Abstract
Candida albicans adheres to host tissue and then proliferates in order to establish a commensal as well as a pathogenic state. Specific adherence to proteins is provided by several surface adhesins of Candida. Two well-studied proteins, Als1p and Als5p, do not require energy for adherence to occur (dead as well as living cells adhere) and have a multiplier effect of cell-cell aggregation that mediates the formation of microcolonies of Candida cells. The entire process is spontaneous, reversible, and stable for physiologically relevant chemical and physical forces. This adherence process is inhibited by the addition of free peptide ligands, including a 23-mer derived from fibronectin (Fn/23) that binds to the adhesins through H bond formation. Adherence was measured by determining the number of yeast cells that adhered to 90-μm-diameter polyethylene glycol (PEG) beads with a 7-mer peptide (KLRIPSV) synthesized on the surfaces of the beads. The concentration of the Fn/23 peptide that inhibited the adherence of cells to the peptide-coated beads by 50% was 4 to 5 μM, and the magnitudes of adherence were similar regardless of the presence or absence of physiologic salt concentrations. The minimum fungicidal concentration of Fn/23 was 2 to 4 μM in water, but there was no killing in physiologic salt concentrations. Peptides from the C and N termini or the center sequence of Fn/23 had no effect on inhibition of adherence and little effect on fungal viability. The fungicidal effect was similar to that seen with 23-, 19-, and 18-mer peptides derived from porcine myeloid cells, a Helicobacter pylori ribosomal protein, and a hybrid of cecropin and magainin, respectively. However, these fungicidal peptides did not inhibit C. albicans adherence to the peptide-coated PEG beads. This dual property of Fn/23, i.e., inhibition of adherence and killing of C. albicans, may provide important adjuvant effects in the treatment of disease caused by this fungus.
Candida albicans is a commensal microorganism of healthy human mucosal surfaces that, in the event of impairment of normal host immunity, can cause trivial to life-threatening opportunistic infections (2, 18). Adherence to host tissue is a prerequisite for establishment of a commensal as well as a pathogenic relationship. The Als proteins are cell surface adhesins capable of forming rapid and extremely stable H bond-dependent associations with host proteins and peptides (5). These associations have been termed stable, reversible, and specific (SRS) adherence and are a property of both Als proteins studied to date, Als1p and Als5p (5). Recognition of ligands by Als1p and Als5p is degenerate (i.e., not highly specific); hence, there is a multitude of potential ligand targets for these adhesins (9). Adherence of C. albicans to a protein target is a spontaneous property of these adhesins. Following adherence, there is a global surface conformational shift of the microorganism, which leads to the spontaneous aggregation of nearby cells into microcolonies (19). This process occurs without expenditure of energy and is a property shared by live as well as dead fungi (5, 19). The aggregation and adherence of cells are reversible upon the addition of formamide or urea or an increase in the pH to 9 or greater, all of which are factors that break hydrogen bonds (4).
Previous work established that an ∼20-mer peptide (formerly available commercially as PepTite 2000 [Telios Corporation, San Diego, Calif.]) is an extremely effective inhibitor of the adherence of C. albicans to immobilized extracellular matrix proteins in vitro (11). This peptide was developed from the cell-binding domain of fibronectin (containing arginine-glycine-aspartic acid [RGD]) and possessed features that allowed for effective adsorption on plastic and other nonbiological surfaces (i.e., it possessed a hydrophobic domain and other sequences to enhance adsorption to charged surfaces). Paradoxically, when PepTite 2000 was placed on plastic, C. albicans could not adhere to the surface (8). In addition, this peptide was shown to be an effective adjuvant in the treatment of disseminated candidiasis in rabbits (11), which was attributed to inhibition of the adherence of yeast cells to host tissue, likely by binding to C. albicans cells and blocking their ability to adhere to proteins or peptides. In those studies, PepTite 2000 was diluted in physiologic salt solutions and had no detectable effect on C. albicans growth or viability.
