Abstract
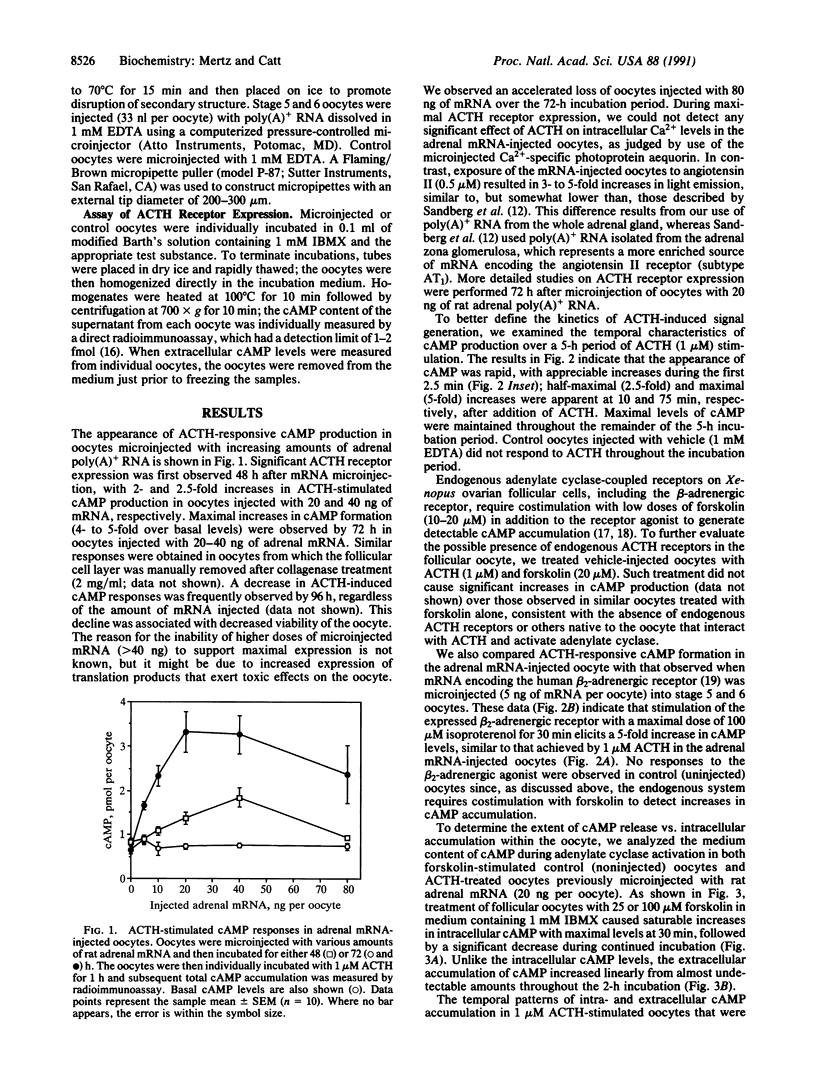
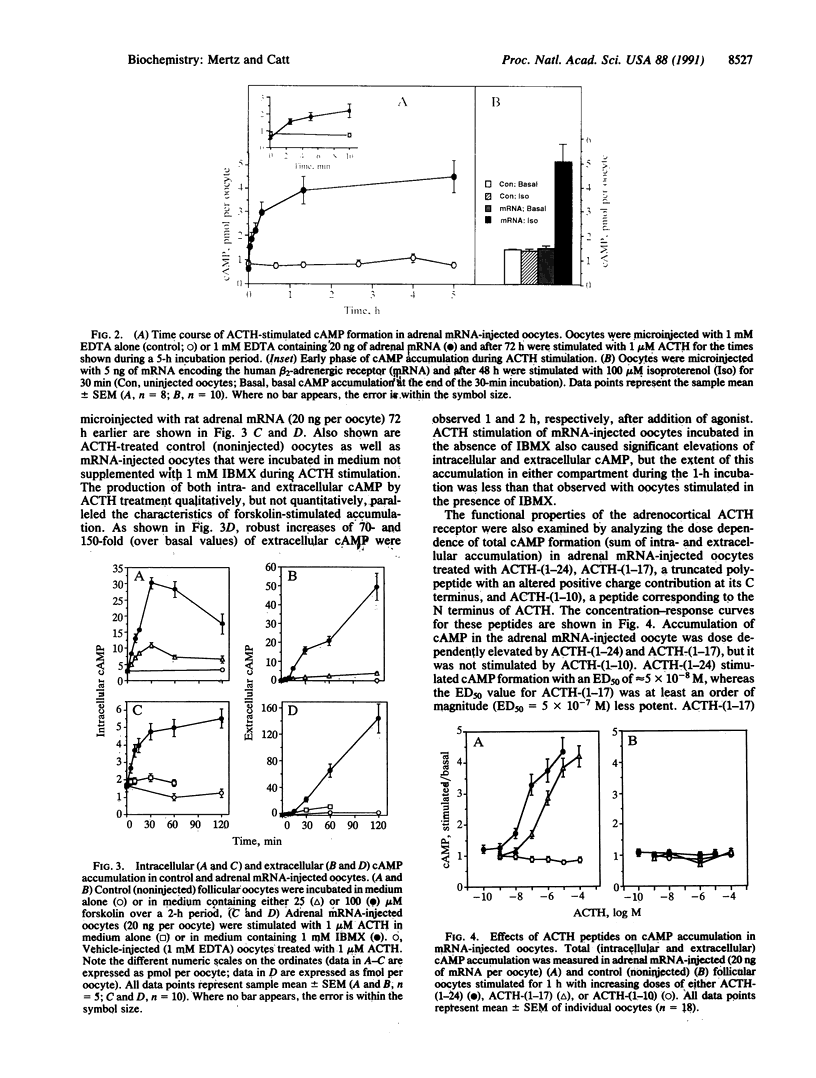
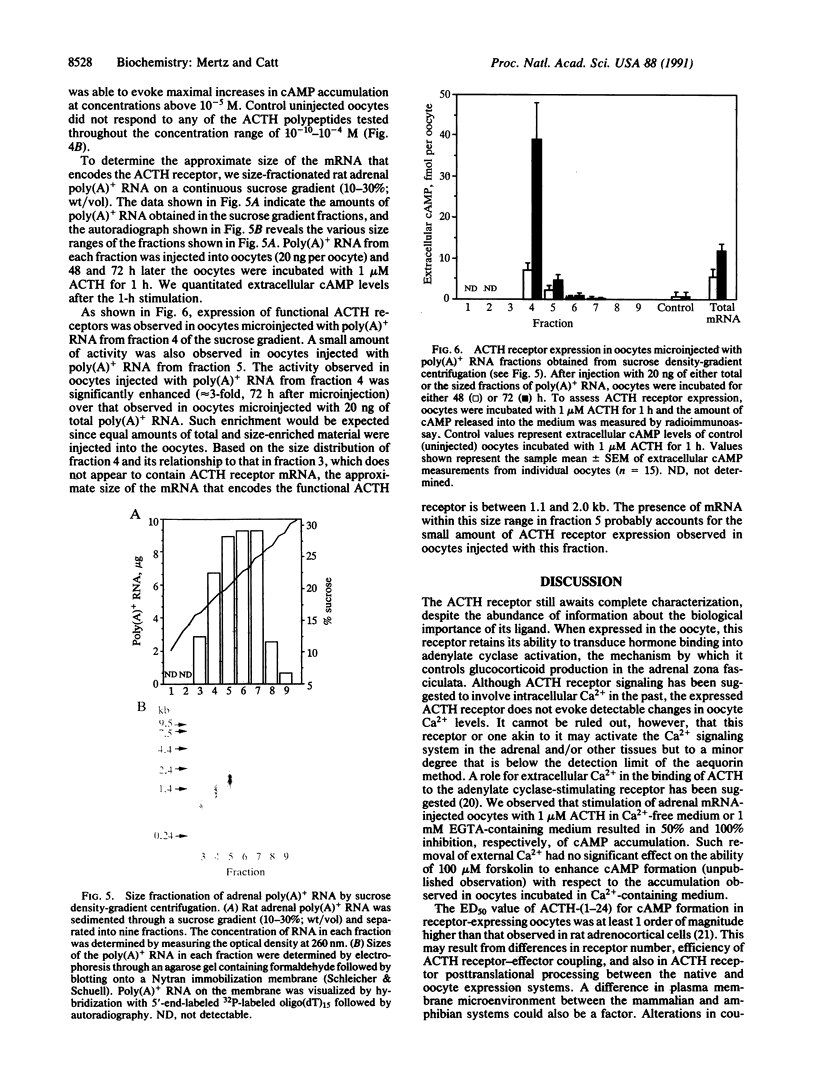
The adrenocorticotropin (ACTH) receptor, which binds corticotropin and stimulates adenylate cyclase and steroidogenesis in adrenocortical cells, was expressed in Xenopus laevis oocytes microinjected with rat adrenal poly(A)+ RNA. Expression of the ACTH receptor in individual stage 5 and 6 oocytes was monitored by radioimmunoassay of ligand-stimulated cAMP production. Injection of 5-40 ng of adrenal mRNA caused dose-dependent increases in ACTH-responsive cAMP production. These were detected at 48 h and reached a maximum 72 h after microinjection of 20-40 ng of adrenal mRNA. In response to 1 microM ACTH, total cAMP production increased within 2.5 min and reached half-maximal and maximal levels (5-fold greater than basal) at 10 and 75 min, respectively, and then remained elevated for up to 5 h. Extracellular cAMP levels were much lower but showed prominent linear increases from almost undetectable levels, with 70- and 150-fold increases evident at 1 and 2 h, respectively. The half-maximal concentration (ED50) for stimulation of cAMP formation was 5 x 10(-8) M ACTH-(1-24); the ED50 for ACTH-(1-17) was 5 x 10(-7) M, and no response was observed with ACTH-(1-10). Size fractionation of rat adrenal poly(A)+ RNA by sucrose density-gradient centrifugation revealed that mRNA encoding the ACTH receptor was present in the 1.1- to 2.0-kilobase fraction. These data indicate that ACTH receptors can be expressed from adrenal mRNA in Xenopus oocytes and are fully functional in terms of ligand specificity and signal generation. The extracellular cAMP response to ACTH is a sensitive and convenient index of receptor expression. This system should permit more complete characterization and expression cloning of the ACTH receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley D. I., Ramachandran J. Characterization of corticotropin receptors on adrenocortical cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7431–7435. doi: 10.1073/pnas.78.12.