Abstract
This report presents evidence that dogs recover from acute canine monocytic ehrlichiosis (CME) after 16 days of doxycycline treatment (10 mg/kg of body weight every 24 h). Blood PCR was as valuable as splenic aspirate PCR for early diagnosis of acute CME. Splenic aspirate PCR was, however, superior to blood PCR for the evaluation of ehrlichial elimination.
Canine monocytic ehrlichiosis (CME) is a tick-borne disease with a global distribution (9). Diagnosis of CME is confirmed by demonstration of morulae in blood smears, serology, culturing of the rickettsiae, and PCR using Ehrlichia canis-specific primers. Tetracyclines are commonly used in the treatment of CME, with doxycycline in particular being the most acceptable and widely used (2, 3).
Blood samples are presently used for PCR evaluation of ehrlichial infection and response to therapy (15). A previous study has demonstrated ehrlichial DNA in splenic aspirates of two subclinically experimentally infected dogs whose blood and bone marrow aspirates were found to be negative for E. canis DNA, suggesting the importance of splenic aspirates in the diagnosis of ehrlichial carriers (6). This finding questioned the validity of previous blood-based PCR studies for evaluating treatment regimens for CME, and the length of doxycycline treatment required for E. canis infection remained uncertain.
The aims of this study were (a) to investigate whether splenic aspirates are superior to blood samples as a sample source for E. canis PCR; (b) to determine whether dogs with acute CME remain persistently infected despite concurrent treatment; and (c) to determine the duration of doxycycline treatment required to eliminate E. canis DNA, as measured by PCR in cases of acute CME.
Five beagle dogs, 4 to 6 years old and negative serologically and by PCR for E. canis, were used in this study. The dogs were artificially infected by intravenous injection of 5 ml of E. canis-infected blood. This blood was drawn from an acutely ill, naturally infected dog. Infection of the donor dog with E. canis was confirmed by serology using the indirect immunofluorescence antibody (IFA) test (11), by blood culture of DH82 cells (4), and by p30-based nested PCR for E. canis (14).
On day 12 postinfection (PI), by which date all of the dogs had become severely ill, the dogs were treated with doxycycline hydrochloride (Doxylin; Dexxon, Or-Akiva, Israel) (10 mg/kg of body weight every 24 h for 60 consecutive days). Blood and ultrasound-guided splenic aspirates from all five dogs for complete blood counts, serology, and PCR were drawn at 14 days preinfection, on the day of inoculation (day 0), on days 7, 10, and 12 PI, and on days 9, 16, 23, 30, 45, and 60 post-treatment initiation (PTx). The study was approved by the Institutional Animal Care and Use Committee of the Agriculture Faculty of the Hebrew University of Jerusalem and complied with all governmental and institutional policies.
The IFA test was performed as described previously (11). For PCR, blood and splenic aspirates were collected in plastic tubes containing EDTA and stored at −80°C until analyzed. DNA was extracted from 200 μl of blood and 50 μl of splenic aspirates by use of a QIAamp DNA Mini kit (QIAGEN, Valencia, Calif.) according to the manufacturer's recommended protocols. The p30-based nested PCR for E. canis for all samples and controls was performed in duplicate as described previously (14). E. canis DNA extracted from cultured E. canis and blood of naturally infected dogs confirmed as having CME by sequencing of 16S rRNA amplicons were used as positive controls. Water and DNA extracted from specific-pathogen-free dog blood were used as negative controls. The presence of E. canis amplicons was demonstrated by agarose gel electrophoresis.
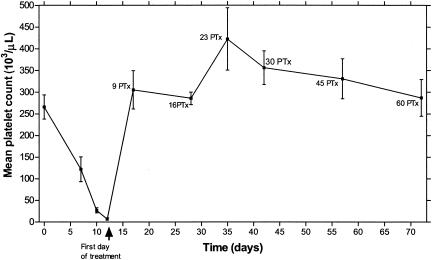
All dogs developed clinical and hematological signs consistent with E. canis infection, including fever (>39.5°C), anorexia, depression, splenomegaly, and thrombocytopenia (<200,000/μl) (Fig. 1). All five dogs became severely ill by days 9 to 12 PI, and treatment with doxycycline was initiated on day 12 PI. All dogs recovered clinically within 72 h PTx. All five dogs seroconverted, and E. canis immunoglobulin G (IgG) antibody titers greater than 1:80 were detected for all dogs on day 12 PI. The dogs remained seropositive during the course of the study. Table 1 presents the blood and splenic PCR results.
FIG. 1.