Once C. albicans adherence has occurred, survival and reproduction become important determinants in maintaining the commensal or pathogenic state. Part of the host defense includes naturally occurring antimicrobial peptides, such as defensins and cathelicidins, which may have potential for use in the treatment of the many presentations of candidiasis (1). These molecules are attractive due to their small size, their simplicity, and the speed with which they kill microorganisms. Killing occurs on contact in low- or no-salt concentrations. One such candidacidal peptide is PMAP-23, derived from porcine myeloid cells (12). This is a 23-mer peptide with several well-characterized derivatives that is broadly microbicidal (14). In this report, we compare the activities of Fn/23 (similar in composition to PepTite 2000, as reported in previous publications [8, 11]), which inhibits Candida adherence as well as kills the microorganism, with the properties of PMAP-23 and other fungicidal peptides that have no measurable effect upon adherence of fungi to proteins or peptides.
MATERIALS AND METHODS
Microorganisms, plasmids, and growth media.
A wild-type C. albicans strain (CA1) originally isolated from a human source was used in these studies and grown in liquid yeast extract-peptone-dextrose (YPD) (6, 10). Saccharomyces cerevisiae YPH499 (MATa ura3-52 lys2-801amber ade2-101ochre trp1-d63 his3-d200 leu2-d1) was obtained from the American Type Culture Collection and used as a heterologous host for the expression of a C. albicans adhesin, Als5p. S. cerevisiae YPH499 or S. cerevisiae YPH499 expressing Als5p were grown in liquid YP-raffinose-galactose media at 28°C prior to adherence assays, as described before (4). The vector of Als5p is a low-copy-number vector, and the gene is expressed from a GAL1 promoter. Cells were thoroughly washed with Tris-EDTA (TE) buffer by centrifugation and suspended at a concentration of ∼109/ml prior to use.
Peptides.
Fn/23 (GRGDSPASSKGGGGSRLLLLLLR) and its derivatives (RGDSPASSKP, SPASSKGGGGSRL, and GGGGSRLLLLLLR) were synthesized using 9-fluorenylmethoxy carbonyl (Fmoc) chemistry on a 72-column synthesizer, and quality assurance was provided by mass spectroscopy and high-performance liquid chromatography (Invitrogen, Carlsbad, Calif.). The PMAP-23 peptide (12, 14), RIIDLLWRVRRPQKPKFVTVWVR-NH2; an 18-mer hybrid of cecropin and magainin, KWKKLLKKPLLKKLLKKL-NH2 (17); and a 19-mer derived from Helicobacter pylori ribosomal protein L1, AKKVFKRLEKLFSKIWNWK-NH2 (13), were synthesized by one author (K. S. Hahm) by the solid-phase method using Fmoc chemistry. An amide 4-methyl benzhydrylamine resin was used as the support to obtain a C-terminal amidate peptide (14). The peptide KLRIPSV was synthesized on 90-μm-diameter polyethylene glycol (PEG) beads using Fmoc chemistry (9). Sequence analysis of peptides attached to the beads confirmed the identity of the peptide (Laboratory for Protein Sequencing and Analyses, Department of Chemistry, University of Arizona, Tucson).
Adherence assay.
For adherence assay purposes, 1 μl of culture concentrate containing ∼106 yeast cells was mixed with approximately 1,000 PEG beads (∼90 μm in diameter) in 1.5 ml of water, TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.0]), or Earle's balanced salt solution without Ca2+, Mg2+, or phenol red (EBSS) and placed in the wells of a six-well culture tray. The PEG beads had the adherent ligand KLRIPSV synthesized on their surfaces. KLRIPSV is a heptamer recognized by Als1p and Als5p as well as by C. albicans (9) and leads to adherence and aggregation of yeast cells on the beads. The tray was agitated horizontally at 150 rpm for 30 min. The tray was removed and examined microscopically, and counts were made of yeast cells that had adhered to PEG beads or an estimate of adherence was made and scored as follows: a score of 0 indicated no adherence, + indicated some adherence of yeast cells (20 to 25/bead), ++ indicated that the bead was almost covered by yeast cells (80 to 150/ bead), and +++ indicated adherence with cell-cell aggregation. Experiments were always done in triplicate and repeated a minimum of three times. In some of the experiments, all ingredients were added simultaneously, whereas in other assays, we were interested in desorption and reagents were added following adherence of the yeast cells to a bead surface.
