D. G., Orkin S. A rapid method for characterizing transgenic mice. Biotechniques. 1990 Jan;8(1):34–34. [PubMed] [Google Scholar]
- Staudt L. M., Lenardo M. J. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]