Abstract
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare but life-threatening reaction to drugs such as carbamazepine and allopurinol. The condition is characterized by skin rashes, fever, hematological disturbances, lymphadenopathy, and organ failure, most probably hepatic dysfunction. To date, only a few cases of valproate-induced DRESS syndrome have been reported.
Case Presentation
We report on the case of a 60-year-old man who had been treated with valproic acid some time before being referred to Kowsar Hospital, Semnan, Iran in December 2015. He was given valproic acid 1000 mg PO, and after 20 days, he had developed widespread rashes, fever, esophagitis, cervical lymphadenopathy, and tender hepatomegaly. Laboratory results at Kowsar showed a drop in hemoglobin, in addition to lymphocytosis, thrombocytopenia, and elevated serum transaminases. DRESS was diagnosed, and corticosteroid therapy was initiated. Administration of the culprit drug to the patient was also stopped. Intravenous immunoglobulin (IVIG) improved the general condition of the patient.
Conclusions
Only a small number of case reports have described valproic acid-induced DRESS syndrome; therefore, the condition is difficult to prevent. Rechallenge with valproic acid should be avoided in patients with a history of reaction to the drug.
Keywords: Rash, Fever, Liver, Valproic Acid, DRESS
1. Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare and life-threatening hypersensitivity reaction to drugs. The incidence of DRESS is about one in 1,000 to one in 10,000 drug exposures (1). The mortality rate associated with the condition is 10 - 20%, which is chiefly due to severe liver dysfunction and renal failure. The condition is characterized by the delayed onset of symptoms, which typically occur two to six weeks after the initiation of treatment. Skin rashes, fever, lymphadenopathy, eosinophilia, and involvement of the liver and kidney are very often seen in patients with DRESS (2). Several mechanisms, for example, a genetic predisposition to immune reactions, and the reactivation of viruses like cytomegalovirus, the Epstein Barr virus, and the herpes virus, are believed to be associated with the pathogenesis of DRESS. Another proposed causative mechanism is the accumulation of toxic metabolites due to a failure in the metabolism of drugs (3). More than 50 drug types have been reported to cause DRESS, which include aromatic anticonvulsants (carbamazepine and lamotrigine), antibiotics, sulfa derivatives, analgesics, and antidepressants (4). DRESS is diagnosed primarily on the basis of the following RegiSCAR criteria: an acute rash, fever above 38°C, lymphadenopathy at two sites, the involvement of at least one internal organ, and abnormalities in the lymphocyte and eosinophil counts (5). The usual treatment for DRESS is discontinuation of the causative drug and the use of corticosteroids. Valproate is an anti-seizure drug and is considered to be the best initial choice. It is frequently used to prevent and treat different kinds of seizures. Gastrointestinal upset, sedation, ataxia, and tremor are the most common toxicities of valproate (6). However, in contrast to phenytoin and lamotrigine, very few studies have reported valproate-induced DRESS syndrome. Here, we describe a case of valproate-induced DRESS syndrome with hepatitis and pleural effusion.
2. Case Presentation
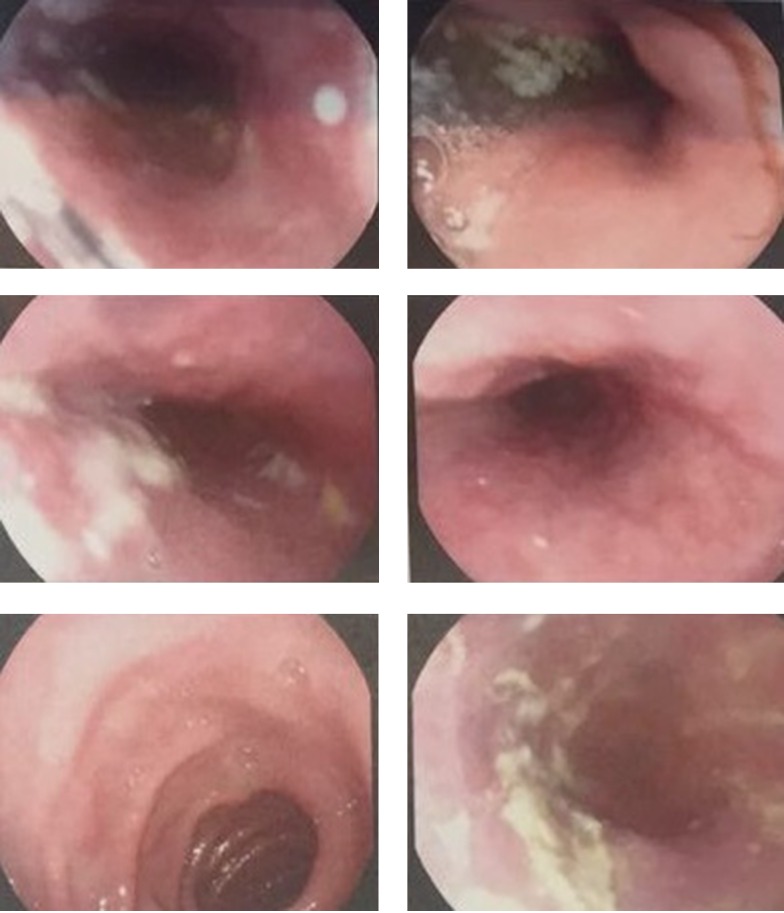
