Abstract
Low levels of thermal degradation products such as carbonyls (formaldehyde, acetaldehyde, acrolein, crotonaldehyde) have been reported in e-cigarette aerosols. The collection and analysis of e-cigarette aerosol carbonyls are often adapted from methods developed for tobacco cigarette smoke. These methodologies are often not sensitive enough to detect low carbonyl levels in e-cigarette aerosols. One objective of this work was to develop and validate a rapid, selective and sensitive ultra-performance liquid chromatography with mass spectrometry method optimized for analysis of carbonyls in e-cigarette aerosols. Aerosols were trapped in 20-puff collections, 4-s durations, 55-mL volumes, 30-s intervals, square wave puff profiles. Collection apparatus involved a linear smoking machine with Cambridge filter pad followed by a glass impinger containing acidified 2,4-dinitrophenylhydrazine. This method showed limits of quantitation and detection of 0.016 and 0.003 µg puff−1, respectively, and run time of 4 min. Six e-cigarettes were evaluated (five devices each). All contained measurable levels of carbonyls. Levels were mostly well below those in conventional cigarettes. However, for some e-cigarettes, formaldehyde levels were above those for tobacco cigarettes (highest at 14.1 µg puff−1). Temperatures related to carbonyl yields in e-cigarette aerosols were explored to better understand carbonyl formation: formation of formaldehyde is low at temperatures below 350°C.
Introduction
E-cigarettes, a type of e-vapor product, are battery-powered devices that contain a heating element and a cartridge filled with a liquid solution. The liquid solution in an e-cigarette, or e-liquid, typically contains tobacco-derived nicotine, water, propylene glycol, glycerin and flavors. The heating element vaporizes the e-liquid that then condenses into an aerosol. When inhaled by the user, the e-cigarette aerosol delivers flavor and nicotine. Because these products use tobacco-derived nicotine and do not burn tobacco, they do not generate or emit smoke; therefore, users inhale and exhale an aerosol and often describe it as “vaping”.
Primarily, the aerosols generated from e-cigarettes are composed of fine particles of liquid and gas phases of the vaporized e-liquid. However, low levels of thermal degradation products such as carbonyls (e.g., formaldehyde, acetaldehyde, acrolein and crotonaldehyde) have been reported in e-cigarette aerosols (1–11). For example, in a study by Uchiyama et al. (2), 9 of the 13 commercial e-cigarette products tested showed detectable levels of carbonyls at variable levels. Goniewicz et al. (1) reported that, of the 15 e-cigarettes in their study, all products contained formaldehyde and acetaldehyde in the aerosols and acrolein was also detected in all but one (1).
It is well known that carbonyls are relatively unstable due to volatility, thermal instability and sensitivity to acidic environments (12). Therefore, it is necessary to derivatize carbonyls as they are collected and minimize the number of puffs per collection (e.g., ≤20 puffs per collection) to minimize analyte loss. Carbonyls are also typically present at trace levels in e-vapor product aerosols, which puts considerable demands on the sensitivity of the analytical methodologies used for their quantitation. Carbonyl concentrations in e-cigarette aerosols are often measured by adapting analytical methodologies designed for tobacco cigarette smoke, such as the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA) recommended method 74 (CRM 74) (13); however, the levels of carbonyls found in e-cigarettes are often far lower than those measured in conventional tobacco cigarettes (1, 14). Therefore, when using the analytical method adapted from the tobacco cigarette method, the levels of collected carbonyls in e-vapor products can be below the limits of detection (<LOD) or below the limits of quantitation (<LOQ) simply because the method lacks the necessary sensitivity.
The analytical method typically used to measure collected carbonyls in cigarette smoke (e.g., CRM 74) uses high-performance liquid chromatography with ultraviolet detection (HPLC-UV). While UV detection is a robust, cost-effective and simple method of analysis, it has limited sensitivity and selectivity, and it is subject to interferences. For example, the flavor components in the e-cigarette aerosol may result in interferences that are unresolved by HPLC-UV caused by co-eluting peaks and can result in overestimating carbonyl concentrations. In addition, the typical derivatization of carbonyls (e.g., 2,4-dinitrophenylhydrazine or DNPH) results in the formation of E and Z stereoisomers. It has been previously reported that these stereoisomers are not formed in racemic amounts and have different UV absorbance maxima (12, 15, 16). If a HPLC-UV method only measures a single isomer, or if the wavelength is not optimized for both isomers, carbonyl concentrations can be underestimated; however, this can be avoided using diode array detectors (12).
While many researchers have adapted cigarette smoke trapping methods to e-cigarette aerosols (e.g., smoking machines and impinge collection), some have explored alternative methods such as sorbent tubes for the collection of e-cigarette aerosols (10, 17, 18). Sorbent tubes have been shown to provide additional sensitivity and simplification (10, 17). Sorbent tubes have also demonstrated comparable trapping efficiencies to traditional smoking machine and impinger trapping (13, 18). However, as discussed by Herrington et al. (17) specialized smoking machines and traditional smoke collection techniques are likely more accurate and reproducible than simple sorbent tube sampling devices. While sorbent tube collections are sensitive, simple and well suited for rapid screening of carbonyls in e-cigarette aerosols, they are subject to variable pressure drops during sampling that influence the puff profiles and thus the composition of the aerosol (10). Due to variable packing densities, it is also often necessary to prescreen sorbent tubes in order to ensure consistent air flows and resulting puff volumes (18). Therefore, for laboratories equipped with traditional smoking machine and impinger trapping, sorbent tube collections should be used for screening purposes only.
