Emerging resistance to chloroquine (CQ) by Plasmodium vivax threatens the health of the hundreds of millions of people routinely exposed to the risk of infection with this organism. CQ has been the first-line therapy for vivax malaria since 1946 (32, 115). Plasmodium falciparum developed resistance to CQ in the 1950s (110), and today it occurs globally (91). Resistance by P. vivax was unknown until 1989, when Australians repatriated from Papua New Guinea failed routine treatment (94). Subsequent reports affirmed that finding, and CQ-resistant P. vivax (CRPV) was reported from Indonesia (8, 35, 99, 100, 111). Reports from Myanmar (76, 82) and India (56, 107) followed. CRPV appeared in travelers from Guyana, South America (88). However, studies in Thailand (38, 72, 103), the Philippines (10), and Vietnam (105) revealed only CQ-sensitive P. vivax. Surveys in Indonesia revealed a low frequency of CRPV in the west (∼10%) (15, 16, 49, 50, 51, 53, 75) and a higher risk in the east (∼45%) (9, 18, 52, 81, 102, 106). This minireview summarizes the present state of knowledge of CRPV as a scientific, clinical, and public health problem. It examines the genesis of CQ therapy for P. vivax and the laboratory and clinical data underpinning the diagnosis of CRPV. The available data showing the global distribution of CRPV are listed. Finally, the clinical data on alternative therapies against CRPV are reviewed.
VIVAX MALARIA AND ANTIMALARIAL THERAPY
Four species in the genus Plasmodium routinely infect humans. P. vivax infects 80 million people annually and accounts for most cases of malaria occurring outside Africa (79). It rarely causes death but inflicts debilitating fever, chills, nausea, vomiting, and myalgia. The prevalence of P. vivax typically ranges from <1 to 25% in areas of Asia and the Americas where the organism is endemic, but it is resurging and now threatens to reencroach where it had been eradicated (7, 28, 31). The chemotherapeutic management of vivax malaria therefore represents an issue of importance to global health.
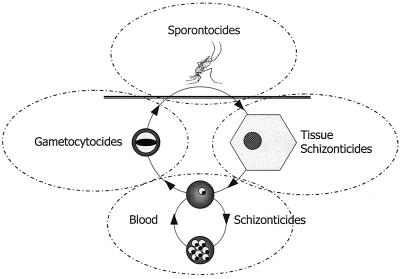
The life cycle of plasmodia defines the chemotherapeutic strategies. These parasites pass through a complex life cycle marked by forms of distinct morphology, function, location, clinical consequence, and susceptibility to antimalarial agents. Figure 1 illustrates the four families of antimalarial drugs defined on the basis of their activities against specific stages in the life cycle. CQ is a blood schizonticide against both P. vivax and P. falciparum. Its activity as a gametocytocide within therapeutic ranges is nonexistent against P. falciparum gametocytes but is potent against P. vivax gametocytes. CQ alone exerts no known sporonticidal or tissue schizonticidal activity.
FIG. 1.
Schematic representing the life cycle of plasmodia and the four families of antimalarial agents. Sporontocidal agents kill forms in the mosquito, including infectious sporozoites. Tissue schizonticides kill parasites developing (schizonts) or quiescent (hypnozoites) in the liver. Blood schizonticides kill the asexual blood forms (trophozoites and schizonts) that cause clinical malaria. The gametocytocides kill or sterilize the sexual forms (gametocytes) that infect mosquitoes.
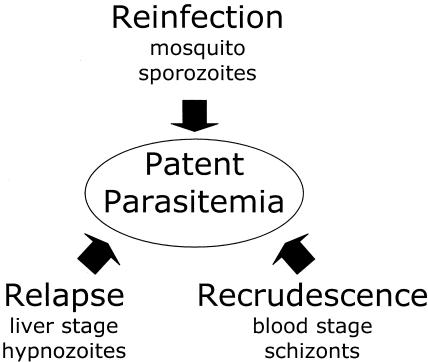
Relapse is an important aspect of the P. vivax life cycle bearing upon chemotherapy and its assessment. Relapse refers to clinical malaria caused by parasites in the bloodstream originating from dormant liver stages called hypnozoites seeded by sporozoites from infectious anopheline mosquitoes (Fig. 1). Relapse may occur weeks to years following the primary episode of parasitemia and clinical disease. Tissue schizonticides, like primaquine, prevent relapse by killing the stages of the organism in the liver. When a parasitemia reappears after blood schizonticidal therapy, it may be a relapse from the liver, a reinfection by a mosquito, or a recrudescence originating from asexual blood-stage parasites that survived therapy (Fig. 2). The emergence of CQ-resistant P. vivax favors the last possibility.
FIG. 2.
The three paths to recurrent parasitemia for malarias caused by P. vivax and P. ovale. Sporozoites from mosquitoes reinfect the livers of humans, which yields merozoites that infect blood. Some sporozoites develop to quiescent hypnozoites in the liver and later cause relapse. Subpatent trophozoites in blood cells mature to schizonts that rupture and release merozoites that infect the blood and cause a recrudescence.
CQ THERAPY
Development.
The first treatment of humans with CQ occurred in 1936 in four syphilis patients in Dusseldorf, Germany, given P. vivax (32). An accounting of the lost records of that trial describes CQ as being “too toxic for practical use in humans.” The Germans investigated sontochin, a methylated analog, which American forces obtained in liberated Algeria in May 1943. Patents for sontochin and CQ were discovered in the United States, and both compounds went to clinical trials. That work, detailed by Wiselogle (114), proved that CQ was more effective and better tolerated.
The early nomenclature of CQ includes SN-7618 and “resochin,” identifiers used by the American and German developers, respectively. The name CQ was formally registered in the United States in March 1946. A month later Loeb et al. (71) published the seminal paper on the activity of CQ against falciparum and vivax malaria. They recommended 1.5 g of base over 48 h for the treatment of acute falciparum or vivax malaria and 0.3 g of base weekly for prophylaxis. These remain the standards for treatment and prophylaxis.
Standard versus effective therapy.
The genesis of recommended therapy constitutes a critical factor now, 60 years later, in defining resistance by P. vivax. What was the minimally effective dose? Loeb et al. (71) provided no data, instead publishing the “Statement Approved by the Board for the Coordination of Malarial Studies.” Most et al. (80) published the first clinical data in November 1946: several hundred American soldiers were treated with 1.0 g over 12 h, 1.5 g over 96 h, or 2.0 g over 7 days. They recommended the use of 1.5 g over 48 h, a regimen not represented in their work.
In 1947, Gordon et al. (58) reported that only 0.8 g (for 6 days) had good efficacy against vivax malaria in 39 subjects. Berliner et al. (20) described total doses from 0.3 to 0.6 g as consistently curing blood-stage P. vivax (McCoy strain) in 10 subjects. However, total doses <0.3 g often failed. Others soon affirmed the sensitivity of P. vivax to substandard regimens down to a 0.3-g total adult dose. Hoekenga (65) described 0.6- or 0.45-g single-dose regimens in Honduras in 1952. Among 100 subjects receiving 0.6 g, only 1 failed the treatment. Among 120 subjects receiving 0.45 g, 5 failed the treatment. In 1950, Butts (27) reported on 202 patients in Central America treated with 0.08 to 1.56 g. Failures occurred only among those receiving <0.3 g. Wilson and Edeson (113) reported on similar findings from Malaysia; among 62 subjects treated with single doses of 0.3 to 0.6 g, none failed. According to Harinasuta as late as 1992 (as cited by Looareesuwan et al. [73]), P. vivax in Thailand remained sensitive to treatment with a single 0.35-g dose. The available data suggest a baseline sensitivity compatible with blood-stage cure of P. vivax with ≥0.3 g of CQ base.
The data from areas of endemicity led to reasoned recommendations for substandard treatments in “immune” populations (22). There was no need (or reliable evidence) to invoke immunity as the basis of efficacy. These regimens had superior efficacies in nonimmune people as well.
RELAPSE
Assessment of the therapeutic effect of CQ requires an understanding of relapse, which is parasitemia originating from latent hypnozoites. The risk of relapse varies with geographic origin. Strains form tropical regions cause relapses more quickly and more often than strains from temperate regions. The rates from a variety of studies have been reviewed elsewhere (12). The risk of relapse with tropical P. vivax often exceeds 50% within a month of the primary attack, and multiple relapses are the rule. Among strains from temperate regions, the risk of relapse typically ranges from 5 to 25%, and multiple relapses are rare.
