Abstract
Human cytomegalovirus (HCMV) is a major human pathogen frequently associated with life-threatening disease in immunosuppressed patients and newborns. The HCMV UL97-encoded protein kinase (pUL97) represents an important determinant of viral replication. Recent studies demonstrated that pUL97-specific kinase inhibitors are powerful tools for the control of HCMV replication. We present evidence that three related quinazoline compounds are potent inhibitors of the pUL97 kinase activity and block in vitro substrate phosphorylation, with 50% inhibitory concentrations (IC50s) between 30 and 170 nM. Replication of HCMV in primary human fibroblasts was suppressed with a high efficiency. The IC50s of these three quinazoline compounds (2.4 ± 0.4, 3.4 ± 0.6, and 3.9 ± 1.1 μM, respectively) were in the range of the IC50 of ganciclovir (1.2 ± 0.2 μM), as determined by the HCMV green fluorescent protein-based antiviral assay. Importantly, the quinazolines were demonstrated to have strong inhibitory effects against clinical HCMV isolates, including ganciclovir- and cidofovir-resistant virus variants. Moreover, in contrast to ganciclovir, the formation of resistance to the quinazolines was not observed. The mechanisms of action of these compounds were confirmed by kinetic analyses with infected cells. Quinazolines specifically inhibited viral early-late protein synthesis but had no effects at other stages of the replication cycle, such as viral entry, consistent with a blockage of the pUL97 function. In contrast to epithelial growth factor receptor inhibitors, quinazolines affected HCMV replication even when they were added hours after virus adsorption. Thus, our findings indicate that quinazolines are highly efficient inhibitors of HCMV replication in vitro by targeting pUL97 protein kinase activity.
Human cytomegalovirus (HCMV) belongs to the family Herpesviridae and is associated with severe forms of human disease (23). Primary acute infection as well as lifelong persistent infection of the host eventually causes multiple pathological consequences which, under unfavorable immunological circumstances, can lead to life-threatening clinical manifestations. At present, clinically available drugs for antiherpesviral therapy are primarily composed of nucleotide and nucleoside or nonnucleotide inhibitors of viral DNA synthesis. The clinical application of these drugs, however, faces severe limitations, such as the induction of adverse side effects and the selection of resistant viruses. Thus, the development of novel antiviral strategies is the focus of investigations worldwide.
The important role of the HCMV UL97-encoded protein kinase (pUL97) for antiviral therapy with ganciclovir (GCV) was recognized a decade ago (15, 26). It is striking that pUL97, which does not phosphorylate natural nucleosides, performs an important pacemaker reaction during conventional therapy, in that pUL97 phosphorylates and thereby activates nucleoside analogues such as GCV and penciclovir (30). pUL97 phosphorylates GCV to its monophosphate form, which subsequently becomes further phosphorylated by cellular enzymes involved in nucleotide metabolism. The resulting GCV triphosphate inhibits viral DNA synthesis in several ways: (i) inhibition of the viral DNA polymerase by competition with the natural nucleoside triphosphate (dGTP) and (ii) chain termination of evolving DNA strands. The latter aspect is the reason why the replication and repair of cellular DNA are also partially affected by phosphorylated GCV, thereby causing cytotoxicity. Thus, pUL97 is necessarily involved in GCV therapy, and virus resistance to GCV frequently results from a mutation in UL97 (7).
Direct inhibitors of the pUL97 protein kinase activity represent promising candidates as novel anti-HCMV drugs. In this respect, it is important that a strong antiviral effect of indolocarbazole compounds (e.g., NGIC-I) on the in vitro replication of HCMV was reported (18, 25, 31). Subsequent detailed investigations of the determinants of virus inhibition led to the validation of pUL97 as an antiviral target (12, 19). However, the excellent antiviral potencies of distinct indolocarbazoles in vitro seemed to be accompanied by relatively unfavorable pharmacological properties in vivo, such as poor pharmacokinetics and bioavailability (M. J. Slater, S. Cockerill, R. Baxter, R. W. Bonser, K. Gohil, E. Robinson, N. Parry, R. Randall, and W. Snowden, 14th Int. Conf. Antivir. Res., abstr. 69, 2001); thus, further preclinical developments await continuation.
Another pUL97-inhibiting compound, 1263W94 (maribavir), which belongs to the chemical class of benzimidazole l-ribosides, has been characterized by several investigators (1, 4, 20). In preclinical and phase I and II clinical studies, maribavir possessed clear antiviral activity (14) and very promising pharmacokinetic profiles (11), accompanied by a low degree of severe adverse effects (27). The main target of action of maribavir was postulated to be pUL97 (1). However, the selection of maribavir-resistant HCMV variants that carried a resistance-conferring mutation, which, surprisingly, mapped to a gene of unknown function (UL27), but that lacked a mutation in UL97 was recently reported (10). This points to a more complex and controversial mode of action of maribavir. Nevertheless, the promising status of an antiviral strategy targeting pUL97 seems to be without doubt (5). In this context, a number of recent publications have contributed to the understanding of the physiological role of pUL97 during HCMV infection. pUL97 is a multifunctional viral protein kinase with profound importance for the efficiency of viral replication (24). Inhibition of pUL97 kinase activity or deletion of the open reading frame for UL97 from the viral genome leads to defects in the early and late events of replication associated with viral DNA synthesis and nucleocytoplasmic capsid export (12, 13, 16, 29).
Here, we describe a novel group of potent inhibitors targeting pUL97. Specific members of the chemical class of quinazolines showed pronounced anti-HCMV activities. Interestingly, the drug gefitinib (Iressa; ZD1839), a protein kinase inhibitor approved for use for antitumor therapy (6), also belongs to this class. The mode of action of selected quinazoline compounds in blocking HCMV replication was confined to inhibition of pUL97 protein kinase activity. Importantly, these compounds were also effective in inhibiting clinical isolates of HCMV resistant to GCV and cidofovir (CDV) therapy. Thus, our data on quinazoline compounds described here provide a starting point for detailed investigations of their pharmacological, toxicological, and antiviral properties in vivo.
