Abstract
A simple, nonisotopic, semiautomated bioassay for the measurement of antimalarial drug levels in plasma or serum based on the quantitation of histidine-rich protein II in malaria culture is presented. The assay requires only small sample volumes and was found to be highly sensitive and reproducible. The results closely paralleled those obtained with isotopic bioassays (R = 0.988, P < 0.001) and high-performance liquid chromatography-electrochemical detection (R = 0.978, P < 0.001).
Bioassays are essentially unspecific, a major advantage for drugs whose metabolites have not been fully identified, a problem not uncommon with antimalarial drugs. They measure the antimalarial activity of the parent compound and all of its active metabolites rather than actual drug levels of a single chemical compound. They have in common the use of Plasmodium falciparum parasite growth to measure drug exposure while differing in the way in which this parameter is assessed (10). This is traditionally achieved by isotopic techniques (3, 8, 9, 11) or microscopic assessment of parasite growth (2, 4). The procedures are similar to those used in drug sensitivity testing (7). Recently developed P. falciparum in vitro growth assays are based on measurement of parasite biomass with a simple sandwich enzyme-linked immunosorbent assay (ELISA) (6). These assays do not involve handling of radioactive material, are semiautomated, require relatively little technical equipment, and are considerably less labor-intensive than morphological assays.
Study samples.
The plasma samples tested originated from two uncomplicated falciparum malaria patients and a healthy Thai volunteer treated with oral sodium artesunate (AS; 100 mg followed by 50 mg orally every 12 h for 5 days). The study protocols were approved by the appropriate ethical review boards, and written informed consent was obtained from all study participants.
Test plates and sample preparation.
The complement-inactivated samples were serially diluted and applied in two columns to 96-well microculture plates at 50 μl/well (i.e., a sample volume of only 200 μl was required for each test to be performed in duplicate). In addition, two columns with serial dilutions of spiked plasma were added to each plate as controls. On each 96-well plate, five unknown samples plus the controls were therefore tested. In addition to the plates with unknown samples, one plate was dosed with six serial dilutions in duplicate of known drug concentrations covering the whole test range (in this case, the starting concentrations were 100, 50, 25, 12.5, 5, and 2.5 ng of dihydroartemisinin [DHA]/ml). The 50% dilution factors from this plate were used to draw the standard curve, which is the basis for the calculation of all drug concentrations (8).
P. falciparum parasites and culture.
The culture and ELISA procedures used largely followed those established for the histidine-rich protein II (HRP2) drug sensitivity assay (6). Synchronized parasitized (clone W2) blood samples from continuous culture were diluted to a 0.1% parasite density and a 1.7% hematocrit. Of this cell medium mixture, 175 μl was added to each well (resulting in a total of 225 μl of fluid per well), and 1.05 ml of the remaining cell medium mixture per plate was mixed with 0.3 ml of drug-free plasma and frozen as negative controls. The plates were then incubated for 72 h in a candle jar or gas mixture (5% CO2, 5% O2, 90% N2) at 37°C before being frozen-thawed.
HRP2 ELISA.
A commercial HRP2 ELISA kit (Malaria Ag CELISA; Cellabs Pty. Ltd., Brookvale, New South Wales, Australia) was used for quantification of HRP2 in the culture samples as described previously (6). However, basically any HRP2-specific ELISA may be used. The complete ELISA takes about 3 h to perform.
Isotopic bioassay and HPLC-ECD.
The isotopic bioassay and high-performance liquid chromatography (HPLC)-electrochemical detection (ECD) were performed in accordance with the standard procedures established at the Armed Forces Research Institute of Medical Sciences (5, 8, 9).
Standard operating procedures and updated information for the HRP2 bioassay are available at http://malaria.farch.net.
Results. A total of 29 samples from three subjects (SA8, SA30, and SAN1) were tested with the new HRP2 bioassay, with an isotopic bioassay, and by HPLC-ECD. For the HPLC-ECD results, drug levels of DHA and AS were measured separately and the results are given as cumulative results for both drugs. The bioassay results are expressed as nanomolar DHA activity equivalents.