Taking into account the issues discussed above, one objective of this work was to develop a sensitive, selective and reproducible method of measuring carbonyls in e-vapor product aerosols collected using traditional smoking machines and impinger trapping followed by ultra-performance LC with mass spectrometry (UPLC-MS) detection. The increased sensitivity of the method over HPLC-UV methods will enable researchers to report accurate concentrations of carbonyls instead of <LOQ or <LOD. Additionally, increased selectivity will prevent over- or under-estimations of carbonyl concentrations. The method includes all carbonyls listed in the United States Food and Drug Administration (FDA) recently established abbreviated list of harmful and potentially harmful constituents (HPHCs) for currently regulated tobacco products (e.g., cigarettes): formaldehyde, acetaldehyde, acrolein and crotonaldehyde (19, 20).
The mechanism for carbonyl formation has been previously described by which glycerol and glycols can form carbonyls under thermal conditions (2, 3, 9, 21, 22). Kosmider et al. (3) recently demonstrated that changing the voltage of select commercially available refillable e-vapor devices (often referred to as “tank systems”) can significantly impact carbonyl formation. They found that as they increased the battery output voltage, and thus the temperature of the heating element, the levels of carbonyls rapidly increased (3). Gillman et al. (9) also demonstrated that for some refillable e-vapor devices, increasing the power applied to the coil resulted in an increase in formaldehyde, acetaldehyde and acrolein in the aerosol.
Gillman et al. (9) recently stated the need for direct measurement of the temperature of the coil during aerosol formation to better understand what coil temperatures lead to carbonyl formation. Geiss et al. (10) recently explored coil temperatures related to carbonyl yields from open tank systems with the mouthpiece, tank and chimney removed. The second objective of the work discussed herein was to further explore the temperatures related to carbonyl yields in cig-a-like e-cigarette devices without disassembly and under controlled puffing conditions.
Experimental
E-cigarette test products
Six commercial e-cigarette products available in the US marketplace (at the time of manuscript preparation, August 2015) were included in the analytical evaluations discussed herein. This included two e-cigarettes produced by Nu Mark LLC (an Altria company) under the MarkTen® brand name (MarkTen® Classic and Menthol with 2.5% approximate nicotine by weight).
Aerosol collection
All aerosol collections were conducted under International Organization for Standardization (ISO) smoking environmental conditions (23) with temperature at 22.0 ± 2.0°C and relative humidity at 60 ± 5%. The protocols for aerosol collection were based upon the Health Canada Intense (HCI) smoking regime (24) puff volume and puff interval. However, a square wave puff profile was needed to reproducibly activate the puff sensors, and ventilation blocking was not applicable to the e-cigarette designs. A 4-s puff duration was the maximum that the smoking machine could accomplish when conducting impinger collections. The regime selected (Table I) also represents the average puffing topography of experienced e-cigarette users to the best of our knowledge at this time (25).
Table I.
Smoking Machine Puff Profile Settings
| Parameter | Setting |
|---|---|
| Square wave | Uniform air transfer throughout puff duration |
| Volumetric air flow | 825 mL min−1 |
| Puff volume | 55.0 ± 0.3 mL |
| Puff duration | 4.00 ± 0.05 s |
| Puff interval | 30.0 ± 0.5 s |
For carbonyl aerosol collection, the devices were puffed in 20-puff increments because the analytes in this chemical class are known to be unstable. If carbonyls were collected with more puffs (e.g., ~100 puffs), it is highly likely that the values would be underestimated on an estimated per puff basis due to analyte loss.