MATERIALS AND METHODS
Cell culture and viruses.
Primary human foreskin fibroblasts (HFFs) and HEK 293 cells were cultivated in minimal essential medium and Dulbecco's minimal essential medium, respectively, containing 5% fetal bovine serum and 100 μg of gentamicin per ml. HCMV AD169 (laboratory strain), recombinant green fluorescent protein (GFP)-expressing AD169 (AD169-GFP) (17), and clinical isolates R2, R3, and R5 were propagated in HFFs. A virus from which UL97 was deleted, AD169delUL97, was kindly provided by G. Pari (University of Nevada, Reno). Stocks of AD169delUL97 were prepared by selection with 200 μM mycophenolic acid and 5 μM xanthine and by plaque purification before use in infection experiments (3, 19, 24). Virus titers were determined by automated GFP quantification for AD169-GFP and plaque titration for AD169 and the other strains. For infection experiments, HFFs were grown to subconfluence (90%) in 12-well plates, incubated for 90 min with a virus stock at the multiplicity of infection indicated in the legend to Fig. 5 at 37°C, and thereafter cultivated in fresh medium. Antiviral drugs were added as described in the legends to Fig. 1 to 5. Transfection of HEK 293 cells was performed by the Lipofectamin Plus method (Invitrogen, Karlsruhe, Germany) in 10-cm dishes at a cell confluence of 75%. The cytotoxicities of the quinazolines were determined in HEK 293 cultures with 0.05% methylene blue solution, as described previously (21).
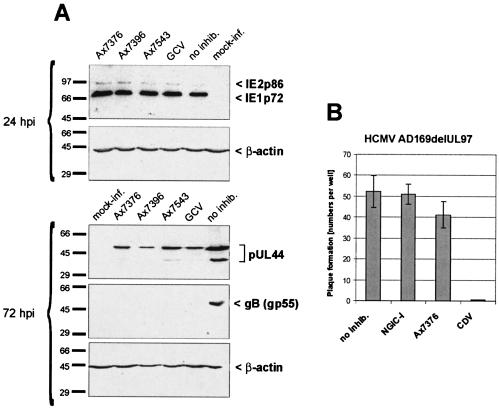
FIG. 5.
Inhibition of HCMV early-late phase by quinazolines and lack of inhibition of HCMV isolates from which UL97 was deleted. (A) HFFs were infected with HCMV AD169 at a multiplicity of infection of 0.02 in six-well plates (or were uninfected as a control [mock-inf.]) and incubated in the presence of the indicated compounds at 20 μM or incubated without inhibitor (no inhib.; DMSO alone). At 24 and 72 h postinfection (hpi), the cells were harvested and the cell lysates were subjected to SDS-PAGE and standard Western blotting procedures. Detection of specific proteins was carried out with the following MAbs: MAb-β-actin (AC-15), MAb810 (IE1p72 and IE2p86), MAb-UL44 (BS510), and MAb-gB (5815-35). (B) Plaque assays were performed with HCMV mutant AD169delUL97, from which the UL97 gene was deleted, in the presence of 0.1% DMSO (i.e., without an inhibitor [no inhib.]), 50 nM NGIC-I, 10 μM Ax7376, or 2 μM CDV, as indicated. Quantification of virus plaques was performed in quadruplicate (infection and counting were each performed in duplicate).
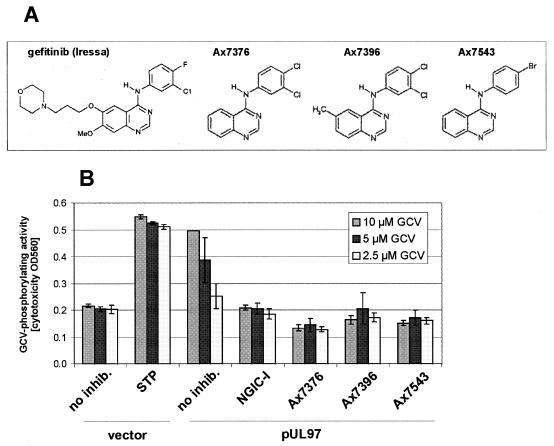
FIG. 1.
Screening of quinazolines by pUL97 in-cell activity assay. (A) Chemical structures. Gefitinib (Iressa) is a well-characterized inhibitor of EGFR kinase approved for use for non-small cell lung cancer therapy. Three related structures of distinct quinazoline derivatives possessing pUL97-inhibiting activities are shown. (B) pUL97 in-cell activity assay. HEK 293 cells were cultivated in 96-well plates and transfected with pcDNA-UL97 or pcDNA3.1 as a vector control (all transfections were performed in triplicate). Thereafter, GCV was added to the culture media at serial concentrations (10, 5, and 2.5 μM) to induce pUL97-dependent cytotoxic effects. Cytotoxicity was quantified after 5 days by measuring the color conversion of the culture media at an optical density of 560 nm (OD560). Inhibition of UL97 kinase activity was tested by incubation with selected quinazoline compounds (Ax7376, Ax7396, and Ax7543; 20 μM each) or the indolocarbazole NGIC-I (100 nM) as a pUL97-specific control inhibitor. Indolocarbazole staurosporine (STP; 100 nM) was used as a cytotoxic control to indicate nonspecific GCV-independent maximum signals.
Plasmid constructs.
Construction of the plasmids pcDNA-UL97, pcDNA-UL97(H469V), and pcDNA-UL97(C603W) was described before (19). Vector pcDNA3.1 was purchased from Invitrogen.
Antiviral drugs.