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheitlin R., Buckley D. I., Ramachandran J. The role of extracellular calcium in corticotropin-stimulated steroidogenesis. J Biol Chem. 1985 May 10;260(9):5323–5327. [PubMed] [Google Scholar]
- Clarke B. L., Bost K. L. Differential expression of functional adrenocorticotropic hormone receptors by subpopulations of lymphocytes. J Immunol. 1989 Jul 15;143(2):464–469. [PubMed] [Google Scholar]
- Garren L. D., Ney R. L., Davis W. W. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Greenfield L. J., Jr, Hackett J. T., Linden J. Xenopus oocyte K+ current. II. Adenylyl cyclase-linked receptors on follicle cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C784–C791. doi: 10.1152/ajpcell.1990.259.5.C784. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Stehle C. J., Finn F. M. Identification of a protein in adrenal particulates that binds adrenocorticotropin specifically and with high affinity. Endocrinology. 1988 Sep;123(3):1355–1363. doi: 10.1210/endo-123-3-1355. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Champigny C., Rabbani S. A., Hendy G. N., Goltzman D. Expression of adenylate cyclase-coupled osseous parathyroid hormone and parathyroid hormone-like peptide receptors in Xenopus oocytes. J Biol Chem. 1991 Mar 15;266(8):4700–4705. [PubMed] [Google Scholar]