In December 2015, a sixty-year-old man was referred to Kowsar Hospital in Semnan, Iran, which is a specialized, government referral hospital. The patient was complaining of skin rash, fever, a distended abdomen, and severe pruritus, which had been going on for 10 days. He had a history of asthma and was using inhaled steroids. His history had not been remarkable until four months prior to his admission. The report showed that the patient had been admitted to another hospital four months previously complaining of a severe headache. A subarachnoid hemorrhage (SAH) was diagnosed by the neurologist and the patient was given valproic acid (Depakene®, Abbvie, US) 500 mg PO once daily to prevent seizures. Twenty days before admission to our hospital, the dose of valproic acid increased to 1000 mg PO once daily. In addition, he was using 26 units of insulin glargine (Lantus®, Sanofi Aventis, France) and 22 units of insulin aspart (Novorapid®, Novo Nordisk, Denmark) for three months. He had no history of drug allergies. On admission to Kowsar Hospital, physical examinations showed that the patient was conscious and well nourished, and had no signs of respiratory distress. He had erythematous maculopapular rashes covering the body, severe pruritus, and no mucosal involvement. He was febrile, with a temperature of 39°C. The patient’s other vital signs were normal. He also had bilateral cervical lymphadenopathy, with lymph nodes of 1 × 2 cm in size that showed slight tenderness. Tender hepatomegaly was observed. Based on these findings, the patient was admitted to the department of internal medicine, section 2, bed number 9. His clinical characteristics are presented in Table 1. The suspected diagnosis of the patient included skin vasculitis, drug eruption, fascioliasis, and Churg-Strauss syndrome, as well as viral infections such as cytomegalovirus and toxoplasmosis. Blood and urine cultures were negative for bacteria. The patient was given an antihistamine and hydrocortisone 30 mg PO on admission day, and the rash and fever subsided. After two days, rash and severe pruritus were seen again with transient fever. The previous treatments, including the insulin and valproic acid, were not stopped. On day 2, prednisolone 45 mg PO was administered. On day 4, purpura and petechia were present on the hands, and a few vesicles were present on the inguinal area and the thigh. Insulin and valproic acid were discontinued. A skin biopsy showed a high infiltration of lymphocytes and scattered eosinophils and neutrophils with focal exocytosis and spongiosis. Mucosal involvement was also present, and esophageal candidiasis was diagnosed by endoscopy (Olympus, Japan) (Figure 1). Fluconazole was initiated to treat the candidiasis (200 mg PO on diagnosis and 100 mg QD thereafter). Due to the patient’s consumption of local vegetables, anthelminthic therapy with albendazole (400 mg PO) was introduced but did not lead to a response. A respiratory examination revealed reduced sounds at both lungs. Due to bilateral costophrenic angle blunting, a chest CT scan (GE Healthcare, USA) was performed, and showed bilateral pleural effusion (Figure 2). Serologies for cytomegalovirus and brucellosis were negative. A significant drop was seen in hemoglobin concentration and platelet numbers, while bone marrow biopsy was negative for blood malignancies. Sonography showed a severe gaseous abdomen suggesting grade 1 fatty liver. According to the RegiSCAR scoring system, the patient had a score of six points, which indicated a definitive diagnosis of DRESS syndrome. On day 10, DRESS was diagnosed. Due to unsuccessful treatments with corticosteroids and a rise in hepatic transaminase to more than 1000 IU/L, intravenous immunoglobulin (IVIG) of 2 gr/kg (Intratect®, Biotest Pharma, Germany) was administered for three consecutive days. On day 13, the general condition of the patient was found to have improved. On day 16, he was discharged with stable laboratory findings and diminished skin rashes (Figure 3). He also remained afebrile. There was considerable improvement in his respiratory symptoms and hepatitis. In addition, the patient’s general condition remained satisfactory for the two-month follow-up period.