Mean and standard error of platelet counts of 5 dogs experimentally infected with E. canis. Day 0 is defined as the inoculation day. All dogs were treated with doxycycline (10 mg/kg of body weight every 24 h for 60 days) starting on day 12 postinfection.
TABLE 1.
Blood and spleen PCR results from five dogs artificially inoculated with E. canisa
| Sample source | PCR result(s) (dog[s])
|
|||||
|---|---|---|---|---|---|---|
| Day 14 PreI-0 | Day 7 PI | Day 10 PI | Day 12 PI/TxD1 | Day 9 PTx | Day 16-60 PTx | |
| Blood | N (1-5) | P (2); N (1, 3-5) | P (1-5) | P (1-5) | N (1-5) | N (1-5) |
| Spleen | N (1-5) | P (2); N (1, 3-5) | P (1-5) | P (1-5) | P (1, 4, 5); N (2, 3) | N (1-5) |
All dogs were treated with doxycycline (10mg/kg of body weight every 24 h for 60 days) starting on day 12 postinfection. PreI, preinfection; PI, postinfection; TxD1, treatment day 1; PTx, post-treatment initiation; N, negative; P, positive.
This study evaluated experimental E. canis infection and recovery and for the first time used simultaneous blood and splenic aspirates for E. canis PCR during the entire course of the study. First detection of ehrlichial DNA occurred on days 7 to 10 PI for all five dogs used in this study. In a previous study, E. canis DNA was detected as early as day 4 after experimental infection with 107 E. canis-infected DH82 cells by use of a less sensitive PCR assay than the one used in the present study (15). The earlier detection of ehrlichial DNA in the former study was probably associated with a larger inoculum size. In the present study, E. canis DNA was first detected on the same day in both blood and spleen samples of each dog, indicating that splenic aspirates were not superior to blood samples for early detection of ehrlichial DNA by PCR. However, ehrlichial DNA could be detected in the spleen after its elimination from the blood for three of the five dogs, indicating that splenic aspirates are superior to blood in the evaluation of response to therapy.
The results of this study support previous results obtained with experimentally infected dogs. In that study, four subclinically infected dogs were found to be carriers 34 months PI by use of splenic sample PCR at a time when blood or bone marrow sample PCR results were negative for two of the four dogs (6). In another study, the spleen of two rhesus macaques, inoculated with the agent of human granulocytic ehrlichiosis (recently reclassified as Anaplasma phagocytophilum), was found to be the last organ to retain the rickettsial DNA (5). The results of those studies and the present study add to the evidence that the spleen harbors ehrlichial DNA for a longer duration than blood. They also support the idea of splenic aspirates as the preferred sample for monitoring residual ehrlichial DNA during therapy. Future studies should investigate whether this phenomenon occurs with other members of the Rickettsiales and/or with other intracellular organisms.
The prolonged detection of E. canis DNA in the spleen during treatment may reflect the presence of a higher number of ehrlichial organisms within the spleen than in blood, and the possibility of false-negative results in blood samples, due to test limitations, cannot be ruled out. However, in light of the high sensitivity of the assay used in the present study, a longer persistence of live organisms in splenic macrophages than in blood monocytes seems more likely. Another possible explanation may be the presence of degraded DNA within splenic macrophages rather than intact live organisms. However, as no culture attempts were performed on splenic aspirates this question remains unresolved until further investigation. In our opinion, in a clinical situation a conservative approach should be employed, and any detection of ehrlichial DNA should be considered a positive result with respect to infection.
Previous reports suggested that dogs remain persistently infected for long periods of time and possibly for their entire lives despite antibiotic treatment (1, 13). Our results indicate that appropriate doxycycline treatment results in full clinical and hematological recovery from the acute stage and in elimination of ehrlichial DNA as measured by PCR. We cannot exclude the possibility that naturally infected dogs or chronically infected dogs may require a longer course of antibiotic treatment due to variable infectious loads, as suggested by a previous study where one of four subclinically and experimentally infected dogs, a persistent carrier for 34 months, remained infected following 6 weeks of treatment with doxycycline (7).