The quinazoline compounds were synthesized by Vichem Kft (Budapest, Hungary) and had a purity >95%. Stock solutions were prepared in dimethyl sulfoxide (DMSO) and were stored at −20°C. Anti-HCMV reference drugs GCV and CDV were purchased from Syntex Arzneimittel/Roche (Aachen, Germany) and Pharmacia & Upjohn S.A. (Luxembourg, Luxembourg), respectively. AG1478 [4-(3-chloroanilino)-6,7-dimethoxyquinazoline] and PD153035 [4-[(3-bromophenyl)amino]-6,7-dimethoxyquinazoline] were obtained from Calbiochem (Bad Soden, Germany).
In-cell activity assay.
The in-cell activity assay screening method for the detection of inhibitors of pUL97 kinase activity was performed as described previously (18, 21). In brief, HEK 293 cells were grown in 96-well plates, transfected with a UL97 expression plasmid or control plasmids, and incubated with medium containing serial concentrations of GCV. Upon phosphorylation of GCV in cells recombinantly expressing pUL97, but not in cells with a control plasmid, cytotoxicity developed, which could be quantified by measuring the color conversion of the phenol red-containing culture medium at a wavelength of 560 nm. The addition of inhibitors of the pUL97 kinase activity to the culture medium immediately after transfection suppressed this cytotoxic effect.
In vitro kinase assay.
The kinase activity of pUL97 was determined in vitro as described previously (18). In brief, after transfection and recombinant expression of pUL97 in HEK 293 cells, the kinase was immunoprecipitated by the use of a FLAG tag-specific antibody (M2; Sigma) and incubated with the mutual kinase substrates histone 2B (H2B) or myelin basic protein (MBP) in the presence of [γ-32P]ATP. Radiolabeled proteins were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. In addition, a panel of radioactive assays for cellular protein kinases (PKC, Akt, RIP, Cdk1, p70S6K, Abl, Src, Lck, Kit, epidermal growth factor receptor [EGFR], PDGF, InsR, Met, ERK1, p38, and JNK) were established. The in vitro kinase reactions were performed at an ATP concentration according to the Km of the respective enzyme for comparison of the inhibitory effects of the compounds, as described previously (8, 21). Incorporation of radioactivity into substrates was quantified by the filter binding assay and scintillation counting (9).
HCMV GFP-based antiviral assay and plaque reduction assay.
Infection assays were performed with HCMV AD169-GFP on HFFs (which had been seeded to subconfluence of approximately 90% and which reached confluence on day 1 to 2 postinfection) in a 12-well format, and GFP was quantified by either automated fluorometry or flow cytometry (fluorescence-activated cell sorter [FACS] analysis), as described elsewhere (17). Plaque reduction assays were performed by the use of a 0.3% agar overlay on infected cells and staining of viral plaques with 1% crystal violet 8 to 12 days postinfection. Antiviral drugs were added to the culture medium or agar overlay either immediately after virus adsorption or at the times indicated below. Since variability of the automated GFP-based antiviral assay was lower than that of the plaque reduction assay in many previous studies, 50% inhibitory concentrations (IC50s) were preferentially determined with the GFP system (17).
Western blot analysis.
Infected HFFs were harvested and lysed before the proteins were denatured. Samples were used for separation by standard SDS-PAGE and Western blot procedures. The following monoclonal antibodies (MAbs) were used for the detection of viral and cellular proteins: MAb-β-actin (AC-15; Sigma), MAb810 for IE2p86 and IE1p72 (Chemicon), MAb-UL44 (BS510; kindly provided by B. Plachter, University of Mainz, Mainz, Germany), and MAb-gB (5815-35; kindly provided by B. Britt, University of Alabama, Birmingham).
RESULTS
Quinazoline compounds are efficient inhibitors of pUL97 kinase activity.
A series of quinazoline compounds was subjected to a primary screening by the pUL97 in-cell activity assay (18, 21) in order to identify potent in vitro inhibitors of the HCMV-encoded protein kinase. Among these, three compounds were highly active in blocking pUL97-specific phosphorylation of the test substrate, GCV, namely compounds Ax7376, Ax7396, and Ax7543. All three compounds are structurally related to the recently approved antitumor drug gefitinib (Iressa) (6) (Fig. 1A). The level of inhibition of GCV phosphorylation was comparable to that of the known inhibitor of pUL97, the indolocarbazole NGIC-I (18, 19) (Fig. 1B). No significant toxicity of Ax7376, Ax7396, and Ax7543 was detected, as determined with vector controls in the absence of pUL97 (data not shown).
Detailed in vitro kinase assays in which recombinant pUL97 was analyzed in the presence of MBP (as a known in vitro substrate) revealed IC50s of 30 ± 15, 30 ± 10, and 170 ± 40 nM for Ax7376, Ax7396, and Ax7543, respectively (Table 1). In order to determine the specificity of action of quinazoline toward pUL97 in comparison to those of other protein kinases, a representative panel of kinases was analyzed in in vitro kinase assays. As shown in Table 1, only the activities of pUL97 and EGFR (Fig. 2C) were drastically reduced by these three compounds, which proves their selectivities in vitro.
TABLE 1.