Early clinical trials often used the Chesson strain of P. vivax, isolated from an American soldier in New Guinea during the war (45). Quinine therapy against Chesson is carefully considered here because it provides a basis for understanding how relapse confounds gauging of the therapeutic response to CQ. Wiselogle (114) provided a comprehensive review of the activity of quinine against P. vivax. In the American clinical trials conducted during World War II, quinine served as the positive control for therapeutic activity. Shannon, in Wiselogle (114), explains the rationale as the fact that quinine is eliminated within 12 h, and thus, a relapse uninhibited by lingering drug is allowed. Shannon further explains the certainty that quinine cured the blood; subjects challenged by blood inoculation and given 12 g of quinine over 12 days were cured without recurrence.
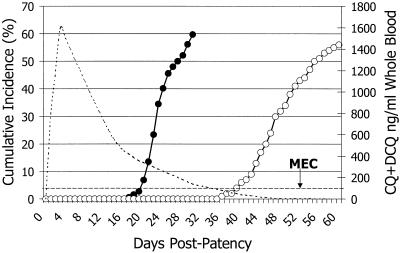
The Chesson sporozoite-challenged subjects treated with quinine represent, as they did 60 years ago, the key to gauging the therapeutic response to CQ by P. vivax. Figure 3 illustrates the relapse rates after quinine treatment. Relapse occurred no sooner than 17 days after the primary attack (5 days after quinine treatment). The median time to relapse was 22 days after the primary attack, and 60% had relapses by 30 days. However, a relapse before 17 days is possible; people harboring multiple broods of hypnozoites accumulated over months or years of exposure may have a relapse at any time. Figure 1 nonetheless suggests that a relapse before 17 days is unlikely among patients experiencing a primary attack after a brief exposure.
FIG. 3.
Cumulative incidence of relapses (left y axis) of P. vivax infections after blood schizonticidal therapy with quinine (solid points) or CQ (hollow points) during clinical trials conducted during the 1940s. Data were gathered from various sources (5, 33, 41, 58, 80, 92, 112, 114). The dotted curve represents hypothetical CQ drug levels (right y axis) declining to below the MEC of approximately 100 ng/ml, marked by the hashed line parallel to the x axis.
RELAPSE AFTER CQ TREATMENT
In the 1940s recurrent parasitemia after effective CQ therapy defined the timing and the risk of relapse for CQ-sensitive P. vivax. Figure 3 illustrates those data. The risk of relapse begins after 35 days, and the rate climbs to 58% by 60 days. What explains the dramatic difference with relapse after quinine treatment? Neither drug alone affects liver stages. The explanation for the difference between the two plots constitutes the rational basis for a clinical diagnosis of resistance.
Figure 3 reveals that the challenge of a relapse commences at 17 days and that >60% of patients have relapses by 35 days (after quinine treatment). The CQ lingering in the bloodstream for up to 35 days after therapy prevents the recurrent parasitemias of relapse. CQ-sensitive parasites attempting development in the bloodstream at 35 days encounter drug levels below the minimal effective concentration (MEC) and relapse occurs. If this interpretation is accurate, standard therapy should result in drug levels close to the MEC at 35 days. Data from other sources confirm this. Berliner et al. (20) measured the MECs for P. vivax in the 1940s. They noted complete cure required ≥10 ng/ml of plasma. Coatney et al. (34) measured CQ levels after a 1.3-g regimen on day 35 and found them to be 8, 9, and 19 ng/ml. These data represent the basis of the widely cited MEC for P. vivax being 10 ng/ml and corroborate the explanation given for the delayed relapses after CQ therapy.
RATIONALE FOR IN VIVO DIAGNOSIS
Biological versus clinical resistance.
A single dose of >0.3 g of CQ or a level in blood plasma >10 ng/ml should achieve complete cure of blood-stage CQ-sensitive P. vivax. An infection that persists in the face of these exposures to drug may be classified biologically resistant. However, no one recommends that vivax malaria be treated with just 0.3 g of CQ. Standard therapy delivers five times that dose, and failure of that regimen constitutes clinical resistance. However, the tidy separation of biological and clinical resistance is difficult with P. vivax. Clinicians wonder if the standard 1.5-g CQ regimen eliminates parasitemia. If not, infection survived the towering drug concentrations following therapy (approximately 200 ng/ml of plasma). This is clinical resistance. In contrast, a parasite emerging from the liver and penetrating waning levels of drug above 10 ng/ml of plasma is biologically resistant. Although spared the immediate spike of drug soon after therapy, relapsing parasites would nonetheless have been killed if they were sensitive (Fig. 3).
Recrudescence versus reinfection and relapse.
Molecular biological assays classify genotypes of P. vivax and distinguish reinfection from relapse (26, 40, 67). However, distinguishing recrudescence from reinfection or relapse represents the critical need in analyzing the therapeutic response. The methods of genetic analysis available at present do not allow this. A genetic match between isolates causing primary and secondary parasitemias does not prove recrudescence because hypnozoites often derive from the genotype of the reference isolate causing parasitemia (40, 67). Mismatch is also ambiguous: it may be a reinfection or a relapse originating from hypnozoites of a distinct genotype or a recrudescence of a minority genotype not originally detected. No method available at present allows the unambiguous classification of P. vivax parasitemias originating from recrudescence, relapse, or reinfection. Multilocus genotyping may ultimately bring clarity to this issue (6).
Sensitive versus resistant.
The detection of CRPV does not require relapse, reinfection, and recrudescence to be distinguished (13). A recurrent parasitemia, regardless of origin, should not occur within 35 days of standard CQ therapy (Fig. 1). Parasitemia before 35 days penetrated an ordinarily lethal exposure to CQ. Parasitemia clearing within 4 days and not reappearing in 35 days may be classified as sensitive.
Clinicians and health policy makers want to know when “resistant” parasites survived therapy or merely penetrated waning levels of drug. The question is not academic. If parasites routinely survive a therapeutic intervention, it must be changed. If parasites instead relapse only after successful therapy, the problem may be attacked with primaquine. The classification explained here allows no such distinction. The analysis nonetheless provides key insights that help guide treatment decisions.
Parasites classified as resistant either persisted for 4 days or cleared but reappeared within 35 days. Persistent parasitemia after 4 days represents the highest grade of clinical resistance, whereas parasitemia recurring late (e.g., 30 days) is probably a relapse in which the isolates penetrated drug levels close to the MEC. The day of recurrence offers a scale upon which relative resistance may be gauged. Parasites penetrating higher drug levels soon after therapy are empirically more resistant than parasites that penetrate lower drug levels later. Clinicians and health policy makers may consider the proportion classified as resistant and the median day of recurrence indicators of the degree of resistance in the parasite population.
EVIDENCE SUPPORTING IN VIVO DIAGNOSIS
Recurrent parasitemia within 35 days of CQ therapy supports a provisional diagnosis of resistance. Confirmation requires proof of adequate compliance to and absorption of therapy by reliable supervision or, ideally, by determination of the levels of drug in blood. Figure 3 illustrates data derived from clinical trials with supervised dosing and ascertainment of drug levels. Counterfeit drug, poor compliance, and emesis may prevent normal drug levels from being achieved. An unambiguous diagnosis of CRPV infection requires the demonstration of parasitemia with ordinarily effective drug levels (>10 ng/ml of plasma).
The blood challenge experiments of Berliner et al. (20) provided a definitive MEC for CQ-sensitive P. vivax. After CQ treatment the relapse pattern with the plasma drug levels (Fig. 3) corroborated the estimate of the MEC. Two issues must be considered today when the MEC is applied to present assessments of CQ treatment effectiveness: (i) the analytical methods of the 1940s and (ii) plasma CQ levels versus whole-blood CQ levels.
Most laboratories have adopted the extraction and high-pressure liquid chromatography procedures described by Patchen et al. (86). This method quantifies CQ and its major metabolite, desethylchloroquine (DCQ), in whole blood collected onto filter paper. This medium spares the need for centrifugation and a cold chain, a key advantage in the setting of the rural tropics.
Early analytical methods did not discriminate between CQ and DCQ (25, 77). The 10-ng/ml MEC represents the sum of the plasma CQ and DCQ concentrations. In vitro studies with CQ-sensitive P. falciparum consistently reveal that CQ and DCQ have roughly equal antimalarial activities (54, 57, 108). In gauging adequate exposure to drug in the bloodstream, the sum of the CQ and DCQ concentrations is the operative statistic. The ratio of the CQ concentration to the DCQ concentration has no clear bearing on the interpretation of this question, except that a very low DCQ concentration relative to the CQ concentration (e.g., the ratio of the CQ concentration to the DCQ concentration > 10) suggests contamination of the sample with CQ dust.
