Abstract
Time-kill and postantifungal effect (PAFE) of amphotericin B, caspofungin, fluconazole, and voriconazole were determined against clinical isolates of Candida guilliermondii, Candida kefyr, and Candida lusitaniae. Azoles displayed fungistatic activity and no measurable PAFE, regardless of the concentration tested. Amphotericin B and caspofungin demonstrated concentration-dependent fungicidal activity, although amphotericin B only produced a significant dose-dependent PAFE against all isolates tested.
Invasive fungal infections are important causes of morbidity and mortality in immunosuppressed patients (10). Although C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis account for the majority of Candida bloodstream infections, recent epidemiologic trends indicate a shift toward infections by the less frequently isolated non-albicans Candida (NAC) species (12). Among NAC species, C. kefyr, C. guilliermondii, and C. lusitaniae are rare causes of invasive infections but are increasingly encountered among severely immunosuppressed patients occurring in nosocomial clusters and/or exhibiting innate or acquired resistance to one or more established antifungal agents, often related to intravascular catheters and breaks in infection control precautions (3-5, 11, 12, 14).
Currently, knowledge of the in vitro pharmacodynamic characteristics of C. kefyr, C. guilliermondii, and C. lusitaniae is poor and limited to amphotericin B (AMB) and voriconazole (VRC) only (7, 15). Therefore, we conducted time-kill and postantifungal effect (PAFE) studies with AMB, caspofungin, fluconazole (FLC), and VRC against bloodstream isolates of C. guilliermondii, C. kefyr, and C. lusitaniae from neutropenic patients.
Antifungal agents.
Stock solutions of AMB (Sigma-Aldrich SRL, Milan, Italy), caspofungin (Merck Sharp & Dohme Italia SpA, Rome, Italy), FLC (Pfizer Inc., New York, N.Y.), and VRC (Pfizer) were prepared in RPMI 1640 medium (Sigma) buffered to a pH of 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma) and stored at −80°C until use. Antifungals were solubilized in sterile water, except AMB in dimethyl sulfoxide (Sigma).
Test isolates.
Six Candida isolates were obtained from the Clinical Microbiology Service, Department of Hematology and Oncology, “Spirito Santo” Hospital, Pescara, Italy, for use in this study: two strains each of C. guilliermondii (337 and 555), C. kefyr (240 and 270), and C. lusitaniae (325 and 447) were selected for testing.
Antifungal susceptibility testing.
The MIC for each isolate was determined, in triplicate, by broth microdilution techniques as outlined by the National Committee for Clinical Laboratory Standards (17). The endpoint was defined as 50% inhibition of visible growth for azoles and complete inhibition of visible growth for AMB and caspofungin.
Time-kill.
Before the time-kill studies were initiated, antifungal carryover effects were examined. Briefly, 100 μl of a standardized suspension (1 × 103 CFU/ml) of each isolate were added to either 900 μl of sterile water with (sample) or without (control) antifungal at 4 and 8 times the MIC. Immediately following the addition of the antifungal, 100 μl was removed and streaked across Sabouraud dextrose agar (SDA) for colony count determination. Antifungal carryover was defined as a reduction in colony counts in samples by >25% compared to control.
Time-kill experiments, conducted in duplicate, began by suspending colonies from a 24-h SDA growth in RPMI 1640 with MOPS (RPMI 1640-MOPS) to approximately 106 CFU/ml. One milliliter of this fungal suspension was then added to 9 ml of RPMI 1640-MOPS without (growth control) or with (test) antifungal at concentrations ranging from 0.125 to 8 times the MIC. The culture was then incubated at 37°C with shaking. At predetermined time points (0, 1, 2, 4, 8, 24, and 48 h), 100 μl samples were removed and serially diluted in cold sterile distilled water, and 100 μl was plated onto SDA for colony counting.
PAFE.
Growth control and test vials were prepared as for time-kill experiments. Following an incubation period of 1 h, antifungal was removed by three sequential centrifugations (3,000 × g, 10 min). The fungal pellet was resuspended in normal saline after the first two centrifugation periods and in 9 ml of warm RPMI 1640-MOPS after the final centrifugation. The resuspended samples were then incubated at 35°C with agitation, and colony counts were performed at 0, 2, 4, 6, 8, and 24 h after the final wash. PAFE experiments were conducted in duplicate.
Analysis.
Fungicidal activity was defined as a ≥3 log10 (99.9%) reduction in CFU per milliliter from the starting inoculum concentration. To quantify the extent of antifungal activity, maximal effect (Emax), the concentration producing 50% of Emax (EC50), the EC90, and the range of the net change (log10 CFU per milliliter) in fungal density were determined for each time point by a sigmoidal Hill three-parameter model with GraphPad Prism (version 4; GraphPad Software Inc., San Diego, Calif.). PAFE was calculated by taking the difference in time required for control and test isolates to grow 1 log10 following drug removal.