Determination of killing.
Yeast cells were placed with the peptide of interest in a chosen buffer and mixed by placing the solution on a rocking platform for 30 min. The microbicidal action of the peptides was determined by staining cells with the vital dye methylene blue (0.05 mg/ml) (live cells exclude the dye [15]). Dye results were confirmed by culture on YPD agar. Mean numbers of CFU of treated yeast cells cultured on YPD agar (expressed as percentages of live cells) were as follows: 100% with no Fn/23 peptide (control), 100% with 0.04 μM Fn/23, 11% with 0.4 μM Fn/23, 4% with 2 μM Fn/23, and 0% with 4 and 8 μM Fn/23. Thus, the minimum fungicidal concentration is between 2 and 4 μM. The dye method is rapid, accurate, and easy to perform; therefore, viability results shown are those obtained by dye study only. Thus, adherence and viability could be monitored simultaneously by microscopy.
Statistical analysis.
The quantitative adherence and viability assays for each variable were performed a minimum of three times. Results are presented as means ± standard deviations and were analyzed by using a paired Student t test, with a P value of <0.05 being considered significant.
RESULTS
Peptides and adherence.
C. albicans (Fig. 1) and S. cerevisiae expressing Als5p readily adhered to and aggregated upon the surfaces of PEG beads coated with the heptamer KLRIPSV. Aggregation occurs through SRS adherence as described above. Prerequisites for SRS adherence include the presence of a sterically accessible peptide backbone in the target peptide or protein with at least five peptide bonds (7). S. cerevisiae not expressing Als proteins (Als1p and Als5p) does not adhere to the peptide-coated PEG beads, and neither C. albicans nor S. cerevisiae expressing Als proteins adhere to beads that lack the peptide. KLRIPSV was chosen as the target peptide because it is recognized by both Als proteins and elicits adherence followed by cell-to-cell aggregation by C. albicans and S. cerevisiae expressing the two Als proteins (9).
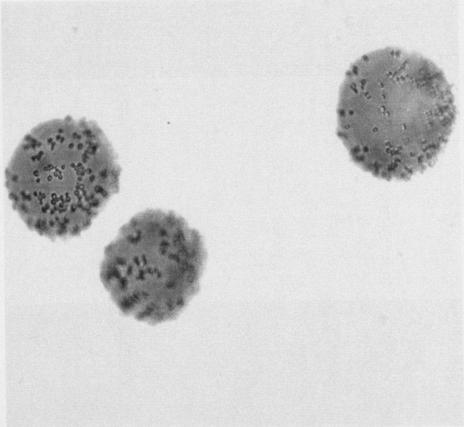
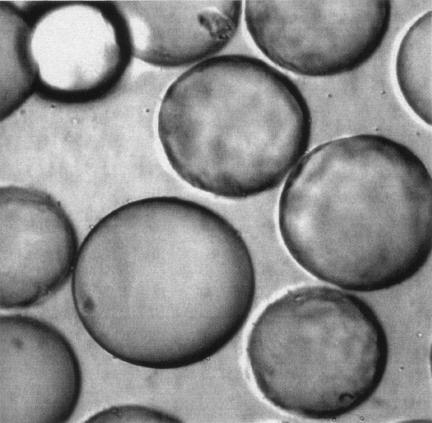
FIG. 1.
(Left) C. albicans adhering to and aggregating on the surfaces of PEG beads with the peptide KLRIPSV synthesized upon their surfaces. (Right) C. albicans, peptide-coated PEG beads, and Fn/23 mixed together. There is no adherence to the beads in the presence of Fn/23 at 100 μg/ml.
