Abstract
This study was performed to investigate placental transfer of nucleoside analogue reverse transcriptase inhibitors (NRTIs) and their concentrations in amniotic fluid when given to human immunodeficiency virus (HIV)-infected pregnant women. A total of 100 HIV type 1-infected mothers receiving antiretroviral therapy, including one or more NRTIs, for clinical indications at the time of delivery were enrolled. Maternal blood samples and amniotic fluid were obtained during delivery or cesarean section, and paired cord blood samples were obtained by venipuncture immediately after delivery. Drug concentrations were measured by using high-performance liquid chromatography. A significant relationship between concentrations in maternal and cord plasma samples was found for zidovudine, lamivudine, stavudine, and didanosine. The ratio between the concentrations in cord and maternal plasma samples (R) was high for zidovudine (R = 1.22), its glucuronide metabolite (3′-azido-3′-deoxythymidine-β-d-glucuronide) (R = 1.01), stavudine (R = 1.32), lamivudine (R = 0.93), and abacavir (R = 1.03) and was low for didanosine (R = 0.38). The ratio between the concentrations in amniotic fluid and cord plasma samples was high for zidovudine (R = 2.24), its glucuronide metabolite (R = 2.83), stavudine (R = 4.87), and lamivudine (R = 3.99) and was lower for didanosine (R = 1.14). These findings indicate that most NRTIs cross the placenta by simple diffusion and are concentrated in the amniotic fluid, probably through fetal urinary excretion. The efficacy or toxicity of NRTIs may vary according to placental transfer.
Treatment recommendations for pregnant women infected with human immunodeficiency virus (HIV) type 1 have been based on the belief that therapies of known benefit to women should not be withheld during pregnancy unless they have known adverse effects on the mother, fetus, or infant and unless these adverse effects outweigh the benefit to the woman (13). There is also evidence from observational studies that combination antiretroviral therapies are more effective than zidovudine monotherapy in reducing the risk of mother-to-child HIV transmission (4, 24). For these reasons, the majority of HIV-infected women in the industrialized countries are treated during pregnancy with a combination of antiretroviral regimens, usually consisting of two nucleoside analog reverse transcriptase inhibitors (NRTIs) and a protease inhibitor (20).
However, the data about the safety of antiretroviral drugs are limited, with the exceptions of zidovudine and, to a lesser extent, lamivudine. A study conducted in France reported that several infants with perinatal exposure to either zidovudine-lamivudine or zidovudine alone developed persistent mitochondrial dysfunction (2). The causal relation between NRTI exposure and mitochondrial disease remains controversial (14). Follow-up is still insufficient to address the effect that exposure to zidovudine or other antiretroviral agents in utero might have on long-term risk for neoplasia or organ system toxicities in children (5, 8).
The placental transfer and pharmacodynamics in the fetal compartment are important to consider when prescribing drugs during pregnancy. Zidovudine has been widely used and studied in pregnancy, and high cord-to-maternal plasma drug concentration ratios have been consistently reported (15, 26). Similar results have been reported with lamivudine (12, 14). There have been few clinical studies concerning didanosine or stavudine (25) and no report concerning abacavir.
The present study was performed to compare the placental transfers and amniotic fluid concentrations of the commercially available nucleoside analogue reverse transcriptase inhibitors used in a clinical setting.
MATERIALS AND METHODS
The study enrolled 100 HIV-infected pregnant women who were receiving NRTIs at the time of delivery and who were enrolled with informed consent in the French Perinatal Cohort, as approved by the institutional review board. The women were delivered at Cochin Saint Vincent de Paul Hospital between 1 April 1999 and 28 February 2002. Women were eligible for participation in this study if they had received an antenatal antiretroviral drug regimen including an NRTI at the time of delivery and had maternal blood, cord blood, and amniotic fluid samples available. Women could receive any licensed antiretroviral agents for prophylaxis of HIV transmission and treatment of their HIV infection. Every woman received zidovudine intravenously during labor for prevention of mother-to-child transmission of HIV. The dose used was 2 mg/kg of body weight intravenously over a 1-h period, followed by a continuous infusion of 1 mg/kg/h intravenously until delivery. The clinical guidelines recommended continuing other antiretroviral drugs orally at the usual hours, with the exception of stavudine, because of its incompatibility with zidovudine.