The level of CQ-DCQ in whole blood corresponding to 10 ng/ml of plasma must be estimated to obtain a MEC in whole blood. Several studies measured CQ and DCQ concentrations in whole blood and plasma (2, 21, 47, 59, 96). The ratio of the concentration in whole blood to the concentration in plasma ranged from 5 to 10 (median, 8), yielding an estimated MEC in whole blood of 75 to 150 ng/ml. These values agree with those occurring 30 to 35 days after therapy, when relapse commences (Fig. 1). Rombo et al. (95) deduced an MEC of 90 ng/ml of whole blood for P. vivax among subjects taking CQ prophylaxis. A 100-ng/ml MEC for CQ-DCQ in whole blood against CQ sensitive P. vivax was adopted (13).
Recurrent parasitemia within 35 days of therapy with ≥100 ng of CQ-DCQ per ml in whole blood demonstrates resistance to CQ. A test duration of 28 days was recommended (13) to accord with the standard World Health Organization test for P. falciparum. The in vivo assessment represents a direct and often convenient means of detecting resistance.
OTHER APPROACHES TO DIAGNOSING RESISTANCE
Experimental animals.
Some nonhuman primate host-adapted strains of P. vivax have been evaluated. Collins and colleagues (36, 37) studied two strains of P. vivax acquired from patients in Indonesia who failed CQ therapy. The therapeutic profiles of CQ among well-characterized strains in humans provide a basis for the classification of wild isolates as sensitive or resistant in animal models. Collins et al. (36) discussed the rationale for classifying the CQ susceptibilities of strains of P. vivax in Aotus or Saimiri monkeys. They point to variations in the minimal therapeutic doses for strains known to be sensitive to CQ in humans: Vietnam Palo Alto (>18 mg), Achiote (10 mg), and Chesson (9 mg). CQ-resistant Indonesian strain CDC I failed to be eradicated with 15 mg and was classified as “possibly even more resistant than the Vietnam Palo Alto strain.” The other strain from Indonesia failed to be eradicated with 30 mg and was classified as resistant (37).
In vitro methods.
The diagnosis of resistance to P. falciparum in vitro has been standard procedure since the 1970s. Although P. vivax has not been cultivated continuously, it develops for periods sufficient to assess the therapeutic response. Methods for doing so have been described since the 1970s (24, 55, 90, 93), and there is renewed interest in these techniques (60, 68, 98, 103, 104). No standard criteria for classifying in vitro responses as sensitive or resistant yet exist. However, many isolates have been characterized in Thailand, where the clinical responses to CQ treatment remain uniformly sensitive. This provides a baseline for in vitro sensitivity: ∼50 ng/ml consistently inhibits development by 50%. In vitro testing for CRPV may prove useful among well-equipped laboratories.
Molecular probes.
No genetic mutations have been linked to resistance to CQ by P. vivax. Nomura et al. (83) investigated mutations in the P. vivax ortholog of the crt gene of P. falciparum, which has been linked to CQ resistance. The mutations incriminated in P. falciparum crt did not occur among CRPV isolates, and no other mutations in that gene correlated with the phenotype. The genetic determinants of resistance to CQ apparently differ between P. vivax and P. falciparum. Continuous cultivation allowed the search for crt mutants in P. falciparum, and similar progress for P. vivax may be difficult; but genomic analyses (46) may ultimately yield genetic determinants of resistance.
Prophylaxis and cross-sectional studies.
Among 94 study subjects taking supervised CQ prophylaxis (5 mg/kg of body weight weekly) in Indonesian New Guinea for 18 or 52 weeks, 29% developed P. vivax infections (11, 48), a rate indistinguishable from that for a placebo group (48). Among 41 subjects in the same region evaluated in two other studies (8, 81), 61% developed vivax malaria. Vivax malaria occurring during supervised prophylaxis proves resistance to CQ.
Cross-sectional analyses of CQ levels may help gauge endemic resistance. Collection of blood on dried filter paper and stained blood films (and later analysis in a laboratory) allow assessment of hundreds of people with just a single day on-site, whereas in vivo assessments require at least 1 month on-site. This approach requires caution, however. A patient reporting to a clinic with concentrations in blood greater than the MEC may have recently self-administered drug, and even sensitive P. vivax may take 4 days to clear. Nonetheless, the proportion of patients infected with P. vivax and having concentrations in blood greater than the MEC provides an estimate of the risk of resistance (14).
GEOGRAPHIC RANGE OF RESISTANCE
Oceania.
The data from Oceania come from just six infections acquired in Papua New Guinea, and no new report has appeared in the past 10 years. Nonetheless, the risk of therapeutic failure is considered high on the basis of the weight of the data from Indonesian New Guinea (see below and Table 1). Moreover, unpublished data (99a) revealed therapeutic failure rates from 0 to 33% in the late 1980s. Surveys of therapeutic responses to CQ throughout Oceania are needed.
TABLE 1.
Summary of recently reported CQ sensitivity or resistance by P. vivax
| Region | Country | Area | Yr | No. of infections examined | No. of resistant infectionsa | Use of drugb | Reference |
|---|---|---|---|---|---|---|---|
| Oceania | Papua New Guinea | East New Britain | 1989 | 2 | 2 | Rx | 94 |
| Not specified | 1989 | 1 | 1 | Rx | 111 | ||
| Madang/Lae | 1991 | 1 | 1 | Rx | 35 | ||
| New Britain | 1992 | 1 | 1 | Rx | 35 | ||
| Madang and Lae | 1992 | 2 | 2 | Rx | 99 | ||
| Southeast Asia | Indonesia | Arso, Papua | 1992 | 24 | 16 | Px | 8 |
| Arso, Papua | 1992 | 1 | 1 | Rx and Px | 8 | ||
| Nias, Sumatra | 1992 | 1 | 1 | Rx and Px | 100 | ||
| Arso, Papua | 1993 | 46 | 10 | Rx | 81 | ||
| Arso, Papua | 1993 | 17 | 9 | Px | 81 | ||
| Arso, Papua | 1995 | 50 | 35 | Rx | 9 | ||
| Arso, Papua | 1995 | 54 | 12 | Px | 11 | ||
| Arso, Papua | 1995 | 40 | 15 | Px | 48 | ||
| Menoreh Hills, Java | 1996 | 14 | 0 | Rx | 15 | ||
| Nias, Sumatra | 1996 | 21 | 3 | Rx | 16 | ||
| Nabire, Papua | 1996 | 34 | 18 | Rx | 18 | ||
| Arso, Papua | 1997 | 49 | 42 | Rx | 102 | ||
| Western Lombok | 1997 | 20 | 0 | Rx | 49 | ||
| Western Borneo | 1998 | 54 | 12 | Rx | 53 | ||
| Northern Sulawesi | 1998 | 11 | 1 | Rx | 51 | ||
| Lesser Sundas | 1998 | 1 | 1 | Rx | 61 | ||
| Gag Island, Papua | 1999 | 38 | 0 | Rx | 52 | ||
| Jayapura, Papua | 2001 | 17 | 5 | Rx | 106 | ||
| Nias, Sumatra | 2002 | 28 | 6 | Rx | 50 | ||
| Menoreh Hills, Java | 2002 | 77 | 14 | Rx | 75 | ||
| Malaysia | Sabah, Borneo | 1996 | 1 | 1 | Rx and Px | 1 | |
| Urban composite | 1998 | 60 | 6 | Rx | 66 | ||
| Myanmar | Mine Pyin and Pwint Phyu | 1993 | 2 | 2 | Rx | 82 | |
| Mingaladon | 1995 | 50 | 7 | Rx | 76 | ||
| Thailand | Bo Rai | 1991 | 77 | 0 | Px | 23 | |
| Chantaburi | 1995 | 57 | 0 | Rx | 103 | ||
| Urban composite | 1999 | 886 | 4 | Rx | 72 | ||
| Sa Kaeo | 2002 | 26 | 0 | Rx | 38 | ||
| Vietnam | Na Trang | 2000 | 23 | 0 | Rx | 105 | |
| Not specified | 2002 | 120 | 28 | Rx | 87 | ||
| Philippines | Palawan | 1996 | 21 | 0 | Rx | 10 | |
| South Asia | Iran | Bandar Abbas | 2002 | 39 | 0 | Rx | 60 |
| India | Bombay | 1995 | 2 | 2 | Rx | 56 | |
| Bombay | 1995 | 7 | 1 | Rx | 89 | ||
| Calcutta | 1995 | 1 | 1 | Px and Rx | 107 | ||
| Mathura | 1996 | 1 | 1 | Rx | 44 | ||
| Bombay | 2000 | 1 | 1 | Rx | 69 | ||
| Bombay | 2003 | 1 | 1 | Rx | 42 | ||
| South America | Guyana | Interior | 1996 | 3 | 3 | Rx | 88 |
| Interior | 2002 | 32 | 0 | Rx | 17 | ||
| Colombia | Llanos Orientales/Uraba | 2001 | 27 | 3 | Rx | 101 | |
| Cali | 2002 | 44 | 0 | Rx | 30 | ||
| Brazil | Manaus | 1999 | 1 | 1 | Rx | 4 | |
| Rondonia | 2000 | 73 | 0 | Rx | 109 | ||
| Belem | 2003 | 30 | 0 | Rx | 74 | ||
| Peru | Amazonia | 2003 | 177 | 4 | Rx | 97 |
Classified as resistant by the reporting authors.