Previous work with magnetic beads coated with proteins or peptides has demonstrated that SRS adherence is not affected by the addition of saccharides and nonionic detergent, and thus lectin-like and hydrophobic interactions are not the predominant forces in this form of adherence. On the contrary, SRS adherence is reversed only upon the addition of factors which are known to break hydrogen bonds, such as formamide, high pH, and urea (4). We tested whether Fn/23 could inhibit C. albicans adhesive interactions. Table 1 shows that this peptide inhibited both adherence to beads and cell-to-cell aggregation when it was added with the ligand beads and also reversed aggregate formation to a large extent if it was added after the interactions had already formed. Formamide was similarly a very effective inhibitor of adherence, completely abrogating the interaction and displaying desorptive activity as well. The addition of formamide results in a dissociation of yeasts from the peptide-coated PEG beads, and if the formamide is washed away from the yeasts, then the same yeast will then readily adhere and aggregate on the peptide-coated PEG beads when resuspended with the beads, which demonstrates that adherence is reversible (9). Inhibition of adherence with the Fn/23 peptide occurred with S. cerevisiae expressing Als5p as well (Table 1).
TABLE 1.
Effects of different treatments on the adherence of C. albicans and S. cerevisiae expressing Als5p to PEG beads with KLRIPSV synthesized on their surfaces
| Chemical agent (concn) | Scorea with:
|
||
|---|---|---|---|
| C. albicans + peptide-coated PEG beads + reagent | C. albicans + peptide-coated PEG beads + yeast cells + addition of reagent after 30 min (desorption) | S. cerevisiae expressing Als5p + peptide-coated PEG beads + reagent | |
| None (control) | +++ | +++ | +++ |
| Fn/23 (100 μg/ml) | 0 | + to ++ | 0 |
| Formamide (50% [vol/vol]) | 0 | 0 to + | 0 |
0, no adherence; +, adherence of some yeast cells (20 to 25/bead); ++, bead almost covered by yeast cells (80 to 150/bead); +++, adherence with cell-cell aggregation.
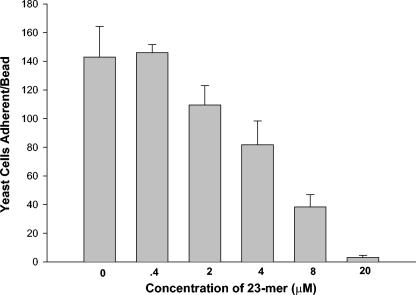
We then asked the question of whether other oligomers, even some derived from Fn/23, could inhibit the adherence of C. albicans to KLRIPSV-coated PEG beads. We divided Fn/23 into sequences possessing only the N and C termini and the central sequence of the peptide and compared the inhibitory actions of these peptides to that of Fn/23 as well as to those of PMAP-23, a well-characterized microbicidal peptide (12), a peptide derived from a hybrid of cecropin and magainin, and a peptide from an H. pylori ribosomal protein (Table 2). Only the intact parent Fn/23 molecule possessed the property of inhibition of adherence. Even peptides derived from Fn/23 did not share this property. The concentration of Fn/23 that inhibited adherence by 50% was determined to be about 4 to 5 μM (Fig. 2).
TABLE 2.
Adherence of C. albicans to PEG beads coated with KLRIPSV in the presence or absence of various peptides
| Peptide (100 μg/ml) | Score for adherence to PEG beads coated with KLRIPSVa |
|---|---|
| None (control) | +++ |
| GRGDSPASSKGGGGSRLLLLLLR | 0 |
| ..........GGGGSRLLLLLLR | +++ |
| ....SPASSKGGGGSRL...... | +++ |
| RGDSPASSKP............. | +++ |
| RIIDLLWRVRRPQKPKFVTVWVR-NH2 | +++ |
| AKKVFKRLEKLFSKIWNWK-NH2 | +++ |
| KWKKLLKKPLLKKLLKKL-NH2 | +++ |
0, no adherence; +, adherence of some yeast cells (20 to 25/bead); ++, bead almost covered by yeast cells (80 to 150/bead); +++, adherence with cell-cell aggregation.