Protein kinase selectivity panela
| Quinazoline | % Kinase activity
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ser/Thr
|
Tyr
|
Tyr and Ser/Thr
|
|||||||||||||||
| pUL97 | PKC | Akt | RIP | Cdk1 | p70S6K | Abl | Src | Lck | Kit | EGFR | PDGF | InsR | Met | ERK1 | p38 | JNK | |
| Ax7376 | 10 ± 3 | 88 ± 21 | 84 ± 14 | 86 ± 12 | 57 ± 2 | 88 ± 5 | 71 ± 17 | 80 ± 4 | 89 ± 15 | 95 ± 9 | 0 ± 2 | 100 ± 3 | 63 ± 11 | 98 ± 16 | 100 ± 10 | 59 ± 9 | 84 ± 6 |
| Ax7396 | 3 ± 2 | 102 ± 8 | 74 ± 2 | 88 ± 10 | 56 ± 4 | 99 ± 2 | 87 ± 10 | 100 ± 21 | 98 ± 8 | 94 ± 9 | 0 ± 5 | 100 ± 2 | 64 ± 7 | 100 ± 17 | 108 ± 3 | 40 ± 14 | 74 ± 10 |
| Ax7543 | 4 ± 2 | 91 ± 5 | 97 ± 8 | 100 ± 8 | 52 ± 3 | 95 ± 2 | 85 ± 4 | 100 ± 12 | 87 ± 7 | 101 ± 5 | 2 ± 2 | 100 ± 5 | 57 ± 20 | 100 ± 21 | 88 ± 6 | 100 ± 9 | 97 ± 15 |
Activity of a selected panel of protein kinases (Ser/Thr, specificity for serine/threonine; tyr, specificity for tyrosine; tyr and ser/thr, dual specificity) was quantified by the use of an in vitro kinase assay. The percentage of kinase activity (mean values±standard deviations) in the presence of 10 μM compound compared to that in the presence of 0.1% DMSO (100% activity) is shown. The averages of two independent experiments performed in duplicate are depicted.
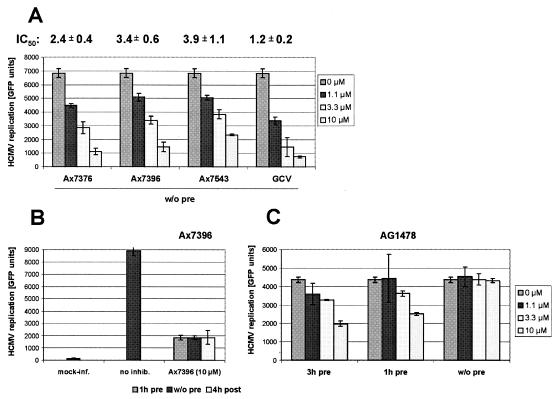
FIG. 2.
HCMV GFP-based antiviral assay for the determination of IC50s. For measurements of viral replication by the automated GFP fluorometry assay, HFFs were grown in 12-well plates and infected with 0.25 GFP-forming tissue culture infective doses of HCMV AD169-GFP. The quinazoline compounds and reference compound GCV were diluted in culture medium to the concentrations indicated (1.1, 3.3, or 10 μM) and added to the cell cultures, either immediately after virus adsorption without preincubation (w/o pre), after preincubation for 1 or 3 h (1h pre and 3h pre, respectively), or 4 h after virus adsorption (4h post). Infection rates (GFP-positive cells) and the lack of cytotoxicity of the compounds (confluent cell layers) were monitored daily by microscopy. At 7 days postinfection, cell layers were harvested, lysed, and subjected to automated fluorometry analysis of the GFP signals. Each panel shows the data for determinations performed in quadruplicate (infection in duplicate, GFP measurement in duplicate). Mock-inf., uninfected; no inhib., infected and incubated with DMSO solvent alone; Ax7376, Ax7396, Ax7543, and AG1478, infection and incubation with one of the three quinazolines or the EGFR inhibitor, respectively, at the indicated concentrations; GCV, infection and incubation with 20 μM GCV.
Selected quinazolines block replication of HCMV laboratory strain AD169.
We next analyzed the effects of the quinazolines on the replication of HCMV AD169-GFP, which expresses GFP as a reporter (HCMV GFP-based antiviral assay [17]). The level of AD169-GFP replication in HFFs was reduced to 4.8% by 10 μM Ax7396, whereas the untreated control had 57.6% GFP-positive cells, as detected by FACS analysis for GFP (Table 2). The level of inhibition was comparable to that obtained with the standard therapy drug, CDV (6.7% infected cells). Propidium iodide counterstaining, used as a marker for cell viability, envisaged that neither Ax7396 nor CDV caused considerable cytotoxic effects: low levels of cells stained negative (for both propidium iodide and GFP), i.e., 18.9 and 12.9% for Ax7396 and CDV, respectively, compared to 14.7 and 11.2%, respectively, for the control cells. The poor cytotoxicities of the quinazolines for human cells were confirmed by methylene blue staining experiments (21). Again, a low level of toxicity for HEK 293 cells with quinazoline treatment was demonstrated, i.e., viabilities of 100% at 1 μM and 100% at 100 μM for Ax7396, viabilities of 100% at 1 μM and 77% at 100 μM for Ax7376, and viabilities of 100% at 1 μM and 75% at 100 μM for Ax7543.
TABLE 2.
GFP-based flow cytometric determination of anti-HCMV effects of quinazolinesa
| Cell type | % Living HCMV-infected cells
|
% Living mock-infected cells (no inhibitor) | ||
|---|---|---|---|---|
| No inhibitor | Ax7396 (10 μM) | CDV (2 μM) | ||
| GFP+ PI+ | 57.6 ± 2.8 | 4.8 ± 0.9 | 6.7 ± 1.0 | 0.4 ± 0.1 |
| GFP− PI− | 11.2 ± 1.2 | 18.9 ± 1.4 | 12.9 ± 1.7 | 14.7 ± 2.0 |
HFFs were infected with HCMV AD169-GFP and treated with the indicated inhibitors, as described in the legend to Fig. 1. At 6 days postinfection, the cells were harvested, fixed with formaldehyde, and used for the determination of virus-positive cells by GFP-based FACS analysis. The portion of living cells was determined by propidium iodide (PI) staining. GFP+ PI+, virus-positive viable cells; GFP− PI−, virus-negative nonviable cells. Each panel was tested in quadruplicate (infection in duplicate, measurement in duplicate).