Rx, standard treatment; Px, standard prophylaxis.
East Asia.
Six East Asian nations have reported data on CRPV: Indonesia, Malaysia, Myanmar, Thailand, Vietnam, and Philippines. The data from Indonesia span 1992 to 2002 and reveal a high risk in the eastern archipelago. The risk in the far eastern province of Papua is highest. Among 41 subjects taking supervised prophylaxis, 61% developed vivax malaria (8, 81), as did 29% (the same rate as the placebo group) in two other studies (11, 48). Among 88 subjects treated up to 1995, 41% had a response consistent with resistance (8, 81). Among 282 subjects evaluated since then, 39% were infected with resistant isolates (9, 11, 48, 52, 102, 106). However, all 38 subjects evaluated on a small, isolated island in Papua had infections with sensitive organisms (52). In western Indonesia two separate surveys at Nias, near northern Sumatra, found 9 inadequate responses among 49 subjects (18%) (16, 50). A survey of 54 subjects in Indonesian Borneo revealed that 22% of the isolates were CRPV (53). Surveys at Lombok (west of Bali) and central Java in the early 1990s found no resistance among isolates from 20 and 14 subjects, respectively (15, 49). However, the site in central Java was reevaluated in 2001, and among 77 subjects, 14 (18%) were infected with CRPV (75). A traveler to Flores in the Lesser Sundas archipelago had CRPV infection (61).
CPRV is endemic in Malaysia, Myanmar, and Vietnam. A traveler to Sabah in Malaysian Borneo (1) presented with vivax malaria in Sweden with ordinarily protective CQ levels in blood. Among a series of 60 patients hospitalized in Kuala Lumpur (1983 to 1992), 6 were described as infected with CRPV (66). CRPV has been confirmed in Myanmar: Myat-Phone-Kyaw et al. (82) presented two case reports, and a survey of 50 patients (76) revealed that 14% were infected with CRPV. A study of 23 subjects in Vietnam in 1995 revealed no CRPV (105), but recently, 28 of 113 (25%) subjects had recurrent P. vivax infections by day 28 after treatment (87).
There is no evidence of CPRV from Thailand, Cambodia, Laos, China, the Korean Peninsula, or the Philippine archipelago. However, Thailand represents the only one of these nations in which adequate surveys have been conducted (38, 72, 103): 1,046 subjects were reliably evaluated, and only 4 (0.4%) had recurrent parasitemia within 28 days after infection (and these responded to a second round of CQ therapy). No reports of CQ efficacy against P. vivax were found from Cambodia, Laos, China, or the Koreas. At Palawan in the Philippines, Baird et al. (10) evaluated 21 P. vivax infections and none recurred within 28 days.
South Asia.
Only seven cases of CRPV have been reported from India (42, 44, 56, 89, 69). The only other study from southern Asia comes from Iran: Hamedi et al. (60) evaluated 39 subjects, and none had a recurrent parasitemia within 28 days.
South America.
Phillips et al. (88) reported on the presence of CPRV in three travelers to Guyana repatriated to Canada. Baird et al. (17) evaluated 32 subjects in Guyana with vivax malaria, and none had recurrent parasitemia within 28 days. Likewise, Castillo et al. (30) and Villalobos-Salcedo et al. (109) evaluated 44 and 73 subjects in Columbia and Brazil, respectively, and none had recurrent parasitemia within 28 days. Machado et al. (74) evaluated 30 subjects infected near Belem in Brazil; the infection was cleared from all subjects by day 4, and none had a recurrence by day 30. However, Soto et al. (101) described three cases of CRPV infection among 27 subjects in Colombia, and Alecrim et al. (4) described a single case from Amazonia. Ruebush and colleagues (97) confirmed four cases of CRPV infection among 177 (2%) infected subjects evaluated in the Amazon region of Peru. CRPV apparently occurs in the New World but at a low frequency (risk, probably <5%).
ALTERNATIVE THERAPIES
Mefloquine.
Some authorities recommend mefloquine for therapy for CRPV (29, 78). No clinical data yet support that recommendation. Mefloquine proved effective against CQ-resistant P. falciparum and was effective against CQ-sensitive P. vivax (3, 43, 62). Good efficacy against CRPV seems a reasonable supposition, and Collins et al. (37) demonstrated that mefloquine had good efficacy against an Indonesian CRPV strain in Aotus monkeys. However, work by Nomura et al. (83) points to different mechanisms of resistance between the two species, and caution is warranted.
Indirect evidence suggests that mefloquine may be efficacious against CRPV. Ohrt et al. (85) demonstrated the complete efficacy of mefloquine for prophylaxis against the CRPV strain known to occur in northeastern Indonesian New Guinea. However, they also found that daily doxycycline had complete efficacy against CRPV, and Taylor et al. (106) showed doxycycline monotherapy to have only 33% efficacy against CRPV. Clinical trials of mefloquine against CRPV are needed.
Halofantrine, CQ plus doxycycline, or primaquine.
Taylor et al. (106) evaluated CQ and doxycycline combined in Indonesia and found 71% efficacy. This was superior to the 29 and 33% efficacies of the respective monotherapies against vivax malaria but was inferior to the 91% efficacy of the combination against P. falciparum. Baird et al. (9) evaluated halofantrine monotherapy and CQ combined with primaquine against P. vivax in Indonesian New Guinea. CQ combined with primaquine (10 mg/kg over 2 weeks or 2.5 mg/kg over 48 h) provided superior efficacy in 79 patients (87%) relative to the efficacy of CQ monotherapy in 50 patients (30%). Halofantrine monotherapy cured all 19 subjects treated, although there was one recurrence on day 28 after the end of treatment.
Phillips et al. (88) used CQ (25 mg/kg over 2 days) and primaquine (2.5 mg/kg over 2 days) in three patients with CRPV infections acquired in Guyana. They described this therapy as inadequate because two patients had recurrent parasitemia after 6 weeks. However, the abbreviated primaquine regimen was not intended to prevent relapse but to clear the bloodstream, and it apparently achieved this in all three patients. The best combination of CQ plus primaquine may be 0.5 mg of primaquine/kg daily for 14 days or 1.0 mg of primaquine/kg daily for 7 days. This regimen clears the bloodstream of CRPV and would prevent relapse. It should not be used for patients likely to be infected with P. falciparum as well, because it has no efficacy against that type of infection (19).
Malarone.
Lacy et al. (70) evaluated Malarone (250 mg of atovaquone and 100 mg of proguanil daily for 3 days; GlaxoSmithKline, London, United Kingdom) in 16 subjects with P. vivax infections in Indonesian New Guinea. They also received 0.5 mg of primaquine/kg daily for 14 days. All subjects cleared the fever and parasitemia by day 3 and remained free of parasitemia for 28 days. Malarone combined with primaquine is the only available therapy with proven efficacy (>90%) against CRPV, but that finding is from the results of a study with just 16 patients.
Tafenoquine.
Tafenoquine (GlaxoSmithKline) is an 8-aminoquinoline analog of primaquine that has potent schizonticidal activity against P. vivax and P. falciparum in tissue and blood and that is now in clinical trials. This drug has been demonstrated to be effective against CRPV in nonhuman primates (39, 84).
Sulfadoxine-pyrimethamine.
Recent work by Hastings and Sibley (63) suggests that P. vivax may be susceptible to antifolate inhibitors of dihydrofolate reductase (DHFR). DHFR mutations in P. vivax are apparently responsible for the lack of activity among the antifolates. Hastings et al. (64) also found that quadruple mutations in dihydrofolate reductase corresponded to the therapeutic failure of sulfadoxine-pyrimethamine treatment among patients with P. vivax infections acquired in Indonesia. When quadruple P. vivax dhfr mutants do not occur, sulfadoxine-pyrimethamine may be useful against CRPV, but clinical trials are needed.