FIG. 2.
Inhibition of adherence by Fn/23 of C. albicans to PEG beads with the peptide KLRIPSV synthesized upon their surfaces. Fn/23 was added to the yeast cells and incubated for 30 min before exposure to the beads.
Killing of fungi.
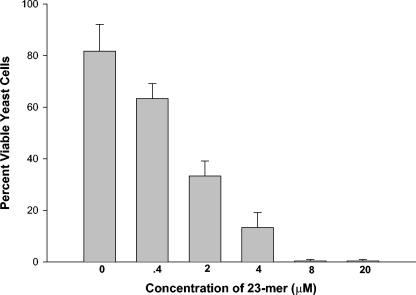
We had noticed that C. albicans yeast cells mixed with the Fn/23 peptide, when suspended in TE buffer or water, aggregate in pairs and in small clusters and appear to have extruded part of their contents. We therefore tested for fungicidal activity. C. albicans yeast cells mixed with the peptide could not be cultured and took up methylene blue dye avidly. The concentration-dependent nature of the fungicidal action of Fn/23 was then determined by incubating cells treated with Fn/23 for 15 to 30 min with methylene blue dye (Fig. 3). All yeast cells were killed by a concentration of 4 to 8 μM Fn/23. The dye method allowed one to observe microscopically cell death and the presence or absence of adherence simultaneously. However, the method was not as sensitive as a CFU-counting technique for detecting cell death. For example, the mean numbers of CFU of Fn/23-treated yeast cells cultured on YPD agar (expressed as percentages of live cells) were as follows: 100% with no Fn/23 peptide (control), 100% with 0.04 μM Fn/23, 11% with 0.4 μM Fn/23, 4% with 2 μM Fn/23, and 0% with 4 and 8 μM Fn/23. Thus, the minimum fungicidal concentration, as determined by the CFU-counting technique, was between 2 and 4 μM.
FIG. 3.
Killing of C. albicans by Fn/23 in TE buffer. Yeast cells were mixed with Fn/23 for 15 min and stained with methylene blue, and their viability was determined microscopically (live cells exclude the vital dye).
The killing action of Fn/23 was, however, abolished in the presence of physiologic salt concentrations (Table 3), a feature common to other microbicidal peptides. However, washing C. albicans cells exposed to the Fn/23 peptide in physiologic salts in water or TE buffer led to their immediate deaths as determined by their inability to exclude methylene blue.
TABLE 3.
Viability of C. albicans in the presence of Fn/23 in physiologic salt solutions, water, and TE buffer
| Suspension for C. albicans + 100 μg of Fn/23 per ml | Mean % viability (determined with methylene blue dye) ± SD |
|---|---|
| EBSS (control) | 99 ± 1 |
| 0.75% EBSS | 98 ± 1 |
| 0.50% EBSS | 97 ± 3 |
| 0.25% EBSS | 74 ± 3a |
| H2O | 0a |
| TE buffer | 0a |
Results were significantly different from those for the control (P < 0.05 by the paired Student t test).
To see if the fungicidal activity of Fn/23 was independent of its antiadhesion properties, we conducted a series of washing experiments. C. albicans cells expressing several Als adhesins or Als-expressing S. cerevisiae cells were treated with Fn/23 in EBSS under conditions leading to complete inhibition of adherence and aggregation. The peptide was then removed from the adhesins by incubation at pH 11 to dissociate the peptide. The cells regained adhesiveness to ligand-coated beads when washed cells were added to beads; however, the C. albicans yeast cells were killed. Thus, Fn/23 binds to the Als adhesins as well as to other components of the cell. Exposure of C. albicans cells to Fn/23 leads to an irreversible commitment to killing in low-ionic-strength media.