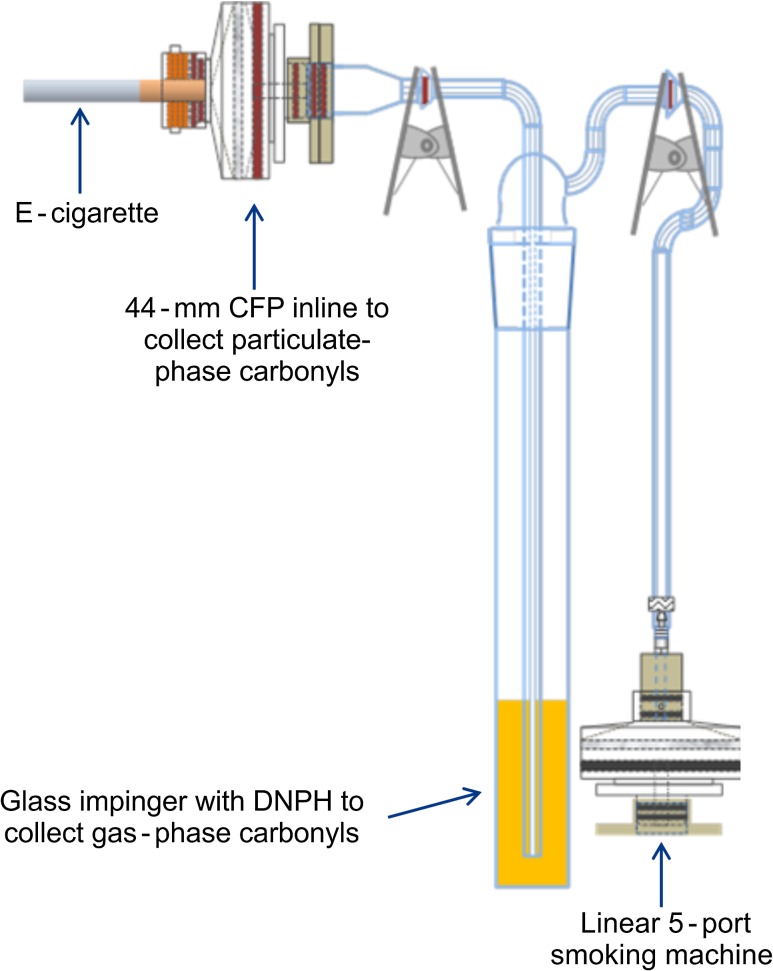
The aerosol samples were collected directly into a collection train (Figure 1) consisting of a 44-mm Cambridge filter pad (CFP) in a custom holder (Phil Gunn Machine, Inc., Richmond, VA) for aerosol collection that was designed to deliver aerosol directly to the pad from e-cigarettes with a 8.0- to 10.5-mm diameter, which was located in front of a glass impinger [24/40 bottle with standard insert (1.6 mm delivery orifice) containing 12/5 ball and socket (part # 030496, Research Glass, Richmond, VA)] containing 30 mL DNPH (Aldrich Chemical Company, Milwaukee, WI) trapping solution [17.5 mM DNPH in optima grade acetonitrile (Fisher Scientific, Atlanta, GA) with 1.5% 1.82 M perchloric acid (Fisher Scientific)]. All samples were collected on a linear 5-port smoking machine (KC Automation, Richmond, VA). Prior to each collection, a leak check and puff volume check were performed with the impinger connected to the smoking machine. Immediately prior to aerosol collection, a clearing puff was taken for each port in order to clear any DNPH trapping solution that may have transferred to the impinger tube. This ensured that each port had the correct puff volume for the first puff.
Figure 1.
The collection train used to directly collect aerosol samples for the determination of carbonyls by UPLC-MS. The single impinger configuration shown consists of a 44-mm CFP in front of the glass impinger containing a DNPH trapping solution.
Following 20 puff collections, the CFP was removed from its holder and the residual condensate in the holder was wiped with the CFP. The impinger was then disconnected, and the CFP was inserted into the DNPH trapping solution within the impinger and vortexed for 5 s. One milliliter of aerosol extract was then transferred to an amber autosampler vial containing 50 µL of internal standard working solution plus pyridine (to stop the derivatization). The internal standard working solution contained 2 µg mL−1 of the hydrazone derivative of formaldehyde-d2 and acetaldehyde-d3 (Aldrich Chemical Company, Milwaukee, WI) in DNPH trapping solution containing 5% certified ACS grade pyridine (Fisher Scientific). Formaldehyde-d2 and acetaldehyde-d3 are derivatized with DNPH during preparation of the stock solution. The hydrazone derivative of formaldehyde-d2 served as the internal standard for the hydrazone derivative of formaldehyde, and the hydrazone derivative of acetaldehyde-d3 served as the internal standard for the hydrazone derivative of acetaldehyde, acrolein and crotonaldehyde. Using this DNPH derivatization method, all forms of the aldehydes are trapped as the hydrazone derivative that prevents this method from differentiating between the carbonyl form of these aldehydes and other forms such as hemiacetals (9, 26).
Chromatographic and mass spectrometry conditions
This method was developed and validated to quantitatively determine the concentration of formaldehyde (CAS #50-00-0), acetaldehyde (CAS #75-07-0), acrolein (CAS #107-02-8) and crotonaldehyde (CAS #4170-30-3) (Supelco, Bellefonte, PA). The method of analysis was modified for e-cigarette aerosol from CRM 74 (13) for cigarette smoke analysis by implementation of the aerosol collection procedure discussed above and the addition of UPLC-MS conditions described below.
The aerosol extracts were analyzed for the respective hydrazones using ACQUITY® UPLC with MS (Waters Quattro Premier) (Waters Corp., Milford, MA) detection. Calibration standards included formaldehyde-DNPH derivative (CAS #1081-15-8), acetaldehyde-DNPH derivative (CAS #1019-57-4), acrolein-DNPH derivative (CAS #888-54-0) and crotonaldehyde-DNPH derivative (CAS #1527-96-4) (Supelco). The calibration range was 0.010–4.00 µg mL−1, which corresponds to 0.016–6.30 µg puff−1 based on a 20-puff collection. Table II contains the details of this 4-min analysis; UPLC mobile phase gradient parameters are shown in Table III.