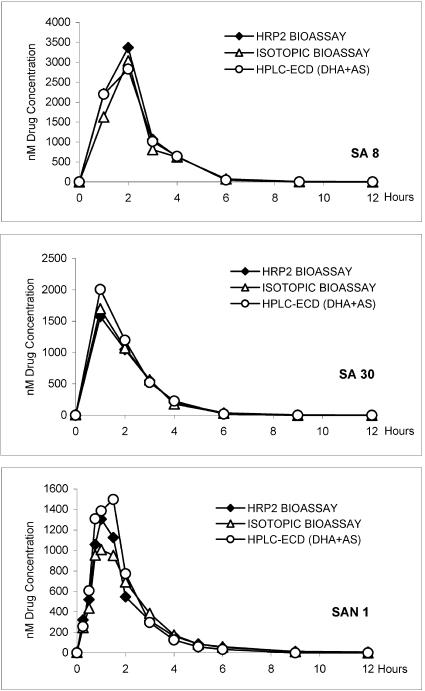
Peak drug levels (2,829 to 3,368, 1,574 to 2,006, and 1,008 to 1,497 nM for SA8, SA30, and SAN1, respectively) were reached within the first 2 h after drug administration (Fig. 1). The drug concentrations showed a steep decrease in the following hours, reaching concentrations below 100 nM before 6 h. The HPLC-ECD results showed an initial smaller peak for AS within the first hour, while DHA peaked somewhat later. The cumulative results for both drugs (AS plus DHA) closely followed those of the bioassays.
FIG. 1.
Drug profiles over a period of 12 h for two malaria patients (SA8 and SA30) and one healthy volunteer (SAN1) tested with the HRP2 bioassay, with an isotopic bioassay, and by HPLC-ECD. Results of both bioassays are given as nanomolar DHA activity equivalents, and HPLC-ECD results are given as cumulative concentrations of DHA and AS.
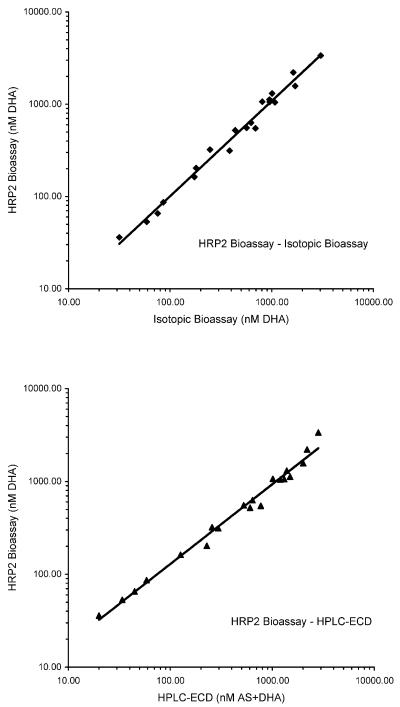
In a correlation analysis, the pooled results obtained with the HRP2 bioassay showed a distinct linear association with those obtained by HPLC-ECD (n = 29; R = 0.978; P < 0.001) and the isotopic bioassay (n = 29; R = 0.988; P < 0.001) (Fig. 2).
FIG. 2.
Scatter plots of the linear associations of drug levels determined by the HRP2 bioassay and HPLC-ECD (n = 29; R = 0.978; P < 0.001), as well as the HRP2 bioassay and an isotopic bioassay (n = 29; R = 0.988; P < 0.001).
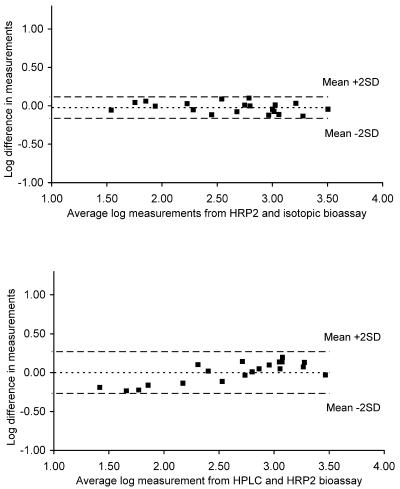
When Bland-Altman plots were used to measure the agreement of the results obtained with the HRP2 bioassay and the HPLC-ECD, the mean difference on a log scale was 0.00, with limits of agreement of −0.269 and 0.271 (Fig. 3). For the agreement between the HRP2 bioassay and the isotopic bioassay, the mean difference was −0.02 (limits of agreement, −0.164 and 0.118).
FIG. 3.
Bland-Altman plots of the agreement of plasma level measurements from the HRP2 bioassay and the isotopic bioassay (mean difference on a log scale, −0.02; limits of agreement, −0.164 and 0.118), as well as from the HRP2 bioassay and HPLC-ECD (mean difference on a log scale, 0.00; limits of agreement, −0.269 and 0.271).
Conclusions. A major problem in assessing antimalarial drug levels by conventional methods, such as HPLC or mass spectrometry, is that these methods require an extensive understanding of the drug's metabolites (1). In recent years this known issue led to the development of bioassays that are based on measurement of the antimalarial activity of all of the drug-related compounds found in body fluids after the administration of antimalarial drugs (2, 8).