Cord blood samples were collected at delivery by venipuncture into heparinized tubes after the cord was carefully wiped to avoid potential contamination with amniotic fluid or maternal blood. Maternal blood was drawn at the same time. Amniotic fluid samples were obtained at the time of membrane rupture in the case of planned cesarean deliveries and whenever possible in other deliveries, with precautions taken to avoid contamination with maternal blood. Amniotic fluid samples that contained visible contamination with blood were discarded. Plasma was isolated by centrifugation.
All of the drugs were measured in a 100-μl plasma sample by high-performance liquid chromatography. An internal standard was used with each analytical procedure. Zidovudine and its glucuronide metabolite zidovudine G were measured simultaneously, extracted by using a solid-phase extraction procedure on Bond Elut C18 (conditioning step, methanol and phosphate-buffered saline buffer; washing step, phosphate-buffered saline buffer; elution step, methanol), and separated isocratically on a Satisfaction C8 Plus column (250 by 3 mm) with a mobile phase consisting of buffer (25 mM potassium phosphate, pH 2.9) and methanol (80:20, vol/vol) at a flow rate of 0.5 ml/min. UV absorbance at 267 nm was used for detection. The lower limit of quantification (LOQ) was 0.05 mg/liter. The mean interassay precisions at the lowest concentration of the quality controls were 10 and 13.9%, and inaccuracies at the LOQ (percent deviation from the expected value) were 4.2 and 7.5%, for zidovudine and its metabolite, respectively. Overall recovery from plasma was 89% for zidovudine and 82% for zidovudine G. Lamivudine, stavudine, and didanosine were measured simultaneously, extracted by solid-phase extraction on Bond Elut C18, and separated on a Satisfaction C8 Plus column (250 by 3 mm) with a gradient (flow rate, 0.5 ml/min) of solvent A (water with 0.01% trifluoroacetic acid) and solvent B (acetonitrile) as follows: 98% solvent A and 2% solvent B for 10 min and 90% solvent A and 10% solvent B for 20 min. UV absorbance at 270 nm was used for detection of lamivudine and stavudine, and UV absorbance at 254 nm was used for didanosine. The LOQs were 0.05 mg/liter for lamivudine and stavudine and 0.025 mg/liter for didanosine. Mean interassay precisions at the low quality controls were 10% (lamivudine), 12.7% (stavudine), and 15% (didanosine), and inaccuracies at the LOQ were 4.5% (lamivudine), 0.67% (stavudine), and 0% (didanosine). Overall recoveries were 65% (lamivudine), 81% (stavudine), and 76% (didanosine). Abacavir was extracted with terbutylmethylether and separated isocratically on a Satisfaction C8 Plus column (250 by 3 mm) with a mobile phase consisting of buffer (10 mM potassium phosphate, pH 7.2) and acetonitrile (82:18, vol/vol) at a flow rate of 0.5 ml/min. UV absorbance at 280 nm was used. The LOQ was 0.025 mg/liter. Mean interassay precision at the low quality controls was 14%, and accuracy at the LOQ was 1%. Overall recovery was 60%. Each blood and amniotic fluid sample was accompanied by the exact sampling time and the hours of the last antiretroviral dose intakes before delivery. The maternal-fetal transfer was evaluated by the ratio of the concentration in fetal plasma to that in maternal plasma.
Descriptive statistics (median and range) were reported for all five NRTIs measured in maternal, cord, and amniotic samples. Linear regression analysis was used to correlate cord and maternal plasma samples. Quantitative values were compared by using the t test.