Table II.
Instrument Settings for Analysis of Carbonyls in E-Cigarette Aerosol by UPLC-MS
| Parameter | Specification |
|---|---|
| Analysis settings | |
| Injection volume | 1 µL |
| Flow rate | 0.5 mL min−1 |
| Run time (solvent manager) | 4.0 min |
| Sample manager settings | |
| Sample compartment temperature | 10°C |
| Column temperature | 45°C |
| Weak wash | Mobile phase Aa |
| Strong wash | Mobile phase Bb |
| 5-µL injection loop | Partial loop with needle overfill |
| Run time | 4.0 min |
| Selected ions monitored for quantitation (analyte) (m/z) | |
| Formaldehyde-DNPH | 209.1 |
| Acetaldehyde-DNPH | 223.1 |
| Acrolein-DNPH | 235.1 |
| Crotonaldehyde-DNPH | 249.2 |
| Formaldehyde-d2 | 211.1 |
| Acetaldehyde-d3 | 223.1 |
| Mass spectrometer settingsc | |
| Ionization mode | Negative electrospray |
| MS mode | Single MS with SIR |
| Capillary voltage | 0.5 kV |
| Cone voltage | 20 V |
| RF lens | 0.5 |
| Source temperature | 110°C |
| Desolvation temperature | 375°C |
| Desolvation gas | Probe 600 L h−1, Cone 20 L h−1 |
RF, Radio frequency; SIR,Single ion recording.
aUPLC mobile phase A = 98:2 10 mM ammonium acetate:methanol.
bUPLC mobile phase B = 90:10 acetonitrile:1-propanol.
cThese settings may require optimization for the particular instrument on which the analysis will be performed.
Table III.
UPLC Mobile Phase Gradient Parameters
| Time (min) | Flow (mL min−1) | A (%) | B (%) | Curve |
|---|---|---|---|---|
| 0.0 | 0.5 | 65 | 35 | Initial |
| 2.0 | 0.5 | 40 | 60 | 6 |
| 2.5 | 0.5 | 40 | 60 | 6 |
| 2.7 | 0.5 | 65 | 35 | 6 |
Heater coil temperature measurements
The effect of heater temperature on formaldehyde formation was determined using prototype e-cigarettes and a ThermaCam model SC6000HS infrared camera (FLIR Systems Inc., Wilsonville, OR) equipped with an indium-antimonide detector measuring in a spectral range of 3.0–5.0 µm at an optical resolution of 640 × 512 to measure coil temperature. A DC power supply was used instead of the standard e-cigarette battery to deliver voltage to the heater of each prototype, allowing for testing at heater temperatures exceeding the typical level used for commercial products. The voltages applied were 3.7, 3.9 and 4.1 V. To measure the temperature of prototype cartridge heater coils, the mouthpiece was removed and the camera was placed in direct line of sight of the heater coil. The e-cigarette was puffed using positive pressure from a smoking machine discharge stroke, matching the parameters of the puffing regime described in Table I. During puffing, the thermal response of the heater coil was recorded with the infrared camera at a frame rate of 30 Hz. The maximum heater coil temperature during the puff was determined through the instrument software. For coil temperatures exceeding 365°C, a 0.2 neutral density filter was attached externally to the camera lens. Subsequent temperature values were measured using calibrations developed previously against a Mikron M360 (LumaSense Technologies, Inc., Santa Clara, CA) blackbody calibration source.
Gas-phase formaldehyde determination
In order to determine the relationship between heater coil temperature and formaldehyde generation, a FTIR spectrometer (Midac Corp., Westfield, MA) equipped with a 3-meter White cell was used to perform the following procedure. First, the maximum heater coil temperature was determined at the voltage of interest by a single puff measurement using the infrared camera. Then, the e-vapor unit was puffed five times in succession, using the same DC voltage, into the FTIR cell in order to fill the cell. Prior to entering the FTIR cell, the vapor was passed through a CFP to remove particulate. After five puffs were taken, a spectrum was collected, and the amount of formaldehyde present was estimated for comparative purposes. Next, the maximum coil temperature from a single puff measurement at the same DC voltage was measured again. At this point, the measurement cycle at the voltage of interest was completed. The data generated were a formaldehyde concentration bracketed by two temperature values at a given voltage. The two temperature values were averaged to produce a single temperature-formaldehyde data point at the given voltage. To complete the measurements for a single prototype, the voltage was raised, and the measurement procedure was repeated. A total of 10 replicates were analyzed in this fashion. The e-liquid formulation used in these prototypes was 34% propylene glycol, 51% glycerin and 15% water with 1.5% nicotine by weight. The puffing parameters were 4-s duration, 55-mL volume and 30-s interval.