Comparative measurements of viral replication by automated fluorometry demonstrated that the quinazolines Ax7376, Ax7396, and Ax7543 inhibit HCMV AD169-GFP in a dose-dependent manner, with IC50s of 2.4 ± 0.4, 3.4 ± 0.6, and 3.9 ± 1.1 μM, respectively (Fig. 2A). These IC50s were in the same range as those of GCV (1.2 ± 0.2 μM). Notably, there was hardly any difference in the degree of inhibition when the compounds Ax7396 (Fig. 2B) and Ax7376 or Ax7543 (data not shown) were added either 1 h before, immediately after, or 4 h after virus adsorption. This property is profoundly different from that seen with inhibitors of viral entry, such as inhibitors of EGFR. In this context, it is important to stress that EGFR was recently defined as a specific entry receptor for HCMV (28). To rule out the possibility that the inhibitory effects of the quinazolines are due to inhibition of EGFR but not inhibition of pUL97 (Table 1), we performed the HCMV GFP-based antiviral assay in the presence of two EGFR-specific inhibitors, AG1478 and PD153035 (2). A concentration-dependent partial inhibition of HCMV replication was determined when HCMV was preincubated with AG1478 or PD153035 for 1 h and was even more pronounced when an inhibitor was added 4 h prior to virus inoculation. However, importantly, neither anti-EGFR compound showed any inhibitory effect on HCMV replication when the compounds were added after virus adsorption (Fig. 2C; data not shown for PD153035). This indicates that the modes of action of typical EGFR inhibitors and quinazolines are different. In conclusion, quinazolines do not seem to act at the level of viral adsorption and entry but possess a strong anticytomegalovirus activity mainly based on the inhibition of pUL97.
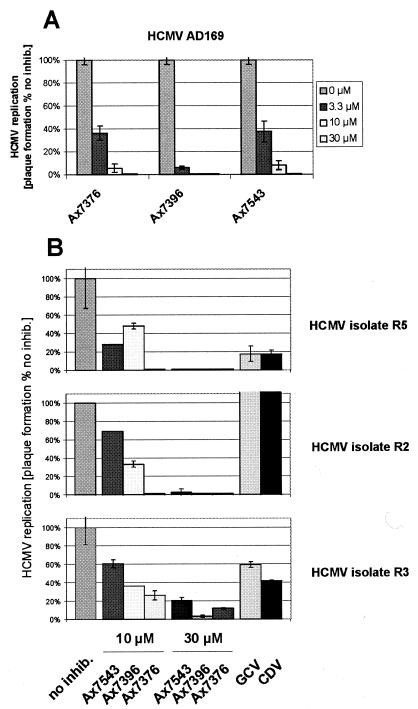
Clinical isolates of HCMV are sensitive to quinazolines.
First, the effects of the quinazolines against laboratory virus strain AD169 were confirmed by the conventional plaque reduction assay (Fig. 3A). The results were similar to those obtained by the GFP-based antiviral assay. Then, for further analysis of the inhibitory potentials of quinazolines, we chose three clinical isolates with individual resistance profiles: R5 is an isolate possessing sensitivity to conventional antiviral therapy drugs, such as GCV and CDV, as illustrated by the results in Fig. 3B (upper panel). Isolate R2 shows a phenotype of high-level resistance to GCV as well as CDV (Fig. 3B, middle panel), while R3 produces an intermediate phenotype with low-level resistance to both drugs (Fig. 3B, lower panel). For isolates R2 and R3, mutations were identified in the UL97 genes by sequence analysis of the cloned fragments, i.e., UL97(H469V) and UL97(C603W), respectively (data not shown). Mutation UL97(C603W) of isolate R3 was proved to confer GCV resistance in vitro (19). The molecular basis for the resistance of isolate R2 is unknown. Importantly, quinazoline treatment strictly inhibited plaque formation by all three viruses. The strongest effects were obtained over a range of concentrations of 10 to 30 μM for Ax7543, Ax7396, and Ax7376 (Fig. 3B).
FIG. 3.
GCV- and CDV-sensitive and -resistant clinical isolates of HCMV are sensitive to quinazolines. HFFs were infected in 12-well plates with HCMV AD169 (A) or clinical isolates R5, R2, and R3 (B) and overlaid with medium-agarose containing the antiviral compounds at the indicated concentrations. At 8 days postinfection, virus plaques were stained and quantified by microscopic counting (infection in duplicate, counting in duplicate). The sensitivities of the virus isolates to the drugs were determined by phenotypic analysis (GCV, 20 μM; CDV, 2 μM) (B), pUL97 in-cell activity assay with cloned UL97 (data not shown), and UL97 gene sequencing (see text). inhib., inhibition.
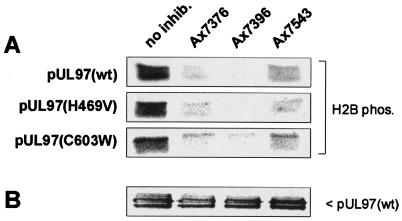
In order to investigate the basis of replication inhibition of these clinical isolates by quinazolines, the respective UL97 gene products were expressed by transfection and were used for in vitro kinase assays (Fig. 4). All three quinazolines (10 μM) exerted a strong inhibitory effect on H2B phosphorylation mediated by pUL97(wild type), pUL97(H469V), or pUL97(C603W), with Ax7396 being the most potent inhibitor. These quinazolines were observed to have similar inhibitory effects on the phosphorylation of MBP by pUL97 and on pUL97 autophosphorylation (data not shown). To exclude the possibility of contamination of the precipitated pUL97 with another cellular kinase, we tested the catalytically inactive mutant pUL97(K355 M) in parallel and did not detect any signals of phosphorylation of H2B or MBP or of autophosphorylation (data not shown). This indicates that mutations in UL97, which are associated with GCV resistance, do not confer cross-resistance to quinazolines, and thus, quinazolines are able to inhibit drug-sensitive and drug-resistant clinical isolates of HCMV.