RESULTS
The mothers' mean age at delivery was 33 years (range, 23 to 43); 54% were African, 43% were European, and 3% were Asian; and 80% were infected through sexual exposure. The median (range) maternal plasma HIV RNA level at delivery was <50 copies/ml (<50 to 36,572 copies/ml), and the proportion of women with a detectable viral load at delivery was 34% (28 of 83). The median (range) CD4 lymphocyte count was 473 × 106/liter (98 × 106 to 1,580× 106/liter). The mode of delivery was cesarean section in 78% of the cases. None of the infants was HIV infected.
The NRTIs received by the 100 women included intravenous zidovudine (n = 100), oral zidovudine (n = 60), lamivudine (n = 67), stavudine (n = 32), didanosine (n = 24), and abacavir (n = 4). The majority of these women (83 of 100) received antiretroviral regimens with two NRTIs, with the most frequently prescribed combination (n = 20) being zidovudine with lamivudine and nelfinavir. Twelve patients were taking a regimen with only one NRTI; these regimens included zidovudine alone (n = 6), one NRTI and nevirapine (n = 5), and one NRTI and two protease inhibitors (n = 1).
Maternal antiretroviral therapy was initiated before the beginning of pregnancy in 27% of cases, during the first trimester in 14% of cases, during second trimester in 25% of cases, and during third trimester in 34% of cases. The median time on treatment with the current NRTI was 3 months (range, 0.23 to 81.7 months) for zidovudine, 4.78 months (range, 0.23 to 81.7 months) for lamivudine, 5.63 months (range, 0.53 to 45.8 months) for stavudine, 4.23 months (range, 0.53 to 37.3 months) for didanosine, and 21.2 months (range, 7.1 to 35 months) for abacavir. The median time elapsed between the last NRTI oral dose and delivery was 5.0 h (range, 0.4 to 22.0 h) for zidovudine, 5.0 h (range, 0.4 to 22 h) for lamivudine, 13.0 h (range, 1.2 to 20 h) for stavudine, 9.0 h (range, 2.1 to 33 h) for didanosine, and 3.5 h (range, 0.4 to 9.0 h) for abacavir.
Median drug concentrations in maternal and cord plasma samples are displayed in Table 1. Cord blood drug concentrations were below the assay limit of detection in 14 of 32 samples (44%) for stavudine and in 15 of 24 samples (62%) for didanosine but were detectable in all samples for zidovudine, zidovudine G, lamivudine, and abacavir.
TABLE 1.
Drug concentrations in maternal and cord plasma samples
| Druga | Maternal plasma
|
Cord plasma
|
Cord plasma/maternal plasma
|
|||
|---|---|---|---|---|---|---|
| n | Median (range) drug concn (mg/liter) | n | Median (range) drug concn (mg/liter) | n | Median (range) drug concn ratio | |
| AZT | 77 | 0.59 (<0.05-3.39) | 97 | 0.70 (0.16-2.27) | 75 | 1.22 (0.18-17.2) |
| AZTG | 94 | 1.60 (<0.05-10.1) | 96 | 1.62 (0.51-6.17) | 90 | 1.01 (0.36-12.1) |
| 3TC | 64 | 0.45 (<0.05-1.66) | 63 | 0.40 (<0.05-1.18) | 59 | 0.93 (0.21-4.03) |
| D4T | 31 | <0.05 (<0.05-0.34) | 32 | 0.04 (<0.05-0.44) | 12 | 1.32 (0.36-2.81) |
| DDI | 24 | <0.05 (<0.05-1.61) | 24 | <0.05 (<0.05-0.46) | 10 | 0.38 (<0.05-2.00) |
| Abacavir | 4 | 0.80 (0.15-1.83) | 4 | 0.83 (0.21-1.80) | 4 | 1.03 (0.92-1.42) |
AZT, zidovudine; 3TC, lamivudine; D4T, stavudine; DDI, didanosine.