We then compared the killing activity of Fn/23 to the killing activities of components of Fn/23 and other known fungicidal peptides. The N or C terminus sequence of amino acids or the sequence from the middle of the peptide had modest effects on the viability of the fungi (Table 4). On the other hand, the 23-mer PMAP-23, peptides derived from cecropin and magainin, and an H. pylori ribosomal protein sequence were fungicidal at concentrations equivalent to that of Fn/23. However, it should be recalled that these other peptides had no effect on the adherence of C. albicans (Table 2) or S. cerevisiae expressing Als5p.
TABLE 4.
Comparison of the killing powers of Fn/23, its derivatives, and other fungicidal peptides
| Peptide at 100 μg/ml in TE buffer | Mean % viability (determined with methylene blue dye) ± SD |
|---|---|
| None (control) | 99 ± 1 |
| GRGDSPASSKGGGGSRLLLLLLR | 0 |
| ..........GGGGSRLLLLLLR | 78 ± 8 |
| .......SPASSKGGGGSRL | 98 ± 1 |
| ....RGDSPASSKP | 80 ± 7 |
| RIIDLLWRVRRPQKPKFVTVWVR-NH2 | 0 |
| AKKVFKRLEKLFSKIWNWK-NH2 | 0 |
| KWKKLLKKPLLKKLLKKL-NH2 | 0 |
DISCUSSION
Antimicrobial peptides are part of the innate host defenses in plants, insects, amphibians, and mammals (16). They have a broad spectrum of activity against bacteria, fungi, and even viruses. The fungicidal action of one of these peptides is believed to occur by the binding of the peptide to the plasma membrane (16, 20), possibly to ergosterol, and in so doing, disrupting the integrity of the membrane and hence the internal homeostasis (3). Macroscopic damage to fungi is a property of several cathelicidin antimicrobial peptides, linear peptides of 23 to 37 amino acids (20) that are larger than defensins and lack cysteine, which confers a β pleat conformation to such peptides. These peptides fold into α helices that are amphipathic (20). The Fn/23 peptide described herein most resembles the cathelicidin antimicrobial peptides in sequence and composition. Exposure of C. albicans to 12.5 μM melittin or PMAP-23 causes gross destruction and rupture of cell membranes and cell walls (14). Similar gross destruction of C. albicans yeast cells led to the serendipitous discovery of the antifungal activity of the Fn/23 peptide, since cells in TE buffer or water exposed to the peptide were misshapen and appeared to extrude cell contents when viewed by light microscopy.
Fn/23 was compared to the activities of other well-characterized antifungal peptides. A peptide derived from an H. pylori ribosomal protein (13), a hybrid of cecropin and magainin (17), and a porcine myeloid cell line (PMAP-23) were chosen for their similarity in number of amino acids to Fn/23. These three peptides have been studied in depth for their broad spectra of activity, modes of killing, and relative antifungal activities (12). Further fungicidal peptides have been synthesized on the basis of the sequence and stereochemistry of PMAP-23 (14). Although these peptides were potent killers, they did not display any antiadhesive qualities.
The cathelicidins, particularly those derived from bovine and porcine myeloid cells, possess other properties besides antimicrobial killing, such as wound healing and angiogenesis (19). It is interesting that Fn/23 not only kills but also inhibits the adherence of C. albicans, two unrelated properties apparently targeting different parts of the fungal cell, i.e., in one case, targeting the Als adhesin cell surface proteins, and in the other case, targeting the cell membrane. These dual properties may prove to be helpful adjuvants in the treatment of some forms of candidiasis, particularly mucosal disease, where a temporary aqueous environment could conceivably be achieved by therapeutic intervention.
Acknowledgments
We thank Min Hahn of the Arizona Cancer Center for the synthesis of the peptide-coated PEG beads.
This research was supported by a grant from the Southeastern Arizona Biomedical Research Foundation, Tucson.