FIG. 4.
In vitro kinase assay with pUL97 from clinical isolates. (A) pUL97(wt) (where wt represents wild type) from isolate R5 or mutants H469V from isolate R2 and C603W from isolate R3 were ectopically expressed in HEK 293 cells by transient transfection, immunoprecipitated with an anti-UL97 antiserum (PepAS 1343 [19]), and tested by in vitro kinase assays with H2B (1.5 μM) as the substrate. Reactions were performed in either the absence (no inhib.) or the presence of one of the quinazoline compounds (Ax7376, Ax7396, or Ax7543; 10 μM each). Samples were separated by SDS-PAGE, and phosphorylated H2B was detected by autoradiography. (B) Lysate controls were taken prior to immunoprecipitation and were used for quantification of the pUL97 expressed by Western blot analysis (anti-UL97 antiserum PepAS 1343). The expression of pUL97(wild type) (as depicted), pUL97(H468V), and pUL97(C603W) was detectable at similar levels.
Experimental selection did not produce quinazoline-resistant viruses.
Resistance formation was analyzed in infection experiments with long-term quinazoline treatment. HCMV AD169-GFP was used to infect HFFs in 12-well plates. Selection of resistant viral clones was performed by the use of agar overlays containing increasing concentrations of quinazoline compounds or GCV as a reference. Each selection interval was continued to the point of formation of GFP-positive viral plaques (approximately 7 days). Plaques were isolated, expanded in culture to produce viral stocks, and subjected to subsequent rounds of selection. Finally, three GCV-resistant clones of AD169-GFP were obtained, whereas selection with none of the quinazoline compounds yielded drug-resistant viruses (Table 3). Transiently, a small number of seemingly resistant plaques—which were, however, not well defined in shape or size—was observed mainly with Ax7543 and Ax7376 treatment; but these virus clones were lost upon repeated selection. A genotypic analysis of GCV-resistant viruses revealed mutation M460I in the UL97 genes of all three clones selected (data not shown). This mutation is a proven GCV resistance-conferring marker and represents one of three main sites of GCV resistance in clinical isolates (7). Thus, while GCV treatment was associated with a rapid onset of resistance formation, quinazoline treatment under the same conditions did not produce resistant viruses.
TABLE 3.
HCMV resistance formation under drug selectiona
| Inhibitor | No. of plaques/agar well
|
|||
|---|---|---|---|---|
| 1.1 μM | 3.3 μM | 10 μM | 10 μMb | |
| Ax7376 | 75 | 8 | ≥5c | 0 |
| Ax7396 | 82 | ≥5c | 0 | 0 |
| Ax7543 | 93 | ≥5c | ≥5c | 0 |
| GCV | 66 | 5 | 3 | 3 |
| None | 80 | >100 | >100 | NDd |
| Mock infection | 0 | 0 | 0 | ND |
HFFs were infected with HCMV AD169-GFP in the presence of the indicated concentrations of drugs for the selection of drug-resistant viruses. The selection intervals for isolation of viral plaques from agar overlays were 7 days. Intermittently, the isolated plaques were expanded in culture without agar with identical drug concentrations (selection at 10 μM was additionally repeated three times). In total, 12 passages of drug selection were performed. A second experiment performed independently confirmed the lack of quinazoline resistance formation.
Repeated three times.
Lack of well-defined plaques.
ND, not determined.
Inhibition of HCMV replication occurs during the early-late phase but not at the level of viral entry.
The results described so far imply that pUL97 is the target for the antiviral activities of the quinazolines. In further studies, we wanted to elucidate the mode of action in more detail. Viral protein production was measured during individual phases of viral replication by Western blotting analysis (Fig. 5A). HFFs were infected with HCMV AD169, treated with 20 μM quinazolines immediately postinfection (or with GCV as a control), and cultivated for 24 or 72 h. At 24 h postinfection, the levels of the viral immediate-early proteins IE2p86 and IE1p72 were determined. No significant changes in protein levels compared to those for the untreated controls were noted as a result of treatment with any of the inhibitors (Fig. 5A, upper panel). The levels of production of viral early protein (pUL44) and early-late protein (gB) were determined at 72 h postinfection. For all quinazolines, the intensities of the two pUL44-specific bands showed that significant inhibition of pUL44 production occurred. (The nature of the pUL44-specific bands is not known; their formation is detectable for various strains of HCMV, and the largest protein species is massively phosphorylated in vitro [16; M. Marschall, unpublished results].) The same inhibitory effect was noted for GCV, which inhibits viral DNA synthesis in a pUL97-dependent manner. Even more pronounced was the inhibitory effects of the quinazolines on the synthesis of the early-late protein gB. All quinazolines blocked gB synthesis to undetectable levels. This finding proves that quinazolines possess inhibitory effects on viral events later than the immediate-early level. Inhibitory effects have consequences on viral protein synthesis during the early stage of HCMV replication and have even more drastic consequences during the late stage of HCMV replication. This is consistent with the specific inhibition of pUL97 protein kinase activity (12, 29).
To confirm this conclusion, we performed an experiment with a mutant of HCMV from which UL97 was deleted (AD169delUL97) (24). As measured by plaque reduction assay, the AD169delUL97 virus possessed a deficiency of replication to about 1 to 5% of that of the parental virus but could eventually be grown to low-titer stocks and used for infection assays (19). In plaque reduction assays, the AD169delUL97 virus was tested for its sensitivities to the quinazoline Ax7376, the indolocarbazole NGIC-I, and CDV (an inhibitor of viral DNA polymerase). Ax7376 and NGIC-I had only little or no effect on the low level of residual replication of the virus deletion mutant, while CDV treatment resulted in complete inhibition (Fig. 5B). This result further indicates that pUL97 is the main target of quinazoline antiviral activity.