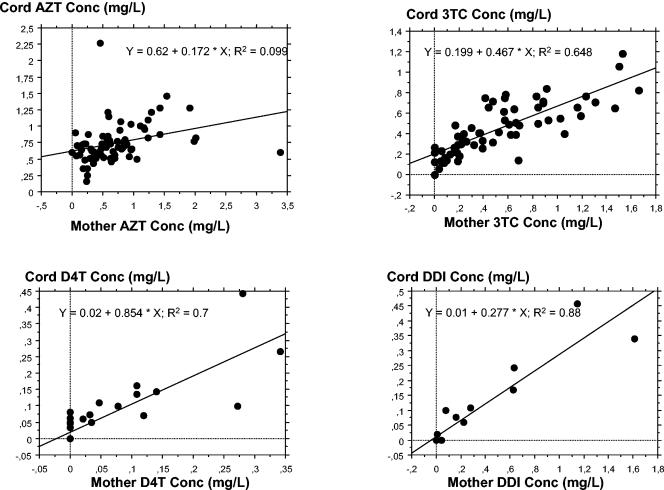
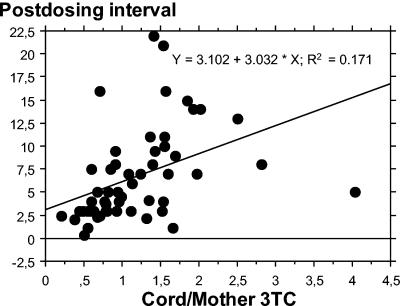
For paired samples with concentrations in maternal samples above the assay limit of quantification, cord-to-maternal plasma drug concentration ratios was determined in order to assess placental transfer. Among the 160 measurable maternal-cord blood sample pairs, transplacental passage was assessed for zidovudine (n = 75), zidovudine G (n = 90), lamivudine (n = 59), stavudine (n = 12), didanosine (n = 10), and abacavir (n = 4). The median cord-to-maternal plasma drug concentration ratio was close to one for zidovudine (R = 1.22; range, 0.18 to 17.2), zidovudine G (R = 1.01; range, 0.36 to 12.1), stavudine (R = 1.32; range, 0.36 to 2.81), lamivudine (R = 0.93; range, 0.21 to 4.03), and abacavir (R = 1.03; range, 0.92 to 1.42) and was small for didanosine (R = 0.38; range, 0.00 to 2.00). There was a significant correlation between maternal and cord plasma drug concentrations for zidovudine (r2 = 0.10; P < 0.01), zidovudine G (r2 = 0.38; P < 0.01), lamivudine (r2 = 0.65; P < 0.01), stavudine (r2 = 0.7; P < 0.01), and didanosine (r2 = 0.88; P < 0.01) (Fig. 1). For abacavir, no significant correlation between maternal and cord plasma drug concentrations was seen. Furthermore, for lamivudine, a significant relationship between cord/maternal drug concentration ratios and postdosing intervals was seen (Fig. 2). For the other drugs, the cord/maternal ratios were not affected by postdosing time intervals.
FIG. 1.
Relationship between concentrations of zidovudine (AZT), stavudine (D4T), lamivudine (3TC), and didanosine (DDI) in cord and maternal plasma samples.
FIG. 2.
Relationship between cord/maternal lamivudine (3TC) concentration ratios in plasma and postdosing intervals.
There were 46 amniotic fluid samples available for zidovudine, 45 for zidovudine G, 27 for lamivudine, 15 for stavudine, 8 for didanosine, and 1 for abacavir. Among these amniotic fluid samples, the proportions with drug concentrations below the detection limit were 1 of 27 for lamivudine, 1 of 15 for stavudine, and 4 of 8 for didanosine, but drug was detectable in all samples for zidovudine (46 of 46), zidovudine G (45 of 45), and abacavir (1 of 1).