CONCLUSIONS
Infections with CQ-sensitive P. vivax were routinely cured with as little as 0.3 g of CQ base, even though 1.5 g has been the recommended therapy since 1946. The clinical failure of standard therapy therefore represents infection with an organism with a high degree of resistance. A persistent or recurrent parasitemia within 14 days of the start of treatment probably represents recrudescence by a highly resistant strain of P. vivax. A recurrent parasitemia between 15 and 35 days after the start of treatment with >100 ng of CQ-DCQ per ml is resistant to CQ, regardless of whether that parasitemia originates from a relapse, a reinfection, or a recrudescence. In general, the day of recurrence correlates inversely with degree of resistance, but isolates that cause recurrences after CQ and DCQ levels fall below the MEC (at about day 35) cannot be classified as sensitive or resistant. When 30 mg of CQ base against P. vivax in Aotus monkeys fails, the organism may be classified as resistant. When 50 ng of CQ base/ml fails to inhibit in vitro schizont development by more than 50%, the isolate may be classified as resistant. CRPV appears to be most common in eastern Indonesia, especially on the island of New Guinea. It appears sporadically elsewhere in Southeast Asia, typically among <15% of strains. No cases of CRPV infection have yet occurred in Thailand. The data supporting alternative therapies for CRPV are scanty. A small trial of Malarone combined with primaquine in Indonesian New Guinea may be the best available evidence of the good efficacy of this agent against CRPV.
Acknowledgments
This work was supported in part by the U.S. Department of Defense Global Emerging Infections Surveillance Program.
The views presented here are my own and do not purport to reflect those of the U.S. Navy or the U.S. Department of Defense.
REFERENCES
- 1.Ahlm, C., J. Wistrom, and H. Carlsson. 1996. Chloroquine-resistant Plasmodium vivax in Borneo. J. Travel Med. 3:124. [DOI] [PubMed] [Google Scholar]
- 2.Akintonwa, A., M. C. Meyer, and P. T. R. Hwang. 1983. Simultaneous determination of chloroquine and desethylchloroquine in blood, plasma, and urine by high-performance liquid chromatography. J. Liquid Chromatogr. 6:1513-1522. [Google Scholar]
- 3.Alcantara, A. K., C. V. Uylangco, R. P. Sangalong, and J. H. Cross. 1985. A comparative clinical study of mefloquine and chloroquine in the treatment of vivax malaria. Southeast Asian J. Trop. Med. Public Health 16:534-538. [PubMed] [Google Scholar]
- 4.Alecrim, M. G. C., W. Alecrim, and V. Macedo. 1999. Plasmodium vivax resistance to chloroquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 32:67-68. [DOI] [PubMed] [Google Scholar]
- 5.Alving, A. S., B. Craige, Jr., C. M. Worton, T. N. Pullman, and L. Eichelberger. 1948. Pentaquine, a therapeutic agent effective in reducing the relapse rate in vivax malaria. J. Clin. Investig. 27(Suppl. 2):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson, T. J. C., B. Haubold, J. T. Williams, J. G. Estrada-Franco, L. Richardson, R. Mollinedo, M. Bockarie, J. Mokili, S. Mharakurwa, N. French, J. Whitworth, I. D. Velez, A. H. Brockman, F. Nosten, M. U. Ferreira, and K. P. Day. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17:1467-1482. [DOI] [PubMed] [Google Scholar]
- 7.Baird, J. K. 2000. Resurgent malaria at the millennium: control strategies in crisis. Drugs 59:719-743. [DOI] [PubMed] [Google Scholar]
- 8.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 9.Baird, J. K., H. Basri, B. Subianto, D. J. Fryauff, P. D. McElroy, B. Leksana, T. L. Richie, S. Masbar, F. S. Wignall, and S. L. Hoffman. 1995. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J. Infect. Dis. 171:1678-1682. [DOI] [PubMed] [Google Scholar]
- 10.Baird, J. K., E. Caneta-Miguel, S. Masbar, D. Bustos, J. A. Abrenica, A. V. O. Layawen, J. M. Calulut, B. Leksana, and F. S. Wignall. 1996. Survey of resistance to chloroquine of falciparum and vivax malaria in Palawan, the Philippines. Trans. R. Soc. Trop. Med. Hyg. 90:413-414. [DOI] [PubMed] [Google Scholar]
- 11.Baird, J. K., D. J. Fryauff, H. Basri, M. J. Bangs, B. Subianto, I. Wiady, Purnomo, B. Leksana, S. Masbar, T. L. Richie, F. S. Wignall, and S. L. Hoffman. 1995. Primaquine for prophylaxis against malaria among nonimmune transmigrants in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 52:479-484. [DOI] [PubMed] [Google Scholar]
- 12.Baird, J. K., and S. L. Hoffman. Primaquine therapy of malaria. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 13.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 14.Baird, J. K., B. Leksana, S. Masbar, Suradi, M. A. Sutanihardja, D. J. Fryauff, and B. Subianto. 1997. Whole blood chloroquine concentrations with Plasmodium vivax infection in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:618-620. [DOI] [PubMed] [Google Scholar]
- 15.Baird, J. K., P. Sismadi, S. Masbar, B. Leksana, Sekartuti, A. Ramzan, and E. Tjitra. 1996. Choroquine sensitive Plasmodium falciparum and P. vivax in central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:412-413. [DOI] [PubMed] [Google Scholar]
- 16.Baird, J. K., M. F. Sustriaya Nalim, H. Basri, S. Masbar, B. Leksana, E. Tjitra, R. M. Dewi, M. Khairani, and F. S. Wignall. 1996. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:409-411. [DOI] [PubMed] [Google Scholar]
- 17.Baird, J. K., T. Tiwari, G. J. Martin, C. L. Tamminga, T. M. Prout, J. Tjaden, P. P. Bravet, S. Rawlins, M. Ferrel, D. Carucci, and S. L. Hoffman. 2002. Chloroquine for the treatment of uncomplicated malaria in Guyana. Ann. Trop. Med. Parasitol. 96:339-348. [DOI] [PubMed] [Google Scholar]
- 18.Baird, J. K., I. Wiady, D. J. Fryauff, M. A. Sutanihardja, B. Leksana, H. Widjaya, Kysdarmanto, and B. Subianto. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627-631. [DOI] [PubMed] [Google Scholar]
- 19.Baird, J. K., I. Wiady, A. Sutanihardja, Suradi, Purnomo, H. Basri, Sekartuti, E. Ayomi, D. J. Fryauff, and S. L. Hoffman. 2002. Short report: therapeutic efficacy of chloroquine combined with primaquine against Plasmodium falciparum in northeastern Papua, Indonesia. Am. J. Trop. Med. Hyg. 66:659-660. [DOI] [PubMed] [Google Scholar]
- 20.Berliner, R. W., D. P. Earle, Jr., J. V. Taggart, C. G. Zubrod, W. J. Welch, N. J. Conan, E. Bauman, S. T. Scudder, and J. A. Shannon. 1948. Studies on the chemotherapy of the human malarias. VI. The physiological disposition, antimalarial activity, and toxicity of several derivatives of 4-aminoquinoline. J. Clin. Investig. 27:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berqvist, Y., and B. Domeij-Nyberg. 1983. Distribution of chloroquine and its metabolite desethylchloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J. Chromatogr. 272:137-148. [DOI] [PubMed] [Google Scholar]
- 22.Black, R. H., C. J. Canfield, D. F. Clyde, W. Peters, and W. H. Wernsdorfer. 1981. In L. J. Bruce-Chwatt (ed.), Chemotherapy of malaria, 2nd ed. World Health Organization monograph series, no. 27. World Health Organization, Geneva, Switzerland.