DISCUSSION
In recent years, several reports have demonstrated the importance of pUL97 for efficient HCMV replication and, thus, suggest that its kinase activity is a promising target for HCMV therapy. In this report, we describe the class of quinazolines as novel pUL97 inhibitors and provide several lines of evidence that these small molecules qualify for use in the development of anti-HCMV drugs: (i) the quinazolines selected were highly potent in blocking the in vitro phosphorylation mediated by pUL97, such as the phosphorylation of various substrates (H2B and MBP) as well as autophosphorylation, with IC50s in the low micromolar range; (ii) the quinazolines did not have the cytotoxic effects of GCV in cells expressing pUL97; (iii) the kinetics of HCMV inhibition and failure to inhibit replication of an HCMV mutant from which UL97 was deleted argue that pUL97 is the target responsible for the anti-HCMV activities of the quinazolines; (iv) clinical isolates of HCMV possess a quinazoline-sensitive phenotype even after they have acquired GCV and CDV resistance-conferring mutations in the UL97 gene; (v) the formation of viral resistance to quinazolines was not observed at the frequency of the formation of resistance to GCV, as analyzed in long-term treatment experiments; and, finally, (vi) of 17 protein kinases tested, only the activities of pUL97 and EGFR were efficiently inhibited by the quinazolines in vitro.
With the help of the master key concept, which uses privileged structures like quinazolines for drug development, it may be possible to develop pUL97-specific kinase inhibitors (22). On the other hand, it might be rather beneficial, with respect to efficacy and resistance formation, for an anti-HCMV drug to block both pUL97 and EGFR, since EGFR represents an HCMV binding and signaling receptor (28). Our own experiments with two EGFR-specific inhibitors (AG1478 and PD153035) did not, however, fully confirm their strong inhibitory effects against HCMV AD169 replication, as previously revealed for AG1478 against the Towne strain of HCMV (28). This may be due to differences in the strains, host cells, and infection conditions used. Most important for the inhibitory effects of EGFR inhibitors seems to be the finding that preincubation of cells with AG1478 prior to HCMV inoculation, which was performed in experiments by Wang et al. (28), appeared to be decisive for the development of an antiviral effect mediated by EGFR inhibitors. Our results support these findings and, furthermore, show that addition of AG1478 or PD153035 at a later time point of infection (e.g., immediately after virus adsorption) did not markedly inhibit HCMV replication, a finding that is in clear contrast to the findings related to the inhibitory properties of quinazolines. This illustrates, on the one hand, that quinazolines exert their main effect at steps later than virus entry and, on the other hand, that EGFR inhibitors eventually have little effect on subsequent intracellular steps of HCMV replication. In this respect, it was interesting that the quinazolines had no or only minor effects on the residual replication efficiency of an HCMV strain from which UL97 was deleted. This finding further confirms the pUL97-directed mode of action of quinazolines.
Experience with drugs previously approved for use for the treatment of infections caused by viruses other than herpesviruses (e.g., zanamivir and oseltamivir for influenza viruses or ribavirin for respiratory syncytial virus) suggests that those drug candidates that do not readily induce the formation of resistant virus should mainly be developed for clinical use. Drugs that target pUL97 might interact with subdomains of the pUL97 kinase domain which are indispensable for its physiological function, such as essential positions of the ATP binding site, the catalytic center, or its substrate recognition sites. This might have important implications for the formation of viral drug resistance. Basically, the mode of resistance formation is expected to be different for GCV and the quinazolines: in the case of GCV resistance, pUL97 remains active in phosphorylating proteins (but specifically lacks an ability to recognize and phosphorylate GCV); in the case of a postulated quinazoline resistance, pUL97 might become catalytically inactive through mutation and the respective viruses might loose their potential to replicate efficiently. This could explain the lack of frequent formation of resistant viruses under experimental conditions, as shown in Table 3. Moreover, for the treatment of HCMV infection, a potential approach is the use of a combination therapy with drugs targeting different target proteins or domains. In this sense, one of the limitations of pUL97 inhibitors is the fact that they would not be suitable for use in conjunction with GCV. However, the use of protein kinase inhibitors with dual specificities, e.g., for pUL97 and, in addition, for a cellular protein kinase which plays an important role in HCMV replication (such as EGFR), might compensate for such limitations. The use of combination therapies with protein kinase inhibitors (e.g., those approved for use for other clinical applications, such as tumor therapy) might also open new possibilities for antiviral treatment.
Crucial criteria other than toxicity and selectivity in the development of a compound into a drug are a favorable absorption, distribution, metabolism, and excretion profile and promising pharmacokinetic and pharmacodynamic properties. In this respect, quinazolines are quite promising, and derivatives of this class are in late stages of clinical development (OSI-744 and Tarceva) or have already been approved (gefitinib [Iressa]) for the treatment of cancer. Indeed, gefitinib also showed a concentration-dependent inhibition of pUL97 activity in in vitro kinase assays and to some degree blocked HCMV replication in HFFs. However, the potency of gefitinib was lower than those of the three quinazolines described here (data not shown).
From the point of view of anti-HCMV drug development, it is important that pUL97 unequivocally be a critical determinant of the efficiency of viral progeny production (24, 29). It is believed that the pUL97 kinase activity not only determines HCMV replication efficiency in cell culture systems but also determines HCMV replication efficiency in vivo and viral pathogenicity. Specifically designed protein kinase inhibitors, some of which are gaining importance for immunosuppression and tumor therapy, could also become interesting tools as novel antiviral treatments. Thus, direct targeting of the pUL97 kinase activity is an attractive antiviral approach which might contribute to the improved treatment of HCMV disease in future.