Median drug concentrations and ratios in amniotic fluid are displayed in Table 2. Median concentrations in amniotic fluid were higher than those in maternal and cord plasma samples for lamivudine (0.45 and 0.41 mg/liter, respectively), zidovudine (0.59 and 0.7 mg/liter, respectively), zidovudine G (1.60 and 1.62 mg/liter, respectively), and stavudine (0.0 and 0.04 mg/liter, respectively). Concentrations of zidovudine and its metabolite in amniotic fluid were higher in mothers receiving oral zidovudine prior to delivery than in mothers received only intrapartum intravenous zidovudine; the median zidovudine and zidovudine G concentrations were, respectively, 1.68 mg/liter (range, 0.89 to 4.33 mg/liter) versus 1.31 mg/liter (range, 0.15 to 2.67 mg/liter) (P < 0.01) and 8.8 mg/liter (range, 3.8 to 26.0 mg/liter) versus 2.9 mg/liter (range, 0.1 to 4.4 mg/liter) (P < 0.01). Median (range) amniotic fluid/cord plasma drug concentration ratios, in samples with cord plasma drug concentrations above the assay limit of detection, were as follows: zidovudine, 2.25 (0.08 to 8.38); zidovudine G, 2.83 (0.06 to 19.6); stavudine, 4.87 (0.98 to 11.9); lamivudine, 3.99 (1.62 to 17.1); and didanosine, 1.14 (1.02 to 3.16). There was only one such ratio for abacavir, 0.89. A significant relationship between amniotic fluid and cord plasma drug concentrations was seen for zidovudine G (r2 = 0.10; P < 0.01), lamivudine (r2 = 0.10; P < 0.01), and stavudine (r2 = 0.22; P < 0.01). The ratio of the amniotic fluid drug concentration to the maternal plasma drug concentration was significantly related to the time elapsed since the last maternal dose for lamivudine but not for stavudine, didanosine, and abacavir.
TABLE 2.
Drug concentrations in amniotic fluid and relationship to concentrations in plasma
| Druga | Amniotic fluid
|
Amniotic fluid/cord plasma
|
Amniotic fluid/maternal plasma
|
|||
|---|---|---|---|---|---|---|
| n | Median (range) drug concn (mg/liter) | n | Median (range) drug concn ratio | n | Median (range) drug concn ratio | |
| AZT | 46 | 1.49 (0.14-4.33) | 46 | 2.25 (0.08-8.38) | 34 | 1.26 (0.17-11.4) |
| AZTG | 45 | 7.29 (0.07-26.0) | 45 | 2.83 (0.06-19.6) | 43 | 3.46 (0.05-27.4) |
| 3TC | 27 | 1.68 (<0.05-6.96) | 26 | 3.99 (1.62-17.1) | 26 | 3.99 (0.83-19.2) |
| D4T | 15 | 0.25 (<0.05-0.7) | 8 | 4.87 (0.98-11.9) | 5 | 6.45 (0.77-28.6) |
| DDI | 8 | 0.03 (<0.05-0.35) | 3 | 1.14 (1.02-3.16) | 3 | 0.44 (0.21-1.21) |
| Abacavir | 1 | 1.6 | 1 | 0.89 | 1 | 0.87 |
AZT, zidovudine; 3TC, lamivudine; D4T, stavudine; DDI, didanosine.
DISCUSSION
These data on the NRTIs zidovudine, lamivudine, stavudine, and didanosine show that they cross the placenta in HIV-infected pregnant women, in agreement with previous studies (12, 14, 15, 25, 26). Our study includes the first preliminary clinical data available on abacavir, which suggest that its placental transfer is also high.