Results
The aerosol samples were collected directly into a collection train (Figure 1) consisting of a 44-mm CFP in front of a glass impinger containing a DNPH trapping solution. For cigarette smoke collection techniques, impingers are typically used to trap constituents in the gas phase, and CFP is used to collect constituents in the particulate phase. CRM 74 (13) uses two impingers and no CFP, as the predominant carbonyl in conventional cigarette smoke is acetaldehyde, which resides primarily in the gas phase. For e-vapor products, formaldehyde is typically found at higher levels than acetaldehyde. Formaldehyde resides in both the gas and liquid phases of an aerosol. During method development, it was observed that ~70% of the formaldehyde is trapped on the CFP and ~30% in the impinger solution.
The UPLC-MS method described herein was fully validated based upon the 2005 International Conference on Harmonization (ICH) guideline “Validation of Analytical Procedures: Text and Methodology Q2(R1)” (27). The calibration curve had a coefficient of determination of >0.995 for all analytes on 3 consecutive days. All calibration points quantified ≤15% from theoretical value. Trapping efficiency for formaldehyde, acetaldehyde and acrolein was 100% when using the collection train with a 44-mm CFP and a single impinger. The use of a second impinger was evaluated during method development, and no formaldehyde, acetaldehyde or acrolein was detected in the second impinge using the aerosol collection parameters described in Table I. Therefore, a single impinger configuration was used (Figure 1). Crotonaldehyde trapping efficiency was not evaluated. The accuracy for formaldehyde recovery was 90.7–106%, acetaldehyde recovery was 92.4–111%, acrolein recovery was 60.5–70.7% (due to the known instability of the DNPH derivative) and crotonaldehyde recovery was 90.7–108%. Instrument precision for all compounds was <5%RSD. The method variability within samples ranged from 1.73 to 12.7%RSD. Intermediate precision over the course of 3 days showed that the method variability within samples ranged from 1.66 to 14.8 %RSD. Evaluation of specificity showed that the DNPH extraction solution contained a low background level of carbonyls, which must be subtracted from sample values. Selected ion monitoring is a highly specific detection technique, thus, additional specificity evaluations were not conducted. The LOD was 0.002 µg mL−1 for each compound, equivalent to 0.003 µg puff−1, and the LOQ was 0.010 µg mL−1 for each compound, equivalent to 0.016 µg puff−1 (using the 20-puff collection). The pyridine addition step is robust within a 30-min window, with <10% change for formaldehyde, acetaldehyde and crotonaldehyde. Acrolein changed up to 13%. Sample extracts are stable for 48 h under refrigerated conditions. Stock solutions and working standards are stable for at least 9 weeks under refrigerated conditions.
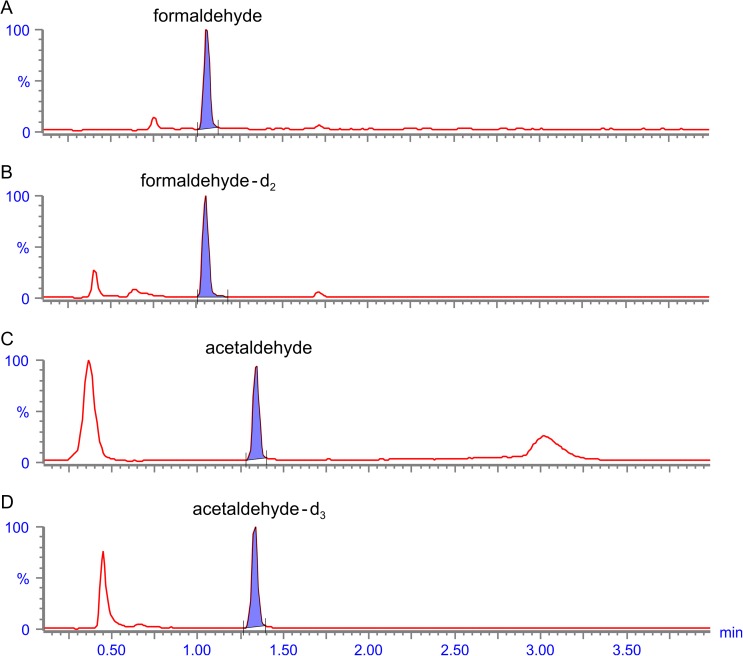
To demonstrate that the method was fit for purpose, six commercial “cig-a-like” e-vapor products (e-cigarettes with rechargeable batteries and disposable cartridges) were evaluated for carbonyls in the aerosol. These commercial products were produced by different manufacturers, and they were selected based upon having e-vapor products that make up a major percentage of convenience-store category sales (28). Not all products from each manufacturer were evaluated, and no specific selection criteria were applied. The products included commercial e-cigarettes available in the US marketplace (at the time of manuscript preparation) produced by Nu Mark LLC (an Altria company) under the MarkTen® brand name. The results from other four commercial products have been de-identified. Table IV shows a summary of the carbonyl data collected from the selected products. Figure 2 shows representative chromatograms of formaldehyde and acetaldehyde detected in the e-cigarette MarkTen® Classic (~2.5% nicotine by weight).