Antifungal susceptibility results.
Median MICs ranged from 0.5 to 4.0 μg/ml for AMB, 0.25 to 128 μg/ml for caspofungin, 0.12 to 2.0 μg/ml for FLC, and 0.12 to 1.0 μg/ml for VRC (Table 1).
TABLE 1.
Microdilution broth results for study isolates
| Test isolate | Median MIC (μg/ml) (n = 6)
|
|||
|---|---|---|---|---|
| AMB | Caspofungin | FLC | VRC | |
| C. guilliermondii 337 | 2 | 128 | 2 | 1 |
| C. guilliermondii 555 | 0.5 | 128 | 2 | 1 |
| C. kefyr 240 | 4 | 0.25 | 0.12 | 0.12 |
| C. kefyr 270 | 4 | 0.25 | 0.25 | 0.25 |
| C. lusitaniae 325 | 4 | 0.5 | 2 | 0.5 |
| C. lusitaniae 447 | 4 | 0.5 | 2 | 1 |
Time-kill results.
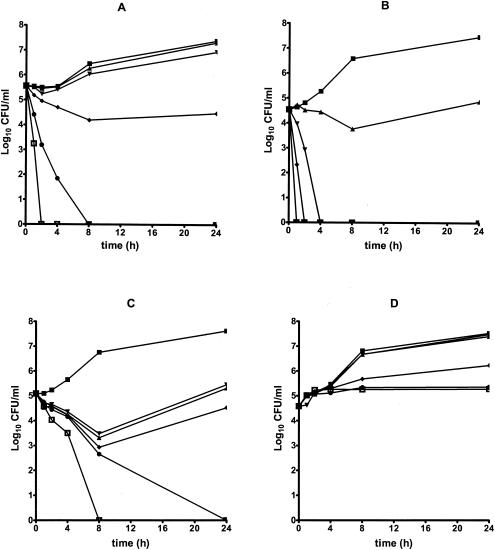
No antifungal carryover has been observed with any of the isolates at the concentrations tested. Time-kill plots representative of those noted in this study are showed in Fig. 1. AMB yielded fungicidal activity at concentrations ≥4 times the MIC against C. guilliermondii isolates, reaching fungicidal activity between 1.1 and 3.1 h. AMB exhibited fungicidal activity beginning at the MIC against both C. kefyr isolates (fungicidal endpoint range, 0.5 to 3.9 h). However, regrowth was observed for strain 240 after 8 h at 1 and 4 times the MIC. AMB showed fungicidal activity against both C. lusitaniae isolates beginning at 0.25 times the MIC (fungicidal endpoint range, 0.6 to 6.2 h). Caspofungin exhibited fungicidal activity against both C. guilliermondii isolates tested at ≥4 times the MIC (fungicidal endpoint range, 0.5 to 13 h). A comparable trend was observed for C. kefyr isolates (fungicidal endpoint range, 2.5 to 11 h), although regrowth was observed for both C. kefyr isolates after 8-h exposition at 0.125, 0.25,r and 1 times the MIC. Caspofungin tested against C. lusitaniae produced fungistatic activity only, except at 8 times the MIC against C. lusitaniae 447 reaching the fungicidal endpoint after 15.6 h. FLC and VRC showed fungistatic activity against all test isolates, regardless of the concentrations tested. Composite ECmax model parameters are summarized in Table 2. The EC90 for both AMB and caspofungin decreased to about the MIC at 8 h and then increased to about 2 times the MIC at 24 h. ECmax increased over time up to 24 h for both antifungals, although AMB yielded fungicidal activity more rapidly than caspofungin did (2 versus 8 h, respectively). The EC90 for both FLC and VRC was approximately 2 times the MIC up to 24 h.
FIG. 1.
Representative time-kill plots for the following: AMB against C. guilliermondii 337 (A) and C. lusitaniae 447 (B); caspofungin against C. kefyr 270 (C); and VRC against C. lusitaniae 325 (D). Antifungals were tested at the following concentrations: 0.125 times the MIC (▴), 0.25 times the MIC (▾), the MIC (⧫), 4 times the MIC (•), and 8 times the MIC (□). ▪, control.
TABLE 2.