- 23.Boudreau, E. F., L. W. Pang, S. Chaikummao, and C. Witayarut. 1991. Comparison of mefloquine, chloroquine plus pyrimethamine-sulfadoxine (Fansidar) and chloroquine as malarial prophylaxis in eastern Thailand. Southeast Asian J. Trop. Med. Public Health 22:183-189. [PubMed] [Google Scholar]
- 24.Brockelman, C. R., P. Tan-Ariya, and D. Bunnag. 1989. Development of in vitro microtest for the assessment of Plasmodium vivax sensitivity to chloroquine. Southeast Asian J. Trop. Med. Public Health 20:41-47. [PubMed] [Google Scholar]
- 25.Brodie, B. B., S. Udenfriend, W. Dill, and G. Downing. 1947. The estimation of organic compounds in biological material. II. Estimation of fluorescent compounds. J. Biol. Chem. 168:319-325. [PubMed] [Google Scholar]
- 26.Bruce, M. C., M. R. Galinski, J. W. Barnwell, G. Snounou, and K. P. Day. 1999. Polymorphism at the merozoite surface protein-3α locus of Plasmodium vivax: global and local diversity. Am. J. Trop. Med. Hyg. 61:518-525. [DOI] [PubMed] [Google Scholar]
- 27.Butts, D. C. A. 1950. Results on 449 cases of naturally acquired malaria treated with chloroquine. J. Natl. Malaria Soc. 9:44-49. [Google Scholar]
- 28.Campbell, C. C. 1997. Malaria: an emerging and re-emerging global plague. FEMS Immunol. Med. Microbiol. 18:325-331. [DOI] [PubMed] [Google Scholar]
- 29.Canada Communicable Disease Report. 2000. Canadian recommendations for the prevention and treatment of malaria among international travelers, vol. 26, suppl. 2. CATMAT, Toronto, Ontario, Canada. [PubMed]
- 30.Castillo, C. M., L. E. Osorio, and G. I. Palma. 2002. Assessement of therapeutic response of Plasmodium vivax and Plasmodium falciparum to chloroquine in a malaria transmission-free area of Colombia. Mem. Inst. Oswaldo Cruz 97:559-562. [DOI] [PubMed] [Google Scholar]
- 31.Chai, J. Y. 1999. Re-emerging Plasmodium vivax in the Republic of Korea. Korean J. Parasitol. 37:129-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coatney, G. R. 1963. Pitfalls in a discovery: the chronicle of chloroquine. Am. J. Trop. Med. Hyg. 12:121-128. [DOI] [PubMed] [Google Scholar]
- 33.Coatney, G. R., W. C. Cooper, and M. D. Young. 1950. Studies in human malaria. XXX. A summary of 204 sporozoite-induced infections by the Chesson strain of Plasmodium vivax. J. Natl. Malaria Soc. 9:381-396. [PubMed] [Google Scholar]
- 34.Coatney, G. R., D. S. Ruhe, W. C. Cooper, E. S. Josephson, and M. D. Young. 1949. Studies in human malaria. X. The protective and therapeutic action of chloroquine (SN 7618) against St. Elizabeth strain of vivax malaria. Am. J. Hyg. 49:49-59. [PubMed] [Google Scholar]
- 35.Collignon, P. 1991. Chloroquine resistance in Plasmodium vivax. J. Infect. Dis. 164:222-223. [DOI] [PubMed] [Google Scholar]
- 36.Collins, W. E., I. K. Schwartz, J. C. Skinner, C. Morris, and V. K. Filipski. 1992. The susceptibility of the Indonesian I/CDC strain of Plasmodium vivax to chloroquine. J. Parasitol. 78:344-349. [PubMed] [Google Scholar]
- 37.Collins, W. E., J. S. Sullivan, D. J. Fryauff, J. Kendall, V. Jennings, G. G. Galland, and C. L. Morris. 2000. Adaptation of a chloroquine-resistant strain of Plasmodium vivax from Indonesia to New World monkeys. Am. J. Trop. Med. Hyg. 62:491-495. [DOI] [PubMed] [Google Scholar]
- 38.Congpuong, K., K. Na-Bangchang, K. Thimasarn, U. Tasanor, and W. H. Wernsdorfer. 2002. Sensitivity of Plasmodium vivax to chloroquine in Sa Kaeo Province, Thailand. Acta Trop. 83:117-121. [DOI] [PubMed] [Google Scholar]
- 39.Cooper, R. D., W. K. Milhous, and K. H. Rieckmann. 1994. The efficacy of WR238605 against the blood stages of a chloroquine-resistant strain of Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 88:691-692. [DOI] [PubMed] [Google Scholar]
- 40.Craig, A. A., and K. C. Kain. 1996. Molecular analysis of Plasmodium vivax from paired primary and relapse infections. J. Infect. Dis. 174:373-379. [DOI] [PubMed] [Google Scholar]
- 41.Craige, B., Jr., A. S. Alving, R. Jones, Jr., C. M. Whorton, T. N. Dullman, and L. Eichelberger. 1947. The Chesson strain of Plasmodium vivax malaria. II. Relationship between prepatent period, latent period, and relapse rate. J. Infect. Dis. 80:228-236. [DOI] [PubMed] [Google Scholar]
- 42.Davis, T. M., D. A. Syed, K. F. Ilett, and P. H. Barrett. 2003. Toxicity related to chloroquine treatment of resistant vivax malaria. Ann. Pharmacother. 37:526-529. [DOI] [PubMed] [Google Scholar]
- 43.Dixon, K. E., U. Pitaktong, and P. Phintuyothin. 1985. A clinical trial of mefloquine in the treatment of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 34:435-437. [DOI] [PubMed] [Google Scholar]
- 44.Dua, V. K., P. K. Kar, and V. P. Sharma. 1996. Chloroquine-resistant Plasmodium vivax malaria in India. Trop. Med. Int. Health 1:816-819. [DOI] [PubMed] [Google Scholar]
- 45.Ehrman, F. C., J. M. Ellis, and M. D. Young. 1945. Plasmodium vivax Chesson strain. Science 101:377. [DOI] [PubMed] [Google Scholar]
- 46.Feng, X., J. M. Carlton, D. A. Joy, J. Mu, T. Furuya, B. Suh, Y. Wang, J. W. Barnwell, and X. Z. Su. 2003. Single-nucleotide polymorphisms and genome diversity in Plasmodium vivax. Proc. Natl. Acad. Sci. USA 100:8502-8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frisk-Holmberg, M., Y. Berqvist, E. Termond, and B. Domeij-Nyberg. 1984. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur. J. Clin. Pharmacol. 26:521-530. [DOI] [PubMed] [Google Scholar]
- 48.Fryauff, D. J., J. K. Baird, H. Basri, I. Sumawinata, Purnomo, T. L. Richie, C. K. Ohrt, E. Mouzin, C. J. Church, A. L. Richards, F. S. Wignall, and S. L. Hoffman. 1995. Randomized placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet 346:1190-1193. [DOI] [PubMed] [Google Scholar]
- 49.Fryauff, D. J., J. K. Baird, D. Candradikusuma, S. Masbar, M. A. Sutamihardja, B. Leksana, S. Tuti, H. Marwoto, T. Richie, and A. Romzan. 1997. Survey of in vivo sensitivity to chloroquine by Plasmodium falciparum and P. vivax in Lombok, Indonesia. Am. J. Trop. Med. Hyg. 56:241-244. [DOI] [PubMed] [Google Scholar]
- 50.Fryauff, D. J., B. Leksana, S. Masbar, I. Wiady, P. Sismadi, A. I. Susanti, H. S. Nagesha, Syafruddin, S. Atmosoedjono, M. J. Bangs, and J. K. Baird. 2002. The drug sensitivity and transmission dynamics of human malaria on Nias Island, North Sumatra, Indonesia. Ann. Trop. Med. Parasitol. 96:447-462. [DOI] [PubMed] [Google Scholar]
- 51.Fryauff, D. J., Soekartono, S. Tuti, B. Leksana, Suradi, S. Tandayu, and J. K. Baird. 1998. Survey of resistance in vivo to chloroquine of Plasmodium falciparum and P. vivax in North Sulawesi, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 92:82-83. [DOI] [PubMed] [Google Scholar]