Table 1. Clinical Characteristics of the Patient.
| Characteristic | Value |
|---|---|
| Age, y | 60 |
| Gender | Male |
| Medications | |
| Valproate | |
| Insulin | |
| Laboratory parameters | |
| Glucose, mg/dl | 120 |
| LDL, mg/dL | 180 |
| HDL, mg/dL | 45 |
| Cholesterol, mg/dL | 189 |
| Bilirubin total, mg, % | 10.9 |
| AST, IU/L | 285 |
| ALT, IU/L | 561 |
| ALP, IU/L | 320 |
| LDH, IU/L | 734 |
| BUN, mg/dL | 16 |
| Creatinine, mg/dL | 1.1 |
| White blood cell count, U/L | 15 × 103 |
| Lymphocyte, U/L | 7.6 × 103 |
| Eosinophil, U/L | 2.1 × 103 |
| HGB, g/dL | 10.9 |
| PLT, U/L | 129 × 103 |
| CRP, mg/dL | 30 |
Figure 1. Endoscopy of the Esophagus Showing the Presence of Candidiasis.
Figure 2. Lung CT scan showing pleural effusion.
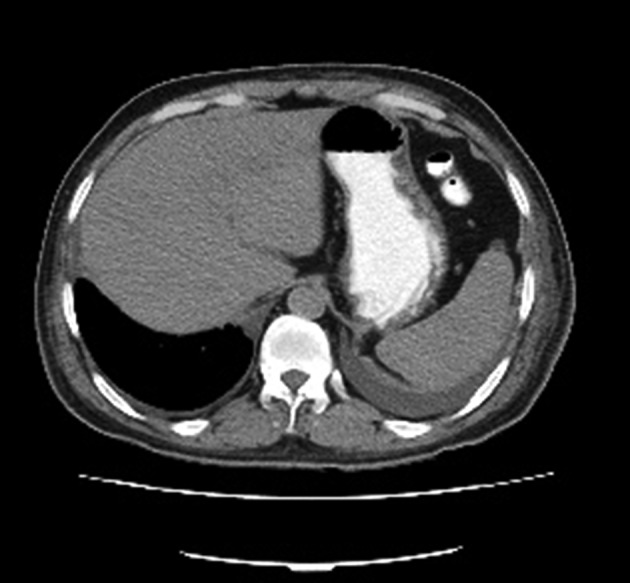
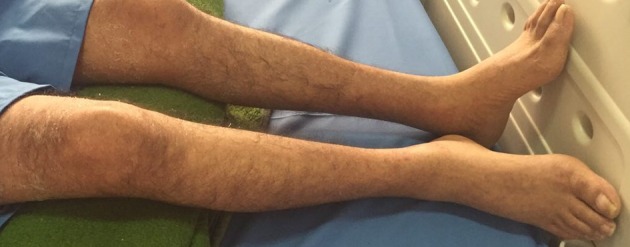
Figure 3. Fading of Skin Rashes After Treatment with IVIG.
3. Discussion
DRESS syndrome is a hypersensitivity reaction to drugs. It is considered a significant complication due to its high rate of mortality. DRESS syndrome, Stevens-Johnson syndrome, and acute generalized exanthematous pustulosis are skin eruptions that can be fatal because they lead to life-threatening organ failures. DRESS syndrome is characterized by sudden skin rashes, fever, blood disturbances, lymphadenopathy, and involvement of the internal organs, most often the liver and the lungs (1, 2). Liver failure is the main cause of death, and it may vary from hepatocellular and cholestatic damage to fulminant hepatic failure (FHF) (3). The pathogenesis of DRESS is multifactorial and remains obscure. The reactivation of viruses, genetic predisposition, and defects in the metabolism of drugs have been proposed as possible factors in the pathogenesis of DRESS syndrome. It has been demonstrated that regulatory T (Treg) cells expand at the acute stage of DRESS, and this functional defect in Treg cells could be responsible for the development of autoimmune diseases that may occur months to years after the resolution of DRESS (3, 7). The causative drugs most commonly associated with DRESS are carbamazepine, phenytoin, allopurinol, sulfasalazine, and abacavir (4). Cacoub’s study showed that of a total of 44 drugs associated with the 172 cases reported between 1997 and 2009, the most frequently reported causative drug was carbamazepine (5). In the vast majority of reported cases of DRESS, aromatic anticonvulsants like carbamazepine and phenytoin have been the culprit drugs. It is suggested that aromatic anticonvulsants can initiate DRESS in individuals with a defect in the epoxide hydrolase enzyme, which can cause an accumulation of arene oxides. This compound is able to trigger various responses (8). Dreesman et al. reported a case of DRESS induced by valproic acid with multiple organ dysfunction (9), while Arevalo-Lorido et al. described a patient with severe DRESS caused by valproate (10). Bota et al. discussed the case of a 25-year-old woman with bipolar disorder who experienced DRESS caused by valproic acid three weeks after the initiation of treatment (11). Similarly, Bin Nakhi et al. described two cases of DRESS onset. In one case, the patient was given carbamazepine, and in the other, the patient received a combination of lamotrigine and valproic acid. For both, the treatments were discontinued and supportive care was administered (12). DRESS is typically diagnosed using the RegiSCAR criteria, and patients must fulfill three of the four main criteria (1). Another method is the Japanese consensus group criteria system, which requires seven of the system’s nine criteria or all of the first five to be met for a diagnosis to be made (3). Case criteria are graded as not met, possibly met, probably met, or definitely met by the RegiSCAR scoring system. Scores of five or more are classified clearly as DRESS syndrome. In