Abstract
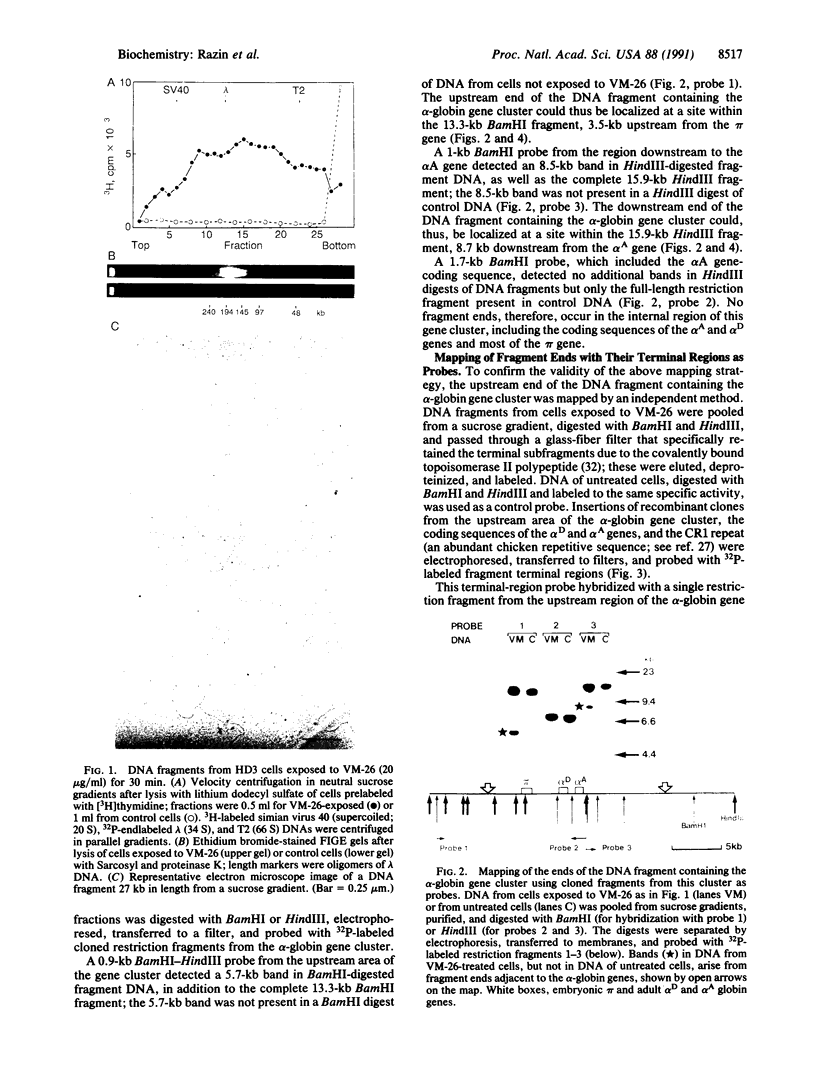
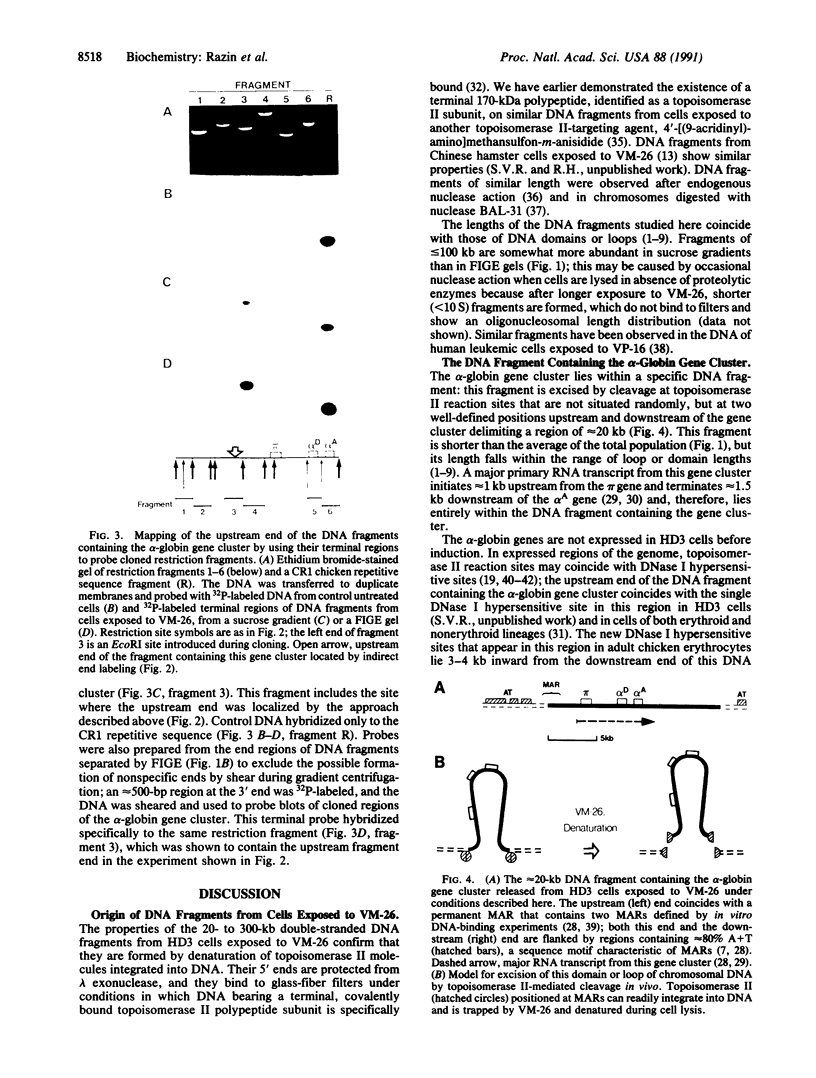
We have mapped the position of the alpha-globin gene cluster in the 20- to 300-kilobase fragments of chromosomal DNA isolated from growing chicken HD3 erythroblastoid cells exposed to 4'-demethylepipodophyllotoxinthenylidene beta-D-glucoside. This epipodophyllotoxin traps functioning topoisomerase II molecules, the denaturation of which cleaves DNA and reveals their reaction sites. The DNA fragments, prepared by centrifugation in sucrose gradients, bind selectively to glass-fiber filters and are protected from lambda 5'-exonuclease, properties compatible with the presence of a topoisomerase II subunit bound to their 5' ends. Restriction enzyme cleavage of the fragments and hybridization with cloned alpha-globin-region probes reveal additional distinctive bands not seen in control DNA, allowing the localization of fragment ends near this gene cluster. The terminal regions of fragments from sucrose gradients or from field-inversion electrophoresis gels were also used to probe cloned regions of the gene cluster. Both approaches show that this cluster of three genes, which is not expressed in these cells, is located at a specific position in a approximately 20-kilobase DNA fragment. The upstream end of this fragment lies in a region that contains a site of DNA attachment to the nuclear matrix mapped by both in vivo and in vitro methods, and its downstream end is flanked by approximately 80% A + T sequences characteristic of matrix-attachment regions. These observations suggest that the DNA fragments are formed because topoisomerase II molecules can specifically and readily integrate into DNA at matrix-attachment regions and that the fragments represent entire DNA loops or domains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Käs E., Laemmli U. K. Preferential, cooperative binding of DNA topoisomerase II to scaffold-associated regions. EMBO J. 1989 Dec 20;8(13):3997–4006. doi: 10.1002/j.1460-2075.1989.tb08582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Blasquez V. C., Sperry A. O., Cockerill P. N., Garrard W. T. Protein:DNA interactions at chromosomal loop attachment sites. Genome. 1989;31(2):503–509. doi: 10.1139/g89-098. [DOI] [PubMed] [Google Scholar]