For zidovudine, lamivudine, and stavudine, the ratio of the drug concentration in the fetal plasma to that in the maternal plasma was about 1, which suggests that these drugs freely crossed the placenta through a passive diffusion mechanism. The physicochemical characteristics of these drugs, i.e., low molecular weight and low protein binding, are compatible with high placental transfer. These findings are consistent with those obtained from animal studies (19), an ex vivo placental perfusion model (1), and human clinical trials (15, 21). For lamivudine, a significant relationship between cord/maternal drug concentration ratios and postdosing intervals was seen, which suggests that accumulation of the compound occurs in the fetal compartment. For didanosine, the ratio of the concentration in the fetal plasma to that in the maternal plasma was the lowest. Low concentrations of didanosine in the maternal circulation at delivery, largely due to once-a-day dosing, are likely to contribute to the low fetal drug concentrations, which is in accord with experimental findings obtained from animal studies (17), the ex vivo placental perfusion model (6, 9), and one clinical trial (25).
The only antiretroviral drugs for which concentrations in amniotic fluid have previously been studied in humans are zidovudine (second trimester) (18) and lamivudine (12). In our study, median concentrations in amniotic fluid were higher than those in maternal and cord plasma samples for lamivudine, stavudine, and zidovudine and its glucuronide metabolite. These high concentrations in amniotic fluid could probably be explained by the fetal urinary excretion of these drugs in the amniotic fluid and slow elimination from the amniotic cavity by the paraplacental route and diffusion across the placental surface. This is consistent with previous animal (23) and clinical (12) studies. Unlike others drugs, zidovudine, which is metabolized to a glucuronide form in the maternal compartment, is rapidly converted in the animal fetus to glucuronide zidovudine (16). Thus, concentrations of intact zidovudine in the fetus are lower, and fetal urinary excretion concerns the glucuronide form of zidovudine.
The comparison of cord-to-maternal drug concentration ratios has several limitations. The results are obtained at delivery and thus cannot be extrapolated to earlier gestational ages. Moreover, cord-to-maternal ratios offer data on only a single point in time. The measured maternal drug concentrations depend on the time between the last maternal dose and delivery, which is usually long. As shown from ex vivo placental perfusion studies, placental transfer is dependent on the drug concentration and is higher at peak concentrations (1, 3). Although we did not find a relationship between the time from last dose to delivery and cord blood drug concentrations (except for lamivudine), few of the maternal levels were peak concentrations. Further studies are required to estimate placental transfer of NRTIs at peak concentrations.
The high concentrations of NRTIs found in the fetal circulation may have beneficial or deleterious consequences. In utero exposure carries a risk of toxicity to the fetus. It should be kept in mind that most of the antiretroviral agents have been introduced relatively recently, and long-term follow up is not available. On the other hand, the efficacy of NRTIs in decreasing the rate of mother-to-child transmission may be at least in part attributable to their ability to provide postexposure prophylaxis in the fetal compartment (22). This would be of importance during late pregnancy and delivery, when most of the exposure occurs (10). However, obtaining low viral replication in the mother, as evidenced by plasma HIV RNA levels below the detection limit, appears to be effective, and may be sufficient, to achieve a low rate of transmission (below 1%) (7).
The high concentrations of antiretroviral drugs that we observed in the amniotic fluid may have important clinical implications. The presence of antiretroviral drugs in the digestive tract may be protective against concomitant exposure to infectious HIV by the oral route. Virus has been detected in oropharyngeal or gastric aspirates from approximately one-third of neonates born to HIV type 1-infected mothers receiving zidovudine (11). Furthermore, antiretrovirals in the amniotic fluid at delivery may amount to an oral loading dose, which would continue to be absorbed in the hours following birth. On the other hand, further investigations will be needed to study whether the high amniotic fluid antiretroviral concentrations have an impact on the long-term safety of children.