Composite Emax model parameters
| Antifungal and time point (h) | EC50 (multiple of MIC) | EC90 (multiple of MIC) | ECmax (log10 CFU/ ml) | Net inoculum change (log10 CFU/ml) |
|---|---|---|---|---|
| AMB | ||||
| 1 | 0.99 | 1.97 | −2.96 | −5.28 to 0.25 |
| 2 | 0.80 | 1.68 | −3.65 | −5.56 to 0.17 |
| 4 | 0.64 | 1.78 | −4.42 | −5.56 to 0.36 |
| 8 | 0.37 | 1.18 | −4.63 | −5.56 to 1.00 |
| 24 | 0.70 | 1.94 | −4.55 | −5.56 to 2.37 |
| Caspofungin | ||||
| 1 | 2.02 | 2.97 | −1.45 | −5.47 to −0.14 |
| 2 | 1.40 | 2.34 | −1.91 | −5.47 to 0.13 |
| 4 | 1.39 | 2.32 | −2.44 | −5.47 to −0.50 |
| 8 | 0.48 | 1.20 | −3.06 | −5.47 to −0.33 |
| 24 | 1.05 | 1.94 | −3.62 | −5.47 to 0.60 |
| FLC | ||||
| 4 | 1.34 | 2.28 | 0.33 | −0.04 to 1.02 |
| 8 | 1.18 | 2.10 | 0.41 | 0.03 to 2.08 |
| 24 | 1.26 | 2.18 | 0.59 | 0.06 to 2.50 |
| VRC | ||||
| 4 | 0.99 | 1.99 | 0.31 | −0.20 to 1.03 |
| 8 | 0.74 | 1.60 | 0.30 | −0.34 to 2.09 |
| 24 | 0.88 | 1.77 | 0.47 | −0.09 to 2.89 |
PAFE results.
Yeasts exposed to FLC and VRC did not produce any measurable PAFE regardless of antifungal concentrations. Caspofungin induced measurable PAFEs for C. lusitaniae 325 only, regardless of concentration tested. In contrast, significant PAFEs were induced by AMB against each of the test isolates. PAFE was generally influenced by concentration in a dose-dependent manner ranging from 1.3 to 9.4 h (mean, 5.5 h), 3.6 to 10 h (mean, 7.8 h), and 9.2 to 14.9 h (mean, 11.2 h) at 0.125, 0.25, and 1 times the MIC, respectively. At 4 and 8 times the MIC, AMB yielded a PAFE of about 13 h for C. lusitaniae 325 only.
Discussion.
According to previous findings (7, 15), AMB was fungicidal against all isolates tested, showing the greatest activity against C. lusitaniae isolates despite the fact that C. lusitaniae is known for rapid development of AMB resistance (4). Caspofungin displayed fungicidal activity against all isolates but C. lusitaniae 325, suggesting that its fungicidal activity is isolate and species specific. Composite ECmax model parameters suggested that the rate of killing of caspofungin and AMB was influenced by increases in the drug concentration. In fact, by comparing the relative EC90s over time, we observed decreased values until 8 h, hence increased up to 24 h, suggesting that higher concentrations result in more-rapid expression of activity. AMB proved to be more potent than caspofungin, as suggested by EC50 data (mean EC50, 0.8 versus 1.3, respectively) against all isolates tested up to 24 h after antifungal exposure. Further, the ECmax of caspofungin and AMB did increase over time, suggesting that maximal activity occurs at the latest time point observed. However, AMB reached the fungicidal endpoint more rapidly than caspofungin did (2 versus 8 h, respectively). The azoles produced fungistatic effects regardless of concentration, supporting previously published data for C. albicans and other NAC species (7, 13). EC50s and EC90s calculated for FLC and VRC suggested that fungistatic activity did not improve with increasing concentrations of the azoles. The increase observed in the EC50s and EC90s of antifungals tested at 24 h may represent an amount of regrowth seen at fungistatic concentrations, too small to be appreciated in time-kill plots but enough to modify the EC50 and EC90.
The information obtained in this study could be further applied to optimize future dosing regimens for this agent. MIC90s of AMB, caspofungin, FLC, and VRC for the NAC species we tested have been previously reported to range from 0.5 to 8.0, 1 to 16, 0.5 to 16, and 0.06 to 0.5 μg/ml, respectively (1, 8, 9, 16, 18-22). Considering mean EC90s calculated from our Emax model (1.4 times the MIC for AMB and 2 times the MIC for caspofungin and both azoles), we predict that close-to-maximal effect (EC90) would be observed if in vivo AMB, caspofungin, FLC, and VRC concentrations of 1 to 16, 1.4 to 22.4, 1 to 32, and 0.12 to 1 μg/ml were achieved, respectively. These values exceed the peak plasma FLC and VRC concentrations (40 and 4 μg/ml, respectively) achieved in human serum following clinically utilized doses (23, 24), suggesting that concentration-independent fungistatic activity is likely to predominate at clinically achieved FLC and VRC concentrations. On the contrary, peak plasma AMB and caspofungin concentrations (2 and 1 μg/ml, respectively) (24) suggest that current dosing strategies for these antifungals should be reevaluated in order to produce higher peak serum levels.