Table IV.
Carbonyl Levels in Selected E-Cigarettes
| E-cigarette name | Formaldehyde (µg puff−1) | Acetaldehyde (µg puff−1) | Acrolein (µg puff−1) | Crotonaldehyde (µg puff−1) |
|---|---|---|---|---|
| Product A | 0.19–14.1 | 0.05–13.61 | <LOQ to 4.11 | <LOD to 0.04 |
| Product B | 0.12–3.13 | 0.05–1.67 | <LOQ to 0.69 | <LOD to <LOQ |
| Product C | 0.21–0.65 | 0.14–0.51 | 0.15–0.61 | <LOD to <LOQ |
| Product D | 0.10–0.22 | 0.29–0.51 | 0.03–0.10 | <LOD to <LOQ |
| MarkTen® Classic | 0.14–0.18 | 0.04–0.06 | <LOQ to 0.02 | <LOD |
| MarkTen® Menthol | 0.07–0.14 | 0.03–0.06 | <LOQ to 0.01 | <LOD |
Figure 2.
Representative chromatograms of aerosol formaldehyde and acetaldehyde detected using the UPLC-MS method in the MarkTen® Classic e-cigarette (~2.5% nicotine by weight) and their respective internal standards (formaldehyde-d2 and acetaldehyde-d3). Chromatograms of selected ion monitoring for m/z (A) 209.1, (B) 211.1, (C) 223.1 and (D) 223.1.
Discussion
All commercial products tested contained formaldehyde and, in most cases, the levels were well below those observed in conventional tobacco cigarettes (~3 µg puff−1) (4). However, for some commercial products, the levels of formaldehyde were greater than those detected in tobacco cigarettes, with the highest level at 14.1 µg puff−1. Levels of formaldehyde detected in MarkTen® Classic and Menthol in this study were consistent with previously published values for this e-cigarette brand using the adapted mainstream cigarette smoke method CRM 74 (13). The previously published MarkTen® values were determined to be below published occupational exposure limits using this aerosol collection regime (8). Acetaldehyde was also measured in all products tested but at levels ranging from 100 to 1,000 times lower than reported in conventional tobacco cigarettes. These results are not inconsistent with previously published data. However, it is difficult to compare reported concentrations of potentially harmful aerosol chemicals (e.g., carbonyls) across currently available e-cigarette studies because the puffing regimes are significantly different. For example, a recent study by Goniewicz et al. (1) used a 1.8-s puff, 10-s interval between puffs and 70-mL puff volume. In another study conducted by Uchiyama et al. (2), a 2-s puff, 30-s interval and 55-mL volume was used. In this study, a 4-s puff, 30-s interval and 55-mL volume was used. The puffing profile used in this study was selected based upon the maximum puff duration that the smoking machine (5-port linear KC Automation smoking machine) could collect for carbonyl analysis in order to maximize the sensitivity of the method.
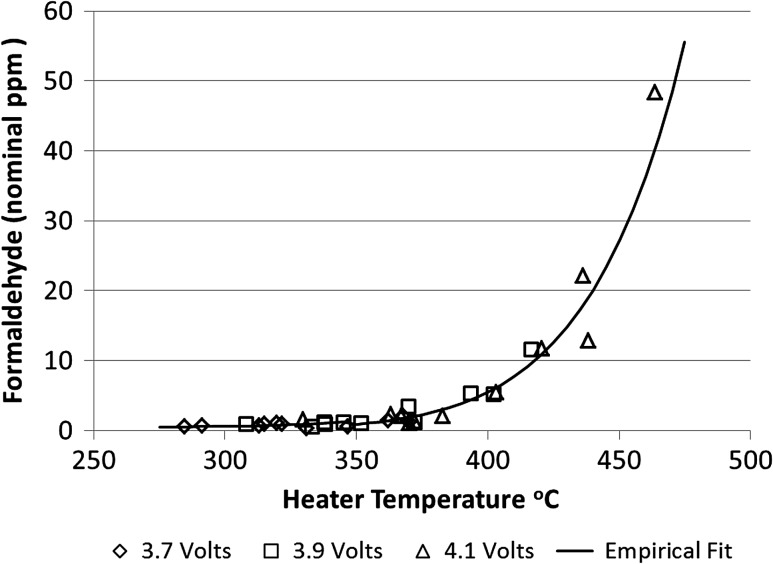
As discussed previously, carbonyls can form from the degradation of glycerol and glycols under thermal conditions (2, 3, 21, 22). To better understand at which temperatures this degradation can occur, the heater voltage of the evaluated prototype device was applied using a DC power supply to raise the heater temperature above the typical level of battery operation. The voltages used were 3.7, 3.9 and 4.1 V. The effect of heater temperature on formaldehyde formation for these prototypes was determined using an infrared camera to measure the maximum heater coil temperature and FTIR to measure gas-phase formaldehyde, and the results are shown in Figure 3. Because the method used for gas phase formaldehyde measurement was designed for comparative purposes only, the measured formaldehyde is reported in nominal p.p.m. The heater temperature range in this analysis was ~275–475°C. We found that the amount of formaldehyde produced is low at temperatures below 350°C, and then it rises steeply with increasing temperature. Based on this finding, the high levels of formaldehyde observed in some e-vapor products were likely due to heater temperatures in excess of 350°C.