- Broders F., Zahraoui A., Scherrer K. The chicken alpha-globin gene domain is transcribed into a 17-kilobase polycistronic RNA. Proc Natl Acad Sci U S A. 1990 Jan;87(2):503–507. doi: 10.1073/pnas.87.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Charron M., Hancock R. Chromosome recombination and defective genome segregation induced in Chinese hamster cells by the topoisomerase II inhibitor VM-26. Chromosoma. 1991 Feb;100(2):97–102. doi: 10.1007/BF00418242. [DOI] [PubMed] [Google Scholar]
- Charron M., Hancock R. DNA topoisomerase II is required for formation of mitotic chromosomes in Chinese hamster ovary cells: studies using the inhibitor 4'-demethylepipodophyllotoxin 9-(4,6-O-thenylidene-beta-D-glucopyranoside). Biochemistry. 1990 Oct 16;29(41):9531–9537. doi: 10.1021/bi00493a006. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Churnikov N. A., Ponomarenko N. A., Airikh L. G. Vydelenie i kharakteristika spetsificheskoi fraktsii khromosomnoi DNK cheloveka--forum DNK. Dokl Akad Nauk SSSR. 1988;303(2):491–493. [PubMed] [Google Scholar]
- Clark R. W., Tseng P. O., Lechuga J. M. A nuclease-derived fragment of metaphase DNA and its relationship to the replicon. Exp Cell Res. 1987 Apr;169(2):296–310. doi: 10.1016/0014-4827(87)90192-3. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A., Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976 Nov;22(2):303–324. doi: 10.1242/jcs.22.2.303. [DOI] [PubMed] [Google Scholar]
- Drake F. H., Zimmerman J. P., McCabe F. L., Bartus H. F., Per S. R., Sullivan D. M., Ross W. E., Mattern M. R., Johnson R. K., Crooke S. T. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987 Dec 5;262(34):16739–16747. [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the closely linked adult chicken alpha-globin genes in recombinant DNAs. Proc Natl Acad Sci U S A. 1980 May;77(5):2596–2600. doi: 10.1073/pnas.77.5.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache G., Razin S. V., Rzeszowska-Wolny J., Moreau J., Targa F. R., Scherrer K. Mapping of structural and transcription-related matrix attachment sites in the alpha-globin gene domain of avian erythroblasts and erythrocytes. Mol Cell Biol. 1990 Oct;10(10):5349–5358. doi: 10.1128/mcb.10.10.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laroche T., Falquet J., Boy de la Tour E., Laemmli U. K. Metaphase chromosome structure. Involvement of topoisomerase II. J Mol Biol. 1986 Apr 20;188(4):613–629. doi: 10.1016/s0022-2836(86)80010-9. [DOI] [PubMed] [Google Scholar]