- 52.Fryauff, D. J., I. Sumawinata, Purnomo, T. L. Richie, E. Tjitra, M. J. Bangs, A. Kadir, and G. Inkokusumo. 1999. In vivo responses to antimalarials by Plasmodium falciparum and Plasmodium vivax from isolated Gag Island off northwest coast of Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 60:542-546. [DOI] [PubMed] [Google Scholar]
- 53.Fryauff, D. J., S. Tuti, A. Mardi, S. Masbar, R. Patipehohi, B. Leksana, K. C. Kain, M. J. Bangs, T. L. Richie, and J. K. Baird. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513-518. [DOI] [PubMed] [Google Scholar]
- 54.Fu, S., A. Bjorkman, D. Ofori-Adjei, B. Wahlin, O. Ericcson, and F. Sjoqvist. 1986. In vitro activity of chloroquine, the two enantiomers of chloroquine, desethylchloroquine and pyronaridine against Plasmodium falciparum. Br. J. Pharmacol. 22:93-96. [PMC free article] [PubMed] [Google Scholar]
- 55.Gajanana, A., and A. N. Raichowdhuri. 1984. Plasmodium vivax: micro in vitro test for assaying chloroquine susceptibility. Trans. R. Soc. Trop. Med. Hyg. 78:416-417. [DOI] [PubMed] [Google Scholar]
- 56.Garg, M., N. Gopinathan, P. Bodhe, and N. A. Kshirsagar. 1995. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans. R. Soc. Trop. Med. Hyg. 89:656-657. [DOI] [PubMed] [Google Scholar]
- 57.Geary, T. G., A. A. Divo, and J. B. Jenson. 1987. Activity of quinoline antimalarials against chloroquine-sensitive and -resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 81:499-503. [DOI] [PubMed] [Google Scholar]
- 58.Gordon, H. H., F. R. Dieuaide, A. Marble, H. B. Christianson, and L. K. Dahl. 1947. Treatment of Plasmodium vivax malaria of foreign origin. Arch. Intern. Med. 79:365-378. [DOI] [PubMed] [Google Scholar]
- 59.Gustafsson, L. L., L. Rombo, G. Alvan, A. Bjorkman, M. Lind, and O. Walker. 1983. On the question of dose-dependent chloroquine elimination of a single oral dose. Clin. Pharmacol. Ther. 34:383-385. [DOI] [PubMed] [Google Scholar]
- 60.Hamedi, Y., M. Nateghpour, P. Tan-ariya, M. Tiensuwan, U. Silachamroon, and S. Looareesuwan. 2002. Plasmodium vivax malaria in southeast Iran in 1999-2001: establishing the response to chloroquine in vitro and in vivo. Southeast Asian J. Trop. Med. Public Health 33:512-518. [PubMed] [Google Scholar]
- 61.Hanna, J. 1993. Chloroquine-resistant Plasmodium vivax: how common? Med. J. Aust. 158:502-503. [PubMed] [Google Scholar]
- 62.Harinasuta, T., D. Bunnag, R. Lasserre, R. Leimer, and S. Vinijanont. 1985. Trials of mefloquine in vivax and of mefloquine plus Fansidar in falciparum malaria. Lancet i:885-888. [DOI] [PubMed] [Google Scholar]
- 63.Hastings, M. D., and C. H. Sibley. 2002. Pyrimethamine and WR99210 exert opposing selection on dihydrofolate reductase from Plasmodium vivax. Proc. Natl. Acad. Sci. USA 99:13137-13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hastings, M. D., K. M. Porter, J. D. Maguire, I. Susanti, W. Kania, M. J. Bangs, C. H. Sibley, and J. K. Baird. 2004. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J. Infect. Dis. 189:744-750. [DOI] [PubMed] [Google Scholar]
- 65.Hoekenga, M. T. 1952. Treatment of malaria with a single dose of amodiaquine or chloroquine. JAMA 149:1369-1371. [DOI] [PubMed] [Google Scholar]
- 66.Jamaiah, I., A. K. Anuar, N. A. Najib, and M. N. Zurainee. 1998. Imported malaria: a retrospective study in University Hospital, Kuala Lumpur, a ten-year experience. Med. J. Malaysia 53:6-9. [PubMed] [Google Scholar]
- 67.Kirchgatter, K., and H. A. del Portillo. 1998. Molecular analysis of Plasmodium vivax relapses using the MSP1 molecule as a genetic marker. J. Infect. Dis. 177:511-515. [DOI] [PubMed] [Google Scholar]
- 68.Kocken, C. H., A. van der Wel, B. Rosenwirth, and A. W. Thomas. 1996. Plasmodium vivax: in vitro antiparasitic effect of cyclosporins. Exp. Parasitol. 84:439-443. [DOI] [PubMed] [Google Scholar]
- 69.Kshirsagar, N. A., N. J. Gogtay, D. Rojgor, S. S. Dalvi, and M. Wakde. 2000. An unusual case of multidrug-resistant Plasmodium vivax malaria in Mumbai (Bombay), India. Ann. Trop. Med. Parasitol. 94:189-190. [DOI] [PubMed] [Google Scholar]
- 70.Lacy, M. D., J. D. Maguire, M. J. Barcus, J. Ling, M. J. Bangs, R. Gramzinski, H. Basri, P. Sismadi, G. B. Miller, J. D. Chulay, D. J. Fryauff, S. L. Hoffman, and J. K. Baird. 2002. Atovaquone/proguanil therapy for Plasmodium falciparum and Plasmodium vivax malaria in Indonesians who lack clinical immunity. Clin. Infect. Dis. 35:e92-e95. [DOI] [PubMed] [Google Scholar]
- 71.Loeb, R. F., W. M. Clark, G. R. Coatney, L. T. Coggeshall, F. R. Dieuaide, A. R. Dochez, E. G. Hakansson, E. K. Marshall, Jr., C. S. Marvel, O. R. McCoy, J. J. Sapero, W. H. Sebrell, J. A. Shannon, and G. A. Carden. 1946. Activity of a new antimalarial agent, chloroquine (SN 7618). JAMA 130:1069-1070. [DOI] [PubMed] [Google Scholar]
- 72.Looareesuwan, S., P. Wilairatana, S. Krudsood, S. Treeprasertsuk, P. Singhasivanon, V. Bussaratid, W. Chokjindachai, P. Viriyavejakul, K. Chalermrut, D. S. Walsh, and J. White. 1999. Chloroquine sensitivity of Plasmodium vivax in Thailand. Ann. Trop. Med. Parasitol. 93:225-230. [PubMed] [Google Scholar]
- 73.Looareesuwan, S., T. Harinasuta, and T. Chongsuphajaisiddhi. 1992. Drug resistant malaria with special reference to Thailand. Southeast Asian J. Trop. Med. Public Health 23:621-634. [PubMed] [Google Scholar]
- 74.Machado, R. L. D., A. F. Filho, V. S. P. Calvosa, M. C. Figueredo, J. M. Nascimento, and M. M. Povoa. 2003. Correlation between Plasmodium vivax variants in Belem, Para State, Brazil and symptoms and clearance of parasitemia. Braz. J. Infect. Dis. 7:175-177. [DOI] [PubMed] [Google Scholar]
- 75.Maguire, J. D., M. D. Lacy, Sururi, P. Sismadi, Krisin, I. Wiady, B. Laksana, M. J. Bangs, S. Masbar, I. Susanti, W. Basuki, M. J. Barcus, H. Marwoto, M. D. Edstein, S. Tjokrosonto, and J. K. Baird 2002. Chloroquine or sulfadoxine-pyrimethamine for the treatment of uncomplicated Plasmodium falciparum malaria during an epidemic in Central Java, Indonesia. Ann. Trop. Med. Parasitol. 96:655-668. [DOI] [PubMed] [Google Scholar]
- 76.Marlar-Than, Myat-Phone-Kyaw, Aye-Yu-Soe, Khaing-Khaing-Gyi, Ma-Sabai, and Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 89:307-308. [DOI] [PubMed] [Google Scholar]
- 77.McChesney, E.W., W. F. Banks, Jr., and J. P. McAuliff. 1962. Laboratory studies of the 4-aminoquinoline antimalarials. II. Plasma levels of chloroquine and hydroxychloroquine in man after various oral dosage regimens. Antibiot. Chemother. 12:583-594. [Google Scholar]
- 78.Medical Letter on Drugs and Therapeutics. 2002. Drugs for parasitic infections, April, p. 6. The Medical Letter, Inc., New Rochelle, N.Y.