Figure 3.
The effect of heater temperature on formaldehyde formation (reported in nominal p.p.m.) as determined using an infrared camera to measure heater coil temperature and FTIR to measure gas-phase formaldehyde.
As stated by Goniewicz et al. (1) and consistent with our observations, the levels of potentially harmful chemicals found in e-cigarettes are far less than those observed in conventional tobacco cigarettes (14). Nevertheless, it is again difficult to make a direct comparison between e-cigarette and conventional cigarette constituent yields because the puffing regimes are significantly different. Conventional cigarettes are typically tested under standardized conditions referred to as ISO, Massachusetts Department of Public Health and/or HCI conditions. As stated above, the methods used for aerosol collection from e-vapor products also vary within the scientific literature. Within the limitations of the aerosol collection machines (e.g., smoking machines), longer puff durations for e-cigarettes result in more aerosol collected.
Previously published studies investigating carbonyls in e-cigarette aerosols typically used analytical methodologies adapted from methods developed for tobacco cigarettes. These methodologies use HPLC-UV and are often not sensitive enough to detect the low levels of carbonyls found in e-cigarette aerosols (e.g., LOQ ≥0.3 µg puff−1). Furthermore, these methods are also subject to interference from e-cigarette flavor systems resulting in a potential for false positive identifications or incorrect quantification of carbonyls. Figure 2 shows representative chromatograms of formaldehyde and acetaldehyde detected in the e-cigarette MarkTen® Classic (~2.5% nicotine by weight) using the UPLC-MS method detailed herein. Albeit these levels were among the lowest observed in this limited data set, the high sensitivity and selectivity of the UPLC coupled to MS detection resulted in well-resolved peaks with minimal matrix interferences and a large signal-to-noise ratio. This UPLC-MS method is far more suitable for the analysis of low levels of carbonyls in e-cigarette aerosols than the HPLC-UV method. This analytical technique also results in faster analysis times and therefore, more rapid laboratory throughput.
Conclusion
With the rapid growth of the e-vapor category and proposed regulation of its products, consensus standardized methods are needed for their evaluation. These methods cannot be simply adapted from cigarette smoke methodologies, as the constituents in e-cigarette aerosols are typically much lower than those observed in conventional tobacco cigarettes. Therefore, more sensitive methodologies must be developed that are suitable (fit for purpose) to measure low-level constituents in e-cigarette aerosols.
In most cases, the HPHCs found in conventional tobacco cigarettes are not observed in e-cigarette aerosols (8). One key reason is the lack of combustion and pyrolysis in the formation of those aerosols. However, as discussed herein, low levels of thermal degradation products such as carbonyls (formaldehyde, acetaldehyde, acrolein and crotonaldehyde) have been found in e-cigarette aerosols. In fact, all commercial products tested in this study contained formaldehyde, acetaldehyde and acrolein. In most cases, the levels were well below those observed in conventional tobacco cigarettes. However, for a few commercial products evaluated in this study, formaldehyde levels above those found in tobacco cigarettes (~3 µg puff−1) (14) were detected, with the highest at 14.1 µg puff−1 (Table IV). It is likely that these elevated levels are due to devices with heater coils that exceed ~350°C during puffing. The data provided in this manuscript demonstrate that there can be significant variability in potentially harmful constituents in this product category.
Acknowledgment
The authors acknowledge the editorial assistance of Eileen Y. Ivasauskas of Accuwrit Inc.
References
- 1.Goniewicz M.L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J. III, et al. ; Levels of selected carcinogens and toxicants in vapour from electronic cigarettes; Tobacco Control, (2014); 23: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchiyama S., Ohta K., Inaba Y., Kunugita N.; Determination of carbonyl compounds generated from the e-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography; Analytical Science, (2013); 29: 1219–1222. [DOI] [PubMed] [Google Scholar]
- 3.Kosmider L., Sobczak A., Fik M., Knysak J., Zaciera M., Kurek J., et al. ; Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage; Nicotine and Tobacco Research, (2014); 16: 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekki K., Uchiyama S., Ohta K., Inaba Y., Nakagome H., Kunugita N.; Carbonyl compounds generated from electronic cigarettes; International Journal of Environmental Research and Public Health, (2014); 11: 11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng T.; Chemical evaluation of electronic cigarettes; Tobacco Control, (2014); 23(Suppl 2): ii11–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tayyarah R., Long G.A.; Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air; Regulatory Toxicology and Pharmacology, (2014); 70: 704–710. [DOI] [PubMed] [Google Scholar]
- 7.Uchiyama S., Inaba Y., Kunugita N.; Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine; Journal of Chromatography A, (2010); 1217: 4383–4388. [DOI] [PubMed] [Google Scholar]
- 8.Flora J.W., Meruva N., Huang C.B., Wilkinson C.T., Ballentine R., Smith D.C., et al. ; Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols; Regulatory Toxicology and Pharmacology, (2016); 74: 1–11. http://dx.doi.org/10.1016/j.yrtph.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Gillman I.G., Kistler K.A., Stewart E.W., Paolantonio A.R.; Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols; Regulatory Toxicology and Pharmacology, (2016). doi:10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Geiss O., Bianchi I., Barrero-Moreno J.; Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours; International Journal of Hygiene and Environmental Health, (2016); 219: 268–277. [DOI] [PubMed] [Google Scholar]
- 11.Jo S.H., Kim K.H.; Development of a sampling method for carbonyl compounds released due to the use of electronic cigarettes and quantitation of their conversion from liquid to aerosol; Journal of Chromatography A, (2016); 1429: 369–373. [DOI] [PubMed] [Google Scholar]
- 12.Miller J.H., Gardner W.P., Gonzalez R.R.; UHPLC separation with MS analysis for eight carbonyl compounds in mainstream tobacco smoke; Journal of Chromatographic Science, (2010); 48: 12–17. [DOI] [PubMed] [Google Scholar]
- 13.CORESTA (2014) CORESTA Recommended Method No 74. Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. http://www.coresta.org/Recommended_Methods/CRM_74-update(July14).pdf (accessed December, 2015).