Acknowledgments
We thank P. Lischka (University of Erlangen-Nürnberg) for critical comments and reading the manuscript. We are grateful to H. Mett for assistance with the kinase assays, B. Klebl (Axxima Pharmaceuticals AG) for helpful discussion, G. Keri and L. Orfi (Vichem Kft) for designing compounds, G. Pari for providing a mutant virus from which UL97 was deleted, Sabrina Auerochs for excellent technical assistance, and B. Fleckenstein (University of Erlangen-Nürnberg) for discussion and support.
This work was supported by BMBF (grant 0312654), IZKF Erlangen, and Bayerische Forschungsstiftung (grant 576/03).
REFERENCES
- 1.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencke, S., A. Ullrich, and H. Daub. 2003. Mutation of threonine 766 in the epidermal growth factor receptor reveals a hotspot for resistance formation against selective tyrosine kinase inhibitors. J. Biol. Chem. 278:15435-15440. [DOI] [PubMed] [Google Scholar]
- 3.Borst, E. M., U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chulay, J., K. Biron, L. Wang, M. Underwood, S. Chamberlain, L. Frick, S. Good, M. Davis, R. Harvey, L. Townsend, J. Drach, and G. Koszalka. 1999. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease. Adv. Exp. Med. Biol. 458:129-134. [DOI] [PubMed] [Google Scholar]
- 5.Coen, D. M., and P. A. Schaffer. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Disc. 2:278-288. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, M. H., G. A. Williams, R. Sridhara, G. Chen, and R. Pazdur. 2003. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 8:303-306. [DOI] [PubMed] [Google Scholar]
- 7.Erice, A. 1999. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 12:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godl, K., J. Wissing, A. Kurtenbach, P. Habenberger, S. Blencke, H. Gutbrod, K. Salassisis, M. Stein-Gerlach, A. Missio, M. Cotten, and H. Daub. 2003. An efficient proteomics method to identify the cellular target of protein kinase inhibitors. Proc. Natl. Acad. Sci. USA 26:15434-15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herget, T., S. A. Oehrlein, D. J. Pappin, E. Rozengurt, and P. J. Parker. 1995. The myristoylated alanine-rich C-kinase substrate (MARCKS) is sequentially phosphorylated by conventional, novel and atypical isotypes of protein kinase C. Eur. J. Biochem. 233:448-457. [DOI] [PubMed] [Google Scholar]
- 10.Komazin, G., R. G. Ptak, B. T. Emmer, L. B. Townsend, and J. C. Drach. 2003. Resistance of human cytomegalovirus to the benzimidazole l-ribonucleoside maribavir maps to UL27. J. Virol. 77:11499-11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koszalka, G. W., N. W. Johnson, S. S. Good, L. Boyd, S. C. Chamberlain, L. B. Townsend, L. C. Drach, and K. K. Biron. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimirob. Agents Chemother. 46:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krosky, P. M., M.-C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krosky, P. M., M.-C. Baek, W. J. Jahng, I. Barrera, R. J. Harvey, K. K. Biron, D. M. Coen, and P. B. Sethna. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalezari, J. P., J. A. Aberg, L. H. Wang, M. B. Wire, R. Miner, W. Snowden, C. L. Talarico, S. Shaw, M. A. Jacobsen, and W. L. Drew. 2002. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob. Agents Chemother. 46:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 16.Marschall, M., M. Freitag, P. Suchy, D. Romaker, R. Kupfer, M. Hanke, and T. Stamminger. 2003. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 311:60-71. [DOI] [PubMed] [Google Scholar]
- 17.Marschall, M., M. Freitag, S. Weiler, G. Sorg, and T. Stamminger. 2000. Recombinant GFP-expressing human cytomegalovirus as a tool for screening of antiviral agents. Antimicrob. Agents Chemother. 44:1588-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2001. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J. Gen. Virol. 82:1439-1450. [DOI] [PubMed] [Google Scholar]
- 19.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle of antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 20.McSharry, J. J., A. McDonough, B. Olson, C. Talarico, M. Davis, and K. K. Biron. 2001. Inhibition of ganciclovir-susceptible and -resistant human cytomegalovirus clinical isolates by the benzimidazole l-riboside 1263W94. Clin. Diagn. Lab. Immunol. 8:1279-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mett, H., K. Hölscher, H. Degen, C. Esdar, B. Felden de Neumann, B. Flicke, T. Freudenreich, G. Holzer, S. Schinzel, T. Stamminger, M. Stein-Gerlach, M. Marschall, and T. Herget. Identification of inhibitors for a virally encoded protein kinase by two different screening systems: in-vitro kinase assay and in-cell activity assay. J. Biomol. Screen, in press. [DOI] [PubMed]
- 22.Müller, G. 2003. Medicinal chemistry of target family-directed masterkeys. Drug Discov. Today 8:681-691. [DOI] [PubMed] [Google Scholar]
- 23.Pass, R. F. 2001. Cytomegaloviruses, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 24.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slater, M. J., S. Cockerill, R. Baxter, R. W. Bonser, K. Gohil, C. Gowrie, J. E. Robinson, E. Littler, N. Parry, R. Randall, and W. Snowden. 1999. Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication. Bioorg. Med. Chem. 7:1067-1074. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 27.Wang, L. H., R. W. Peck, Y. Yin, J. Allanson, R. Wiggs, and M. B. Wire. 2003. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, X., S.-M. Huong, M. L. Chiu, N. Raab-Traub, and E.-S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 29.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann, A., D. Michel, I. Pavic, W. Hampl, A. Lüske, J. Neyts, E. De Clerq, and T. Mertens. 1997. Phosphorylation of aciclovir, genciclovir, penciclovir and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antivir. Res. 36:35-42. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann, A., H. Wilts, M. Lenhardt, M. Hahn, and T. Mertens. 2000. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antivir. Res. 48:49-60. [DOI] [PubMed] [Google Scholar]