REFERENCES
- 1.Andra, J., O. Berninghausen, and M. Leippe. 2001. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med. Microbiol. Immunol. 189:169-173. [DOI] [PubMed] [Google Scholar]
- 2.Calderone, R. A., and P. C. Braun. 1991. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 55:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLucca, A., J. Bland, C. Vigo, T. Jacks, J. Peter, and T. Walsh. 2000. d-Cecropin B: proteolytic resistance, lethality for pathogenic fungi and binding properties. Med. Mycol. 38:301-308. [DOI] [PubMed] [Google Scholar]
- 4.Gaur, N. K., S. A. Klotz, and R. L. Henderson. 1999. Overexpression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect. Immun. 67:6040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaur, N. K., and S. A. Klotz. 2004. Accessibility of the peptide backbone of protein ligands is a key specificity determinant in Candida albicans SRS adherence. Microbiology 150:277-284. [DOI] [PubMed] [Google Scholar]
- 6.Gaur, N. K., and S. A. Klotz. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaur, N. K., R. L. Smith, and S. A. Klotz. 2002. Candida albicans and Saccharomyces cerevisiae expressing ALA1/ALS5 adhere to accessible threonine, serine or alanine patches. Cell Commun. Adhes. 9:45-57. [DOI] [PubMed] [Google Scholar]
- 8.Klotz, S., and R. Smith. 1990. Candida albicans adherence to subendothelial extracellular matrix components is inhibited by arginine-glycine-aspartic acid peptides. Clin. Res. 38:13A. [Google Scholar]
- 9.Klotz, S. A., N. K. Gaur, D. F. Lake, V. Chan, J. Rauceo, and P. N. Lipke. 2004. Degenerate peptide recognition by Candida albicans adhesins Als5p and Als1p. Infect. Immun. 72:2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klotz, S. A., D. J. Drutz, J. L. Harrison, and M. Huppert. 1983. Adherence and penetration of vascular endothelium by Candida yeasts. Infect. Immun. 42:374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotz, S. A., R. L. Smith, and B. W. Stewart. 1992. Effect of an arginine-glycine-aspartic acid-containing peptide on hematogenous candidal infections in rabbits. Antimicrob. Agents Chemother. 36:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, D., D.-H. Kim, Y. Park, H. Kim, H. K. Kim, Y. Shin, and K.-S. Hahm. 2001. Fungicidal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem. Biophys. Res. Commun. 282:570-574. [DOI] [PubMed] [Google Scholar]
- 13.Lee, D., H. Kim, Y. Park, H. Kim, B. Choi, C. Choi, and K.-S. Hahm. 2002. Design of novel analogue peptides with potent antibiotic activity based on the antimicrobial peptide, HP (2-20), derived from N-terminus of Helicobacter pylori ribosomal protein L1. Biochim. Biophys. Acta 1598:185-194. [DOI] [PubMed] [Google Scholar]
- 14.Lee, D., P. Kim, Y. Park, E.-R. Woo, J. Choi, C.-H. Choi, and K.-S. Hahm. 2002. Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem. Biophys. Res. Commun. 293:231-238. [DOI] [PubMed] [Google Scholar]
- 15.Lehrer, R. I., and M. J. Cline. 1969. Interaction of Candida albicans with human leukocytes and serum. J. Bacteriol. 98:996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehrer, R. I., A. K. Lichtenstein, and T. Ganz. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 17.Park, Y., D. G. Lee, S. H. Jang, E. R. Woo, H. G. Jeong, C.-H. Choi, and K.-S. Hahm. 2003. A Leu-Lys-rich antimicrobial peptide: activity and mechanism. Biochim. Biophys. Acta 1645:172-182. [DOI] [PubMed] [Google Scholar]
- 18.Pendrak, M. L., and S. A. Klotz. 1995. Adherence of Candida albicans to host cells. FEMS Microbiol. Lett. 129:103-114. [DOI] [PubMed] [Google Scholar]
- 19.Rauceo, J. M., N. K. Gaur, K.-G. Lee, J. E. Edwards, S. A. Klotz, and P. N. Lipke. 2004. Global cell surface comformational shift mediated by a Candida albicans adhesin. Infect. Immun. 72:4948-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanetti, M. 2003. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:1-10. [DOI] [PubMed] [Google Scholar]