This is the first published study on the PAFE produced by antifungal agents against C. guilliermondii, C. kefyr, and C. lusitaniae. Although no PAFE was induced by the azoles in any of the strains tested, the method we used for removing drug gets the azole concentrations below the sub-MIC levels that have been previously reported by Turnidge et al. (25) to result in a clinically relevant slower growth of the yeasts. AMB produced a significant dose-dependent PAFE for all isolates tested, as has been previously described for other Candida spp. (2, 25). Caspofungin, although it exhibited fungicidal activity as well as AMB, did not induce a measurable PAFE, in contrast with findings of Ernst et al. (6) concerning C. albicans and Cryptococcus neoformans.
In conclusion, our results demonstrate that AMB may be useful for the treatment of infections caused by C. kefyr, C. guilliermondii, and C. lusitaniae. Animal and clinical studies are warranted to define the clinical relevance of our data.
Acknowledgments
This work was supported by a grant from the Ministero della Salute e della Ricerca (MIUR; Italy), grant 1% Sanità-2003. I.S. is the recipient of a grant from the Dipartimento di Scienze Biomediche, Università “G. D'Annunzio,” Chieti, Italy.
We thank Francesca Faricelli for technical assistance.
REFERENCES
- 1.Cheng, M. F., K. W. Yu, R. B. Tang, Y. H. Fan, Y. L. Yang, K. S. Hsieh, M. Ho, and H. J. Lo. 2004. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn. Microbiol. Infect. Dis. 48:33-37. [DOI] [PubMed] [Google Scholar]
- 2.Chryssanthou, E., O. Cars, and J. Sjölin. 2002. New automated method for determining postantifungal effect of amphotericin B against Candida species: effects of concentration, exposure time, and area under the curve. Antimicrob. Agents Chemother. 46:4016-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy, C., M. Nguyen, and V. Yu. 1996. Fungemia caused by Candida lusitaniae. Infect. Med. 13:940, 948-951. [Google Scholar]
- 4.Collin, B., C. J. Clancy, and M. H. Nguyen. 1999. Antifungal resistance in non-albicans Candida species. Drug Resist. Updat. 2:9-14. [DOI] [PubMed] [Google Scholar]
- 5.Dick, J. D., R. R. Rosengard, W. G. Merz, R. K. Stuart, G. M. Hutchins, and R. Saval. 1985. Fatal disseminated candidiasis due to amphotericin B-resistant Candida guilliermondii. Ann. Intern. Med. 102:67-68. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, E. J., K. Yodoi, and M. E. Klepser. 2002. Rates and extent of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 46:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, N. Holliday, and S. B. Killian. 2004. Multicenter comparison of Sensitre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 42:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favel, A., A. Michel-Nguyen, A. Datry, S. Challier, F. Leclerc, C. Chastin, K. Fallague, and P. Regli. 2004. Susceptibility of clinical isolates of Candida lusitaniae to five systemic antifungal agents. J. Antimicrob. Chemother. 53:526-529. (Advance Access published 12 February 2004.) [DOI] [PubMed] [Google Scholar]
- 10.Groll, A. H., and T. J. Walsh. 2002. Invasive fungal infections in the neutropenic cancer patient: current approaches and future strategies. Infect. Med. 19:326-334. [Google Scholar]
- 11.Hadfield, T. L., M. B. Smith, R. E. Winn, M. G. Rinaldi, and C. Guerra. 1987. Mycoses caused by Candida lusitaniae. Rev. Infect. Dis. 9:1006-1012. [DOI] [PubMed] [Google Scholar]
- 12.Hazen, K. C. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 8:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krcmery, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 15.Manavathu, E. K., J. L. Cutright, and P. H. Chandrasekar. 1998. Organism-dependent fungicidal activities of azoles. Antimicrob. Agents Chemother. 42:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell, M. J., S. A. Messer, R. J. Hollis, L. Boyken, S. Tendolkar, D. J. Diekema, M. A. Pfaller, and the International Fungal Surveillance Participant Group. 2003. Evaluation of Etest method for determining fluconazole and voriconazole MICs for 279 clinical isolates of Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:1087-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, R. N. Jones, and the International Fungal Surveillance Participant Group. 2003. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2003. Caspofungin activity against clinical isolates of fluconazole-resistant Candida. J. Clin. Microbiol. 41:5729-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp. including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, and R. N. Jones. 2004. In vitro susceptibilities of rare Candida bloodstream isolates to ravuconazole and three comparative antifungal agents. Diagn. Microbiol. Infect. Dis. 48:101-105. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz, S., D. Milatovic, and E. Thiel. 1997. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br. J. Hematol. 97:663-665. [DOI] [PubMed] [Google Scholar]
- 24.Summers, K. K., T. C. Hardin, S. J. Gore, and J. R. Graybill. 1997. Therapeutic drug monitoring of systemic antifungal therapy. J. Antimicrob. Chemother. 40:753-764. [DOI] [PubMed] [Google Scholar]
- 25.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]