Abstract
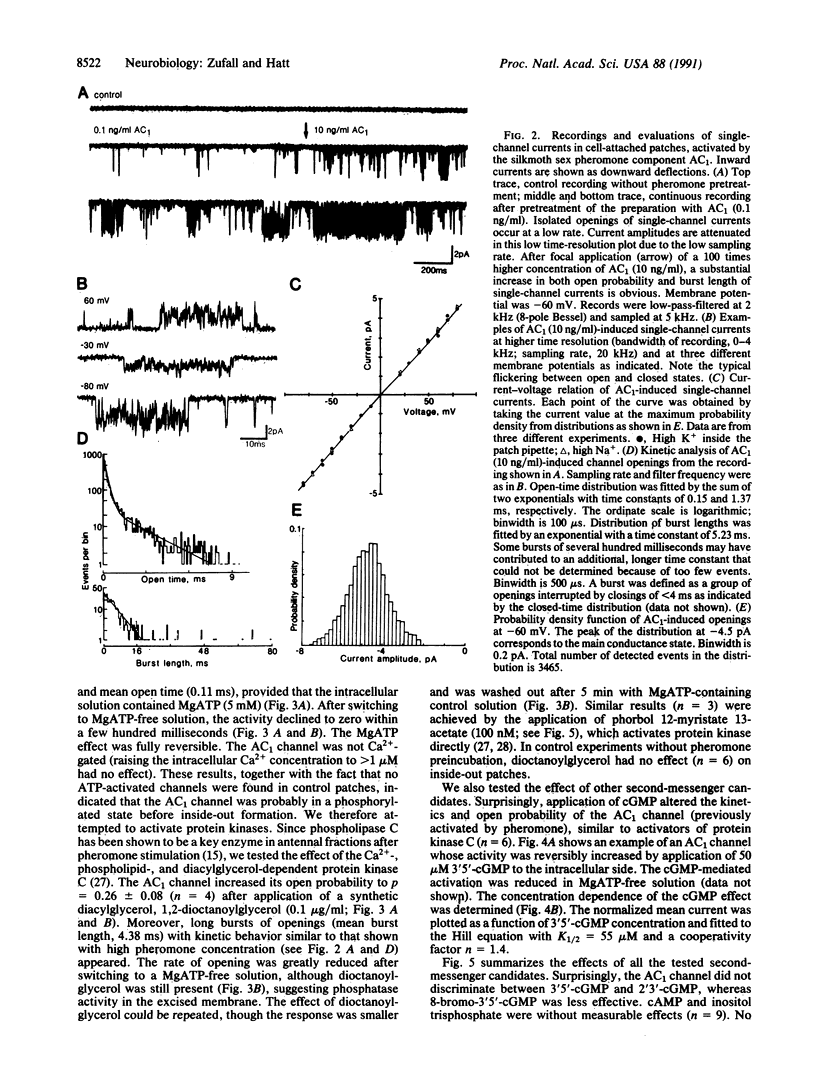
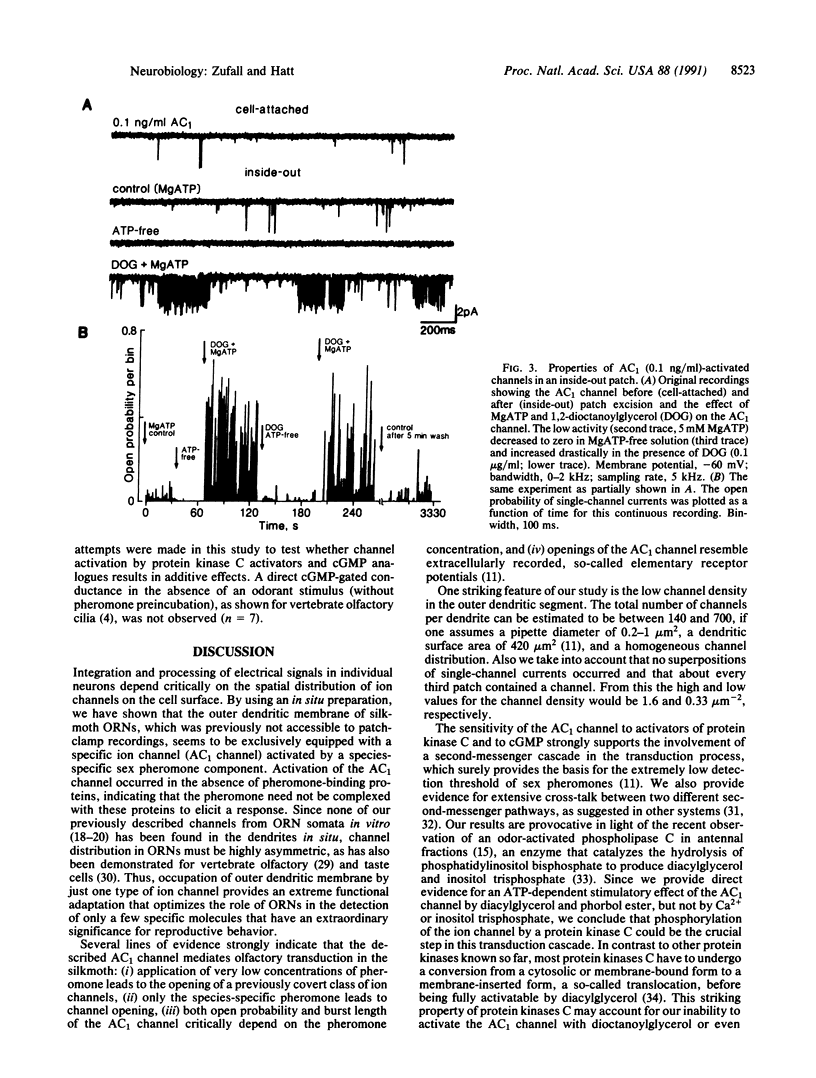
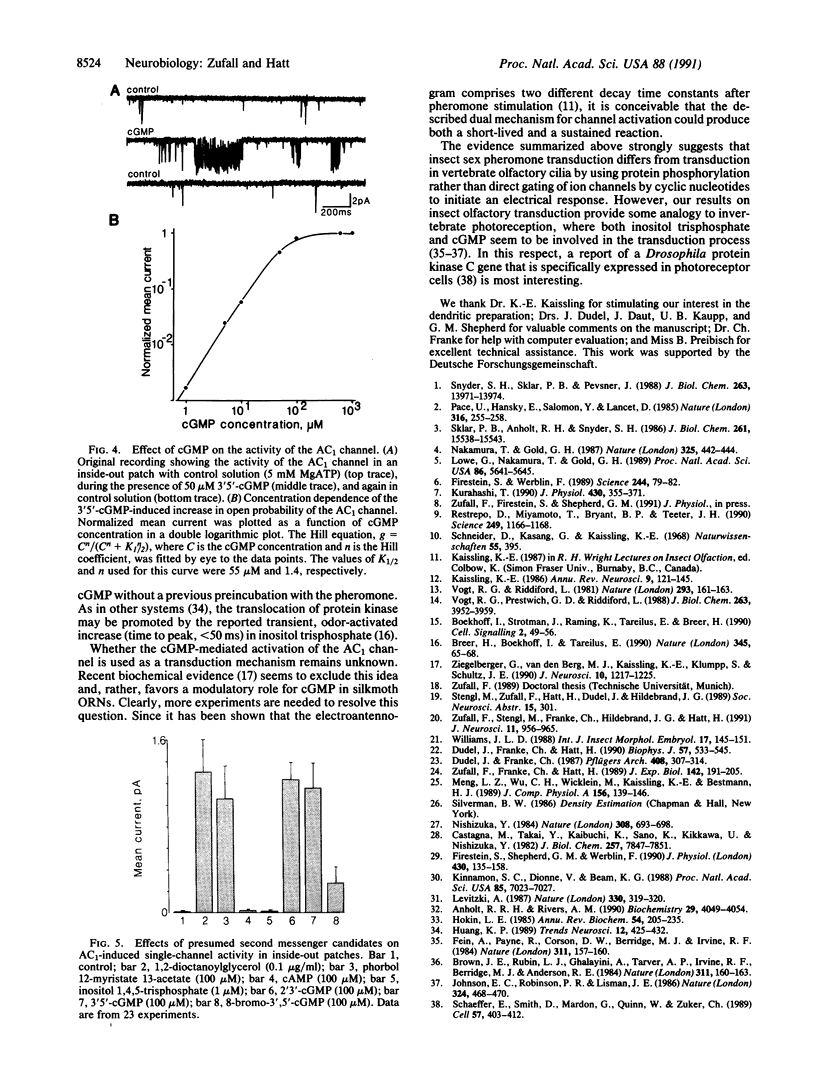
Olfactory transduction is thought to take place in the outer dendritic membrane of insect olfactory receptor neurons. Here we show that the outer dendritic plasma membrane of silkmoth olfactory receptor neurons seems to be exclusively equipped with a specific ion channel activated by low concentrations of the species-specific sex pheromone component. This so-called AC1 channel has a conductance of 56 pS and is nonselectively permeable to cations. The AC1 channel can be activated from the intracellular side by protein kinase C activators such as diacylglycerol and phorbolester and by cGMP but not by Ca2+, inositol 1,4,5-triphosphate, or cAMP. Our results imply that phosphorylation of this ion channel by protein kinase C could be the crucial step in channel opening by sex pheromones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R. R., Rivers A. M. Olfactory transduction: cross-talk between second-messenger systems. Biochemistry. 1990 May 1;29(17):4049–4054. doi: 10.1021/bi00469a004. [DOI] [PubMed] [Google Scholar]
- Boekhoff I., Strotmann J., Raming K., Tareilus E., Breer H. Odorant-sensitive phospholipase C in insect antennae. Cell Signal. 1990;2(1):49–56. doi: 10.1016/0898-6568(90)90032-6. [DOI] [PubMed] [Google Scholar]
- Breer H., Boekhoff I., Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990 May 3;345(6270):65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984 Sep 13;311(5982):160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. II. Dependence of channel open time on glutamate concentration. Pflugers Arch. 1987 Mar;408(3):307–314. doi: 10.1007/BF02181474. [DOI] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Firestein S., Shepherd G. M., Werblin F. S. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. J Physiol. 1990 Nov;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Werblin F. Odor-induced membrane currents in vertebrate-olfactory receptor neurons. Science. 1989 Apr 7;244(4900):79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Huang K. P. The mechanism of protein kinase C activation. Trends Neurosci. 1989 Nov;12(11):425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Johnson E. C., Robinson P. R., Lisman J. E. Cyclic GMP is involved in the excitation of invertebrate photoreceptors. Nature. 1986 Dec 4;324(6096):468–470. doi: 10.1038/324468a0. [DOI] [PubMed] [Google Scholar]
- Kaissling K. E. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci. 1986;9:121–145. doi: 10.1146/annurev.ne.09.030186.001005. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. C., Dionne V. E., Beam K. G. Apical localization of K+ channels in taste cells provides the basis for sour taste transduction. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7023–7027. doi: 10.1073/pnas.85.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T. The response induced by intracellular cyclic AMP in isolated olfactory receptor cells of the newt. J Physiol. 1990 Nov;430:355–371. doi: 10.1113/jphysiol.1990.sp018295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Cross-talk between PKC and cyclic AMP pathways. 1987 Nov 26-Dec 2Nature. 330(6146):319–320. doi: 10.1038/330319c0. [DOI] [PubMed] [Google Scholar]
- Lowe G., Nakamura T., Gold G. H. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Restrepo D., Miyamoto T., Bryant B. P., Teeter J. H. Odor stimuli trigger influx of calcium into olfactory neurons of the channel catfish. Science. 1990 Sep 7;249(4973):1166–1168. doi: 10.1126/science.2168580. [DOI] [PubMed] [Google Scholar]
- Schaeffer E., Smith D., Mardon G., Quinn W., Zuker C. Isolation and characterization of two new drosophila protein kinase C genes, including one specifically expressed in photoreceptor cells. Cell. 1989 May 5;57(3):403–412. doi: 10.1016/0092-8674(89)90915-x. [DOI] [PubMed] [Google Scholar]
- Schneider D., Kasang G., Kaissling K. E. Bestimmung der Riechschwelle von Bombyx mori mit Tritium-markiertem Bombykol. Naturwissenschaften. 1968 Aug;55(8):395–395. doi: 10.1007/BF00593307. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Snyder S. H., Sklar P. B., Pevsner J. Molecular mechanisms of olfaction. J Biol Chem. 1988 Oct 5;263(28):13971–13974. [PubMed] [Google Scholar]
- Vogt R. G., Prestwich G. D., Riddiford L. M. Sex pheromone receptor proteins. Visualization using a radiolabeled photoaffinity analog. J Biol Chem. 1988 Mar 15;263(8):3952–3959. [PubMed] [Google Scholar]
- Ziegelberger G., van den Berg M. J., Kaissling K. E., Klumpp S., Schultz J. E. Cyclic GMP levels and guanylate cyclase activity in pheromone-sensitive antennae of the silkmoths Antheraea polyphemus and Bombyx mori. J Neurosci. 1990 Apr;10(4):1217–1225. doi: 10.1523/JNEUROSCI.10-04-01217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F., Stengl M., Franke C., Hildebrand J. G., Hatt H. Ionic currents of cultured olfactory receptor neurons from antennae of male Manduca sexta. J Neurosci. 1991 Apr;11(4):956–965. doi: 10.1523/JNEUROSCI.11-04-00956.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]