- Hartwig M. Organization of mammalian chromosomal DNA: supercoiled and folded circular DNA subunits from interphase cell nuclei. Acta Biol Med Ger. 1978;37(3):421–432. [PubMed] [Google Scholar]
- Jackson D. A., Dickinson P., Cook P. R. The size of chromatin loops in HeLa cells. EMBO J. 1990 Feb;9(2):567–571. doi: 10.1002/j.1460-2075.1990.tb08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Levy-Wilson B., Fortier C. The limits of the DNase I-sensitive domain of the human apolipoprotein B gene coincide with the locations of chromosomal anchorage loops and define the 5' and 3' boundaries of the gene. J Biol Chem. 1989 Dec 15;264(35):21196–21204. [PubMed] [Google Scholar]
- Marshall B., Ralph R. K. The mechanism of action of mAMSA. Adv Cancer Res. 1985;44:267–293. doi: 10.1016/s0065-230x(08)60029-9. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., Hancock R. Chromosomol DNA fragments from mouse cells exposed to an intercalating agent contain a 175-kdalton terminal polypeptide. Can J Biochem Cell Biol. 1985 Jul;63(7):780–783. doi: 10.1139/o85-099. [DOI] [PubMed] [Google Scholar]
- Razin S. V. DNA interactions with the nuclear matrix and spatial organization of replication and transcription. Bioessays. 1987 Jan;6(1):19–23. doi: 10.1002/bies.950060106. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Rzeszowska-Wolny J., Moreau J., Scherrer K. Lokalizatsiia uchastkov prikrepleniia DNK k iadernomu skeletu v ramkakh domena alpha-globinovykh genov kur v funktsional'no aktivnykh i funktsional'no neaktivnykh iadrakh. Mol Biol (Mosk) 1985 Mar-Apr;19(2):456–466. [PubMed] [Google Scholar]
- Razin S. V., Vassetzky Y. S., Hancock R. Nuclear matrix attachment regions and topoisomerase II binding and reaction sites in the vicinity of a chicken DNA replication origin. Biochem Biophys Res Commun. 1991 May 31;177(1):265–270. doi: 10.1016/0006-291x(91)91977-k. [DOI] [PubMed] [Google Scholar]