our case, the score was seven points, and the condition was therefore classified definitively as DRESS syndrome. Discontinuation of the offending drug is the primary treatment. In addition, corticosteroids are routinely used to suppress systemic symptoms. It is noteworthy that there is no consensus regarding the medical treatments of DRESS. As suggested, systemic corticosteroids are reserved to treat severe DRESS with gross manifestations; however, due to the high likelihood of relapse, most clinicians prefer to use systemic corticosteroids even for mild forms of DRESS (13). At present, the type and dose of corticosteroid, and the duration of treatment are not conclusive. A common regimen is parenteral steroids followed by an oral steroid. Interestingly, our case did not respond even to a high dose of corticosteroids. To avoid fulminant hepatitis and to reduce severe symptoms, IVIG was initiated. Three doses of IVIG improved the general condition of the patient. To the best of our knowledge, only five cases of valproic acid-induced DRESS syndrome have been reported so far. However, while DRESS syndrome is rare, it can be fatal. Therefore, clinicians must be aware of the presence of cross-reactivity between aromatic anticonvulsants and valproic acid. In addition to corticosteroids, the efficacy of other immunosuppressive agents needs to be further investigated for the treatment of DRESS.
Acknowledgments
The authors wish to thank the Kowsar Hospital staff for all of their efforts.
Footnotes
Authors’ Contribution:Mahboubeh Darban managed the case and collected the data and revised the manuscript. Bahador Bagheri collected the data, and drafted and edited the manuscript.
References
- 1.Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145(1):67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156(3):609–11. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 3.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36(1):6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourgeois GP, Cafardi JA, Groysman V, Hughey LC. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66(6):229–36. doi: 10.1016/j.jaad.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–97. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087–100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 7.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27(8):387–93. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Moling O, Tappeiner L, Piccin A, Pagani E, Rossi P, Rimenti G, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir--a hypothesis. Med Sci Monit. 2012;18(7):57–62. doi: 10.12659/MSM.883198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreesman A, Hoorens A, Hachimi-Idrissi S. Multiple organ dysfunction syndrome: infection or hypersensitivity reaction? Eur J Emerg Med. 2010;17(4):228–9. doi: 10.1097/MEJ.0b013e3283311f04. [DOI] [PubMed] [Google Scholar]
- 10.Arevalo-Lorido JC, Carretero-Gomez J, Bureo-Dacal JC, Montero-Leal C, Bureo-Dacal P. Antiepileptic drug hypersensitivity syndrome in a patient treated with valproate. Br J Clin Pharmacol. 2003;55(4):415–6. doi: 10.1046/j.1365-2125.2003.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bota RG, Ligasan AP, Najdowski TG, Novac A. Acute hypersensitivity syndrome caused by valproic Acid: a review of the literature and a case report. Perm J. 2011;15(2):80–4. doi: 10.7812/tpp/10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bin-Nakhi HA, Sadeq S, Pinto RG, Habeeb Y. Anticonvulsant hypersensitivity syndrome: report of 2 cases from Kuwait. Med Princ Pract. 2003;12(3):197–9. doi: 10.1159/000070760. [DOI] [PubMed] [Google Scholar]
- 13.Funck-Brentano E, Duong TA, Bouvresse S, Bagot M, Wolkenstein P, Roujeau JC, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72(2):246–52. doi: 10.1016/j.jaad.2014.10.032. [DOI] [PubMed] [Google Scholar]