- Kobilka B. K., MacGregor C., Daniel K., Kobilka T. S., Caron M. G., Lefkowitz R. J. Functional activity and regulation of human beta 2-adrenergic receptors expressed in Xenopus oocytes. J Biol Chem. 1987 Nov 15;262(32):15796–15802. [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pastan I. ACTH-receptor interaction in the adrenal: a model for the initial step in the action of hormones that stimulate adenyl cyclase. Ann N Y Acad Sci. 1971 Dec 30;185:195–209. doi: 10.1111/j.1749-6632.1971.tb45249.x. [DOI] [PubMed] [Google Scholar]
- Londos C., Rodbell M. Multiple inhibitory and activating effects of nucleotides and magnesium on adrenal adenylate cyclase. J Biol Chem. 1975 May 10;250(9):3459–3465. [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Schulster D. Studies on the binding of 125I-labelled corticotrophin to isolated rat adrenocortical cells. J Endocrinol. 1975 Jan;64(1):175–184. doi: 10.1677/joe.0.0640175. [DOI] [PubMed] [Google Scholar]
- McIntosh R. P., Catt K. J. Coupling of inositol phospholipid hydrolysis to peptide hormone receptors expressed from adrenal and pituitary mRNA in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9045–9048. doi: 10.1073/pnas.84.24.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz L. M., Pedersen R. C. The kinetics of steroidogenesis activator polypeptide in the rat adrenal cortex. Effects of adrenocorticotropin, cyclic adenosine 3':5'-monophosphate, cycloheximide, and circadian rhythm. J Biol Chem. 1989 Sep 15;264(26):15274–15279. [PubMed] [Google Scholar]
- Mizuno T., Okada M., Itoh A., Maruyama T., Hagiwara H., Hirose S. Affinity labeling of ACTH receptors in bovine adrenal cortex membranes. Biochem Int. 1989 Oct;19(4):695–700. [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Steroidogenesis-activator polypeptide isolated from a rat Leydig cell tumor. Science. 1987 Apr 10;236(4798):188–190. doi: 10.1126/science.3563495. [DOI] [PubMed] [Google Scholar]