REFERENCES
- 1.Bawdon, R. E. 1998. The ex vivo human placental transfer of the anti-HIV nucleoside inhibitor abacavir and the protease inhibitor amprenavir. Infect. Dis. Obstet. Gynecol. 6:244-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanche, S., M. Tardieu, P. Rustin, A. Slama, B. Barret, G. Firtion, N. Ciraru-Vigneron, C. Lacroix, C. Rouzioux, L. Mandelbrot, I. Desguerre, A. Rotig, M. J. Mayaux, and J. F. Delfraissy. 1999. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet 354:1084-1089. [DOI] [PubMed] [Google Scholar]
- 3.Casey, B. M., and R. E. Bawdon. 1998. Placental transfer of ritonavir with zidovudine in the ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 179:758-761. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, E. R., M. Charurat, L. Mofenson, I. C. Hansson, J. Pitt, C. Diaz, and K. Hayani. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 29:484-494. [DOI] [PubMed] [Google Scholar]
- 5.Culnane, M., M. G. Fowler, S. S. Lee, G. McSherry, M. Brady, K. O'Donnell, L. Mofenson, S. L. Gortmaker, D. E. Shapiron, G. Scott, E. Jimenez, E. C. Moore, C. Diaz, P. M. Flynn, B. Cunningham, and J. Oleske. 1999. Lack of longterm effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. JAMA 281:151-157. [DOI] [PubMed] [Google Scholar]
- 6.Dancis, J., J. D. Lee, S. Mendoza, and L. Liebes. 1993. Transfer and metabolism of dideoxyinosine by the perfused human placenta. J. Acquir. Immune Defic. Syndr. 6:2-6. [PubMed] [Google Scholar]
- 7.Dorenbaum, A., C. K. Cunningham, R. D. Gelber, M. Culnane, L. Mofenson, P. Britto, C. Rekacewicz, M. L. Newell, J. F. Delfraissy, B. Cunningham-Schrader, M. Mirochnick, J. L. Sullivan, et al. 2002. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 288:189-198. [DOI] [PubMed] [Google Scholar]
- 8.Hanson, I. C., T. A. Antonelli, R. S. Sperling, J. M. Oleske, E. Cooper, M. Culnane, L. A. Fowler Kalish, S. S. Lee, G. McSherry, L. Mofenson, and D. E. Shapiro. 1999. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:463-467. [DOI] [PubMed] [Google Scholar]
- 9.Henderson, G. I., A. B. Perez, Y. Yang, R. L. Hamby, R. S. Schenken, and S. Schenker. 1994. Transfer of dideoxyinosine across the human isolated placenta. Br. J. Clin. Pharmacol. 38:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kourtis, A. P., M. Bulterys, S. R. Nesheim, and F. K. Lee. 2001. Understanding the timing of HIV transmission from mother-to-infant. JAMA 285:709-712. [DOI] [PubMed] [Google Scholar]
- 11.Mandelbrot, L., M. Burgard, J. P. Teglas, J. L. Bénifla, C. Kahn, P. Blot, E. Vilmer, S. Math, G. Firtion, S. Blanche, M. J. Mayaux, and C. Rouzioux. 1999. Frequent detection of HIV-1 in the gastric aspirates of neonates born to HIV-infected mothers. AIDS 13:2143-2149. [DOI] [PubMed] [Google Scholar]
- 12.Mandelbrot, L., G. Peytavin, G. Firtion, and R. Farinotti. 2001. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in HIV infected pregnant women. Am. J. Obstet. Gynecol. 184:153-158. [DOI] [PubMed] [Google Scholar]
- 13.Minkoff, H., and M. Augenbraun. 1997. Antiretroviral therapy for pregnant women. Am. J. Obstet. Gynecol. 176:478-489. [DOI] [PubMed] [Google Scholar]