AS was chosen for this study because this drug is almost entirely metabolized to DHA and both drugs may easily be measured by HPLC-ECD (12). The drug profiles measured with the HRP2 bioassay very closely followed the cumulative curves for AS plus DHA as determined by HPLC-ECD and the isotopic bioassay (8, 9). Both the HRP2 and isotopic bioassays are based on measurement of the metabolic activity of parasites exposed to plasma or serum samples containing drugs with antimalarial activity, and their procedures are closely related to those of drug sensitivity testing. The HRP2 bioassay uses a simple double-site sandwich ELISA to measure HRP2, an essential metabolic product of P. falciparum parasites, to determine parasite growth and its inhibition by antimalarial drugs (6). The major advantage of HRP2-based assays lies in the avoidance of the handling of radioactive material and the fact that these tests require considerably less technical equipment and infrastructure. An additional advantage lies in the long incubation times that also allow the testing of slow-acting drugs with antimalarial activity, such as antibiotics. A 200-μl sample volume is sufficient for duplicate measurements in the HRP2 bioassay, whereas a 1- to 2-ml sample volume is required for HPLC.
What at first sight seems to be the biggest disadvantage of bioassays, their complete lack of specificity, is at the same time also their biggest advantage. Unlike conventional methods for the measurement of drug levels in serum or plasma, bioassays measure all compounds that show antimalarial activity. This allows measurement of the antimalarial activity even of drugs whose metabolites are unknown. Ideally, bioassays should therefore be used to complement and not to replace traditional, highly specific methods, such as HPLC or mass spectrometry. As the kinetics of discrete metabolites are sometimes even more important than those of the parent compound, the HRP2 bioassay has much to offer for the assessment of a critical pharmacodynamic parameter, activity against the parasite.
Acknowledgments
We thank George Watt for providing the samples used in this study and Duangsuda Siriyanonda, Maneerat Rasameesoroj, and Kritsanai Yingyuen for technical assistance.
This work was supported by the U.S. Department of Defense Global Emerging Infection Surveillance program.
REFERENCES
- 1.Karbwang, J., and K. Na-Bangchang. 1993. Clinical pharmacokinetics, p. 37-54. In J. Karbwang and W. H. Wernsdorfer (ed.), Clinical pharmacology of antimalarials. Mahidol University, Bangkok, Thailand.
- 2.Kotecka, B. M., and K. H. Rieckmann. 1993. Chloroquine bioassay using malaria microcultures. Am. J. Trop. Med. Hyg. 49:460-464. [DOI] [PubMed] [Google Scholar]
- 3.Kotecka, B. M., K. H. Rieckmann, T. M. Davis, K. T. Batty, and K. F. Ilett. 2003. Comparison of bioassay and high performance liquid chromatographic assay of artesunate and dihydroartemisinin in plasma. Acta Trop. 87:371-375. [DOI] [PubMed] [Google Scholar]
- 4.Li, X., and K. Rieckmann. 1992. A bioassay for derivatives of qinghaosu (artemisinin). Trop. Med. Parasitol. 43:195-196. [PubMed] [Google Scholar]
- 5.Melendez, V., J. O. Peggins, T. G. Brewer, and A. D. Theoharides. 1991. Determination of the antimalarial arteether and its deethylated metabolite dihydroartemisinin in plasma by high-performance liquid chromatography with reductive electrochemical detection. J. Pharm. Sci. 80:132-138. [DOI] [PubMed] [Google Scholar]
- 6.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II, a novel approach to antimalarial drug susceptibility testing. Antimicrob. Agents Chemother. 46:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noedl, H., C. Wongsrichanalai, and W. H. Wernsdorfer. 2003. Malaria drug-sensitivity testing: new assays, new perspectives. Trends Parasitol. 19(4):175-181. [DOI] [PubMed] [Google Scholar]
- 8.Teja-Isavadharm, P., J. O. Peggins, T. G. Brewer, N. J. White, H. K. Webster, and D. E. Kyle. 2004. A Plasmodium falciparum bioassay for measurement of artemisinin derivatives in plasma or serum. Antimicrob. Agents Chemother. 48:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teja-Isavadharm, P., G. Watt, C. Eamsila, K. Jongsakul, Q. Li, D. Keeratithakul, N. Sirisopana, L. Loesuttiviboon, T. G. Brewer, and D. E. Kyle. 2001. Comparative pharmacokinetic-effect kinetics of oral sodium artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:717-721. [DOI] [PubMed] [Google Scholar]
- 10.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 190:792-794. [DOI] [PubMed] [Google Scholar]
- 11.Traore, B., E. Lazaro, and F. Gay. 1997. A bioassay for evaluating antimalarial activity and for measuring concentration in plasma. Trop. Med. Int. Health 2:929-933. [DOI] [PubMed] [Google Scholar]
- 12.White, N. J. 1994. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S41-S43. [DOI] [PubMed] [Google Scholar]