- Reitman M., Felsenfeld G. Developmental regulation of topoisomerase II sites and DNase I-hypersensitive sites in the chicken beta-globin locus. Mol Cell Biol. 1990 Jun;10(6):2774–2786. doi: 10.1128/mcb.10.6.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge M., Th'ng J., Hamaguchi J., Bradbury E. M. The topoisomerase II inhibitor VM-26 induces marked changes in histone H1 kinase activity, histones H1 and H3 phosphorylation, and chromosome condensation in G2 phase and mitotic BHK cells. J Cell Biol. 1990 Nov;111(5 Pt 1):1753–1762. doi: 10.1083/jcb.111.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T. C., Wang J. C., Liu L. F. In vivo localization of DNA topoisomerase II cleavage sites on Drosophila heat shock chromatin. Mol Cell Biol. 1986 Apr;6(4):985–992. doi: 10.1128/mcb.6.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C. G., Snapka R. M. Patterns of strongly protein-associated simian virus 40 DNA replication intermediates resulting from exposures to specific topoisomerase poisons. Biochemistry. 1990 Dec 11;29(49):10934–10939. doi: 10.1021/bi00501a011. [DOI] [PubMed] [Google Scholar]
- Sperry A. O., Blasquez V. C., Garrard W. T. Dysfunction of chromosomal loop attachment sites: illegitimate recombination linked to matrix association regions and topoisomerase II. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5497–5501. doi: 10.1073/pnas.86.14.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumph W. E., Kristo P., Tsai M. J., O'Malley B. W. A chicken middle-repetitive DNA sequence which shares homology with mammalian ubiquitous repeats. Nucleic Acids Res. 1981 Oct 24;9(20):5383–5397. doi: 10.1093/nar/9.20.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy A., Schedl P., Sander M., Hsieh T. S. Topoisomerase II cleavage in chromatin. J Mol Biol. 1986 Sep 20;191(2):231–246. doi: 10.1016/0022-2836(86)90260-3. [DOI] [PubMed] [Google Scholar]
- Vassetzky Y. S., Jr, Razin S. V., Georgiev G. P. DNA fragments which specifically bind to isolated nuclear matrix in vitro interact with matrix-associated DNA topoisomerase II. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1263–1268. doi: 10.1016/0006-291x(89)92246-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Beug H., Groudine M., Graf T. Temperature-sensitive changes in the structure of globin chromatin in lines of red cell precursors transformed by ts-AEV. Cell. 1982 Apr;28(4):931–940. doi: 10.1016/0092-8674(82)90072-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]
- Zechiedrich E. L., Osheroff N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 1990 Dec;9(13):4555–4562. doi: 10.1002/j.1460-2075.1990.tb07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]