- 14.Moodley, J., D. Moodley, K. Pillay, H. Coovadia, J. Saba, R. van Leeuwen, C. Goodwin, P. R. Harrigan, K. H. Moore, C. Stone, R. Plumb, and M. A. Johnson. 1998. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J. Infect. Dis. 178:1327-1333. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan, M. J., P. J. Boyer, G. B. Scott, W. P. Parks, S. Weller, M. R. Blum, J. Balsley, Y. J. Bryson, et al. 1993. The pharmacokinetics and safety of zidovudine in the third trimester of pregnancy for women infected with human immunodeficiency virus and their infants: phase I acquired immunodeficiency syndrome clinical trials group study (protocol 082). Am. J. Obstet. Gynecol. 168:1510-1516. [DOI] [PubMed] [Google Scholar]
- 16.Patterson, T. A., Z. K. Binienda, G. W. Lipe, M. P. Gillam, W. Slikker, Jr., and J. A. Sandberg. 1997. Transplacental pharmacokinetics and fetal distribution of azidothymidine, its glucuronide, and phosphorylated metabolites in late-term rhesus macaques after maternal infusion. Drug Metab. Dispos. 25:453-459. [PubMed] [Google Scholar]
- 17.Pereira, C. M., C. Nosbisch, H. R. Winter, W. L. Baughman, and J. D. Unadkat. 1994. Transplacental pharmacokinetics of dideoxyinosine in pigtailed macaques. Antimicrob. Agents Chemother. 38:781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pons, J. C., A. M. Taburet, E. Singlas, J. F. Delfraissy, and E. Papiernik. 1991. Placental passage of zidovudine during the second trimester of pregnancy: study by direct foetal blood sampling under ultrasound. Eur. J. Gynecol. Obstet. Biol. Reprod. 40:229-231. [DOI] [PubMed] [Google Scholar]
- 19.Public Health Service Task Force. 2002. Recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women. Safety and toxicity of individual antiretroviral agents in pregnancy. May 23, 2002. http://www.AIDSinfo.nih.gov.
- 20.Public Health Service Task Force. 2003. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. June 16, 2003. http://AIDSinfo.nih.gov. [PubMed]
- 21.Rodman, J. H., P. Flynn, B. Robbins, E. Jimenez, A. D. Bardeguez, J. F. Rodriguez, S. Blanchard, and A. Fridland. 1999. Systemic pharmacokinetics and cellular pharmacology of zidovudine in HIV-1 infected women and newborn infants. J. Infect. Dis. 180:1844-1850. [DOI] [PubMed] [Google Scholar]
- 22.Sperling, R., D. Shapiro, R. W Coombs, J. A. Todd, S. A. Herman, G. D. McSherry, M. J. O'Sullivan, R. B. Van Dyke, E. Jimenez, C. Rouzioux, P. M. Flynn, and J. L. Sullivan. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N. Engl. J. Med. 335:1621-1629. [DOI] [PubMed] [Google Scholar]
- 23.Tuntland, T., A. Odinecs, C. Nosbisch, and J. D. Unadkat. 1998. In vivo maternal-fetal-amniotic fluid pharmacokinetics of zidovudine in the pigtailed macaque: comparison of steady-state and single-dose regimens. J. Pharmacol. Exp. Ther. 285:54-62. [PubMed] [Google Scholar]
- 24.Tuomala, R. E., D. Shapiro, L. M. Mofenson, Y. Bryson, M. Culnane, M. D. Hugues, M. J. Sullivan, G. Scott, A. M. Stek, D. Wara, and M. Bulterys. 2002. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N. Engl. J. Med. 346:1863-1870. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., E. Livingston, S. Patil, R. E. McKinney, A. D. Bardeguez, J. Gandia, P. Clax O'Sullivan, S. Huang, and J. D. Unadkat. 1999. Pharmacokinetics of didanosine in antepartum and postpartum human immunodeficiency virus-infected pregnant women and their neonates: an AIDS Clinical Trials Group study. J. Infect. Dis. 180:1536-1541. [DOI] [PubMed] [Google Scholar]
- 26.Watts, D. H., Z. A. Brown, T. Tartaglione, S. K. Burchett, K. Opheim, R. Coombs, and L. Corey. 1991. Pharmacokinetic disposition of Zidovudine during pregnancy. J. Infect. Dis. 163:226-232. [DOI] [PubMed] [Google Scholar]