- 14.Counts M.E., Morton M.J., Laffoon S.W., Cox R.H., Lipowicz P.J.; Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions; Regulatory Toxicology and Pharmacology, (2005); 41: 185–227. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama S., Ando M., Aoyagi S.; Isomerization of aldehyde-2,4-dinitrophenylhydrazone derivatives and validation of high-performance liquid chromatographic analysis; Journal of Chromatography A, (2003); 996: 95–102. [DOI] [PubMed] [Google Scholar]
- 16.Grosjean E., Green P.G., Grosjean D.; Liquid chromatography analysis of carbonyl (2,4-dinitrophenyl)hydrazones with detection by diode array ultraviolet spectroscopy and by atmospheric pressure negative chemical ionization mass spectrometry; Analytical Chemistry, (1999); 71: 1851–1861. [DOI] [PubMed] [Google Scholar]
- 17.Herrington J.S., Myers C.; Electronic cigarette solutions and resultant aerosol profiles; Journal of Chromatography A, (2015); 1418: 192–199. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson C.T., Wilkinson J., Miller J.H., Flora J.W.; Determination of gas-phase carbonyls in e-cigarette aerosol using a sorbent tube vs. impinger collection; Proceedings of CORESTA SSPT October 2015, Jeju Island, South Korea, (2015). [Google Scholar]
- 19.FDA Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list; Federal Register, (2012); 77: 20034–20037. [Google Scholar]
- 20.FDA (2012) Guidance for Industry. Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke Under Section 904(a)(3) of the Federal Food, Drug, and Cosmetic Act. http://www.fda.gov/downloads/TobaccoProducts/GuidanceComplianceRegulatoryInformation/UCM297828.pdf (accessed June, 2015).
- 21.Paine J.B. III, Pithawalla Y.B., Naworal J.D., Thomas J.; Carbohydrate pyrolysis mechanisms from isotopic labeling: Part 1: the pyrolysis of glycerin: discovery of competing fragmentation mechanisms affording acetaldehyde and formaldehyde and the implications for carbohydrate pyrolysis; Journal of Analytical and Applied Pyrolysis, (2007); 80: 297–311. [Google Scholar]
- 22.Laino T., Tuma C., Curioni A., Jochnowitz E., Stolz S.; A revisited picture of the mechanism of glycerol dehydration; Journal of Physical Chemistry A, (2011); 115: 3592–3595. [DOI] [PubMed] [Google Scholar]
- 23.ISO (2012) ISO Standard 3308, International Organization for Standardization. Routine Analytical Cigarette-Smoking Machine—Definitions and Standard Conditions. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 24.Health Canada (1999) Test Method T-115. Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke. Health Canada, Ottawa, Canada. [Google Scholar]
- 25.Vansickel A.R., Edmiston J., Liang Q., Duhon C., Liu J., Sarkar M.; Characterization of electronic cigarette prototype puff topography in adult exclusive cigarette smokers and adult exclusive electronic cigarette users; Presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT), February 5–8, 2014, Seattle, WA POS3-65, (2014).
- 26.García-Alonso S., Pérez-Pastor R., Sevillano-Castaño M.L.; Determination of glyoxal and methylglyoxal in atmospheric particulate matter by 2,4- dinitrophenylhydrazine derivatisation; Toxicological and Environmental Chemistry, (2006); 88: 445–452. [Google Scholar]
- 27.ICH (2005) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline Q2(R1): Validation of Analytical Procedures: Text and Methodology Q2(R1). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed June, 2015).
- 28.Wells Fargo Securities . (2014) Equity Research: Nielsen C-Store Data-E-Cig Sales Remain Strong. Wells Fargo Securities, LLC, Equity Research Department, Minneapolis, MN.