- Penhoat A., Jaillard C., Saez J. M. Corticotropin positively regulates its own receptors and cAMP response in cultured bovine adrenal cells. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4978–4981. doi: 10.1073/pnas.86.13.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. A., Gutmann N. S., Tsao J., Schimmer B. P. Mutations in cyclic AMP-dependent protein kinase and corticotropin (ACTH)-sensitive adenylate cyclase affect adrenal steroidogenesis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1896–1900. doi: 10.1073/pnas.76.4.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran J., Muramoto K., Kenez-Keri M., Keri G., Buckley D. I. Photoaffinity labeling of corticotropin receptors. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3967–3970. doi: 10.1073/pnas.77.7.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala G. B., Hayashi K., Catt K. J., Dufau M. L. Adrenocorticotropin action in isolated adrenal cells. The intermediate role of cyclic AMP in stimulation of corticosterone synthesis. J Biol Chem. 1979 May 25;254(10):3861–3865. [PubMed] [Google Scholar]
- Sandberg K., Markwick A. J., Trinh D. P., Catt K. J. Calcium mobilization by angiotensin II and neurotransmitter receptors expressed in Xenopus laevis oocytes. FEBS Lett. 1988 Dec 5;241(1-2):177–180. doi: 10.1016/0014-5793(88)81055-x. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Waterman M. R. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- Smith A. A., Brooker T., Brooker G. Expression of rat mRNA coding for hormone-stimulated adenylate cyclase in Xenopus oocytes. FASEB J. 1987 Nov;1(5):380–387. doi: 10.1096/fasebj.1.5.2824269. [DOI] [PubMed] [Google Scholar]
- Spindel E. R., Giladi E., Brehm P., Goodman R. H., Segerson T. P. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol Endocrinol. 1990 Dec;4(12):1956–1963. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]