- 79.Mendis, K., B. J. Sina, P. Machesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64S:97-106. [DOI] [PubMed] [Google Scholar]
- 80.Most, I. M., C. A. Kane, P. H. Lavietes, E. F. Schroeder, and J. M. Hayman. 1946. Chloroquine for treatment of acute attacks of vivax malaria. JAMA 131:963-967. [DOI] [PubMed] [Google Scholar]
- 81.Murphy, G. S., H. Basri, Purnomo, E. M. Andersen, M. J. Bangs, D. L. Mount, J. Gorden, A. A. Lal, A. R. Purwokusumo, S. Harjosuwarno, K. Sorensen, and S. L. Hoffman. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96-100. [DOI] [PubMed] [Google Scholar]
- 82.Myat-Phone-Kyaw, Myin-Oo, Myint-Lwin, Thaw-Zin, Kyin-Hla-Aye, and New-New-Yin. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687. [DOI] [PubMed] [Google Scholar]
- 83.Nomura, T., J. M. Carlton, J. K. Baird, H. A. del Portillo, D. J. Fryauff, D. Rathore, D. A. Fidock, X. Su, W. E. Collins, T. F. McCutchan, J. C. Wootton, and T. E. Wellems. 2001. Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria. J. Infect. Dis. 183:1653-1661. [DOI] [PubMed] [Google Scholar]
- 84.Obaldia, N., III, R. N. Rossan, R. D. Cooper, D. E. Kyle, E. O. Nuzum, K. H. Rieckmann, and G. D. Shanks. 1997. WR238605, chloroquine, and their combinations as blood schizonticides against a chloroquine-resistant strain of Plasmodium vivax in Aotus monkeys. Am. J. Trop. Med. Hyg. 56:508-510. [DOI] [PubMed] [Google Scholar]
- 85.Ohrt, C., T. L. Richie, H. Widjaja, G. D. Shanks, J. Fitriadi, D. J. Fryauff, J. Handschin, D. Tang, B. Sandjaja, E. Tjitra, L. Hadiarso, G. Watt, and F. S. Wignall. 1997. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 126:963-972. [DOI] [PubMed] [Google Scholar]
- 86.Patchen, L. C., D. L. Mount, I. K. Schwartz, and F. C. Churchill. 1983. Analysis of filter paper absorbed finger-stick blood samples for chloroquine and its major metabolite using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 278:81-89. [DOI] [PubMed] [Google Scholar]
- 87.Phan, G. T., P. J. de Vries, B. Q. Tran, H. Q. Le, N. V. Nguyen, T. V. Nguyen, S. H. Heisterkamp, and P. A. Kager. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop. Med. Int. Health 2:858-864. [DOI] [PubMed] [Google Scholar]
- 88.Phillips, E. J., J. S. Keystone, and K. C. Kain. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 23:1171-1173. [DOI] [PubMed] [Google Scholar]
- 89.Potkar, C. N., N. A. Kshirsagar, and R. Kathuria. 1995. Resurgence of malaria and drug resistance in Plasmodium falciparum and Plasmodium vivax species in Bombay. J. Assoc. Physicians India 43:336-338. [PubMed] [Google Scholar]
- 90.Powell, R. D., and E. M. Berglund. 1974. Effects of chloroquine upon the maturation of asexual erythrocytic forms of Plasmodium vivax in vitro. Am. J. Trop. Med. Hyg. 23:1007-1014. [DOI] [PubMed] [Google Scholar]
- 91.Price, R. N., and F. Nosten. 2001. Drug resistant falciparum malaria: clinical consequences and strategies for prevention. Drug Resist. Update 4:187-196. [DOI] [PubMed] [Google Scholar]
- 92.Pullman, T. N., B. Craige, Jr., A. S. Alving, C. M. Whorton, R. Jones, Jr., and L. Eichelberger. 1948. Comparison of chloroquine, quinacrine, and quinine in the treatment of acute attacks of sporozoite-induced vivax malaria (Chesson strain). J. Clin. Investig. 27:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Renapurkar, D. M., V. R. Padhan, N. K. Sutar, R. A. Deshmukh, C. H. Pandit, and S. N. Marathe. 1989. Micro test for assaying sensitivity of Plasmodium vivax in vitro. Chemotherapy (Tokyo) 35:160-163. [DOI] [PubMed] [Google Scholar]
- 94.Rieckmann, H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 95.Rombo, L., Y. Berqvist, and U. Hellgren. 1987. Chloroquine and desethylchloroquine concentrations during regular and long-term malaria prophylaxis. Bull. W. H. O. 65:879-883. [PMC free article] [PubMed] [Google Scholar]
- 96.Rombo, L., O. Ericsson, G. Alvan, B. Lindstrom, and L. L. Gustafsson. 1985. Chloroquine and desethylchloroquine in plasma, serum and whole blood: problems in assay and handling of samples. Ther. Drug Monit. 7:211-215. [DOI] [PubMed] [Google Scholar]
- 97.Ruebush, T. K., II, J. Zegarra, J. Cairo, E. M. Andersen, M. Green, D. R. Pillai, W. Marquino, M. Huilca, E. Arevalo, C. Garcia, L. Solary, and K. C. Kain. 2003. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 69:548-552. [PubMed] [Google Scholar]
- 98.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Sattabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schuurkamp, G. J., P. E. Spicer, R. K. Kereu, P. K. Bulugol, and K. H. Rieckmann. 1992. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 86:121-122. [DOI] [PubMed] [Google Scholar]
- 99a.Schuurkamp, G. J. T. 1992. Ph.D. dissertation. University of Papua New Guinea.
- 100.Schwartz, I. K., E. M. Lackritz, and L. C. Patchen. 1991. Chloroquine-resistant Plasmodium vivax from Indonesia. N. Engl. J. Med. 324:927. [DOI] [PubMed] [Google Scholar]
- 101.Soto, J., J. Toledo, P. Gutierrez, M. Luzz, N. Llinas, N. Cedeno, M. Dunne, and J. Berman. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90-93. [DOI] [PubMed] [Google Scholar]
- 102.Sumawinata, I. W., Bernadeta, B. Leksana, A. Sutamihardja, Purnomo, B. Subianto, Sekartuti, D. J. Fryauff, and J. K. Baird. 2002. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 68:416-420. [PubMed] [Google Scholar]
- 103.Tan-ariya, P., K. Na-Bangchang, T. Tin, L. Limapibul, C. R. Brockelman, and J. Karbwang. 1995. Clinical response and susceptibility in vitro of Plasmodium vivax to the standard regimen of chloroquine in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:426-429. [DOI] [PubMed] [Google Scholar]
- 104.Tasanor, O., H. Noedl, K. Na-Bangchang, K. Congpuong, J. Sirichaisinthop, and W. H. Wernsdorfer. 2002. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 83:49-61. [DOI] [PubMed] [Google Scholar]
- 105.Taylor, W. R., H. N. Doan, D. T. Nguyen, T. U. Tran, D. J. Fryauff, E. Gomez-Saladin, K. C. Kain, D. C. Le, and J. K. Baird. 2000. Assessing drug sensitivity of Plasmodium vivax to halofantrine or chloroquine in southern, central Vietnam using an extended 28-day in vivo test and polymerase chain reaction genotyping. Am. J. Trop. Med. Hyg. 62:693-697. [DOI] [PubMed] [Google Scholar]
- 106.Taylor, W. R., H. Widjaja, T. L. Richie, H. Basri, C. Ohrt, E. Tjitra, E. Taufik, T. R. Jones, K. C. Kain, and S. L. Hoffman. 2001. Chloroquine/doxycycline combination versus chloroquine alone and doxycycline alone for the treatment of Plasmodium falciparum and Plasmodium vivax malaria in northeastern Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 64:223-228. [DOI] [PubMed] [Google Scholar]
- 107.Van Den Abbeele, K., E. Van Den Enden, and J. Van Den Ende. 1995. Combined chloroquine and primaquine resistant Plasmodium vivax malaria in a patient returning from India. Ann. Soc. Belge Med. Trop. 75:73-74. [PubMed] [Google Scholar]
- 108.Verdier, F., J. Le Bras, F. Clavier, and I. Hatin. 1984. Blood levels and in vitro activity of desethylchloroquine against Plasmodium falciparum. Lancet i:1186-1187. [DOI] [PubMed] [Google Scholar]
- 109.Villalobos-Salcedo, J. M., M. S. Tada, E. Kimura, M. J. Menezes, and L. H. Pereira-da-Silva. 2000. In-vivo sensitivity of Plasmodium vivax isolates from Rondonia (western Amazon region, Brazil) to regimens including chloroquine and primaquine. Ann. Trop. Med. Parasitol. 94:749-758. [DOI] [PubMed] [Google Scholar]
- 110.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 111.Whitby, M., G. Wood, J. R. Veenendaal, and K. Rieckmann. 1989. Chloroquine-resistant Plasmodium vivax. Lancet ii:1395. [DOI] [PubMed] [Google Scholar]
- 112.Whorton, C. M., E. Yount, Jr., R. Jones, Jr., A. S. Alving, T. N. Pullman, B. Craige, Jr., and L. Eichelberger. 1950. Studies in human malaria. The Chesson strain of Plasmodium vivax malaria. III. Clinical aspects. J. Infect. Dis. 80:237-249. [DOI] [PubMed] [Google Scholar]
- 113.Wilson, T., and J. F. B. Edeson. 1954. Studies on the chemotherapy of malaria. III. The treatment of acute malaria with chloroquine. Med. J. Malaya 9:114-131. [PubMed] [Google Scholar]
- 114.Wiselogle, F. Y. 1946. A survey of antimalarial drugs, 1941-1945, 2 vols. J. W. Edwards, Ann Arbor, Mich.
- 115.World Health Organization. 2001. The use of antimalarial drugs. Report of a WHO informal consultation. Report WHO/CDS/RBM/2001.33. World Health Organization, Geneva, Switzerland.