The most currently used therapeutic protocol for CME is doxycycline (10 mg/kg of body weight daily) for 28 days, as recommended by the Ehrlichia Consensus Statement from the Infectious Disease Study Group of the American College of Veterinary Internal Medicine (12). A report from an earlier study showed that doxycycline (10 mg/kg daily) for 7 days was ineffective at eliminating experimentally induced E. canis infection from the blood and tissues of three of five dogs (10). In another study, doxycycline (5.6 to 6 mg/kg twice daily) used for 14 days was effective in eliminating experimentally induced E. canis from the blood of eight acutely infected dogs (2). In the present study, no ehrlichial DNA could be detected in any of the blood or spleen samples from days 16 PTx onwards, suggesting the successful elimination of the rickettsia. The high sensitivity of the PCR assay used further supports our assumption. Our results suggest that the duration of doxycycline treatment for dogs suffering from acute CME should be reduced to 16 days. Shorter treatment will reduce costs, the probability of side effects, and the risk of antibiotic resistance.
Platelet counts of beagle dogs experimentally infected with E. canis have been shown to remain low for weeks and months when the dogs are left untreated (6, 8, 16). After initiation of doxycycline treatment, platelet counts returned to the normal reference range (200,000 to 500,000/μl) within 9 to 16 days and increased to levels greater than the preinfection levels until day 23 PTx, suggesting a rebound effect. Thereafter, the platelet counts decreased towards the preinfection levels (Fig. 1). The return of posttreatment platelet counts to within the normal reference range was faster than the disappearance of DNA from splenic aspirates for two of the five dogs. This finding suggests that although an increase in platelet counts to within the normal reference range is a positive predictor for therapeutic success, it does not indicate recovery or support discontinuation of treatment before completion of the full doxycycline course.
In conclusion, this study presents evidence that dogs recover from acute CME with adequate doxycycline treatment and that they do not remain carriers. The results suggest that the duration of doxycycline treatment for acute CME should be reduced to 16 days instead of the presently recommended 28 days. The present study also indicates the importance of the use of splenic aspirates in determining ehrlichial elimination and treatment success.
REFERENCES
- 1.Bartsch, R. C., and R. T. Greene. 1996. Post-therapy antibody titers in dogs with ehrlichiosis: follow-up study on 68 patients treated primarily with tetracycline and or doxycycline. J. Vet. Int. Med. 10:271-274. [DOI] [PubMed] [Google Scholar]
- 2.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 42:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhles, W. C., D. L. Huxsoll, and M. Ristic. 1974. Tropical canine pancytopenia: clinical, hematologic and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J. Infect. Dis. 130:357-367. [DOI] [PubMed] [Google Scholar]
- 4.Dawson, J. E., Y. Rikihisa, S. A. Ewing, and D. B. Fishbein. 1991. Serologic diagnosis of human ehrlichiosis using two Ehrlichia canis isolates. J. Infect. Dis. 163:564-567. [DOI] [PubMed] [Google Scholar]
- 5.Foley, J. E., N. W. Lerche, J. S. Dumler, and J. E. Madigan. 1999. A simian model of human ehrlichiosis. Am. J. Trop. Med. Hyg. 60:987-993. [DOI] [PubMed] [Google Scholar]
- 6.Harrus, S., T. Waner, I. Aizenberg, J. E. Foley, A. M. Poland, and H. Bark. 1998. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J. Clin. Microbiol. 36:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrus, S., T. Waner, I. Aizenberg, and H. Bark. 1998. Therapeutic effect of doxycycline in experimental subclinical canine monocytic ehrlichiosis: Evaluation of a 6-week course. J. Clin. Microbiol. 36:2140-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrus, S., T. Waner, A. Keysary, I. Aroch, H. Voet, and H. Bark. 1998. Investigation of splenic functions in canine monocytic ehrlichiosis. Vet. Immunol. Immunopathol. 62:15-27. [DOI] [PubMed] [Google Scholar]
- 9.Huxsoll, D. L., H. L. Amyx, I. E. Hemelt, P. K. Hildebrandt, R. M. Nims, and W. S. Gochenour. 1972. Laboratory studies of Tropical canine pancytopenia. Exp. Parasitol. 31:53-59. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal, Z., and Y. Rikihisa. 1994. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J. Clin. Microbiol. 32:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keysary, A., T. Waner, M. Rozner, J. E. Dawson, R. Zass, C. K. Warner, K. L. Biggie, and S. Harrus. 1995. Isolation, in vitro propagation and genetic characterization of Ehrlichia canis from dogs in Israel. Vet. Parasitol. 62:331-340. [DOI] [PubMed] [Google Scholar]
- 12.Neer, T. M., E. B. Breitschwerdt, R. T. Greene, and M. R. Lappin. 2002. Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. J. Vet. Int. Med. 16:309-315. [DOI] [PubMed] [Google Scholar]
- 13.Skotarczak, B. 2003. Canine ehrlichiosis. Ann. Agric. Environ. Med. 10:137-141. [PubMed] [Google Scholar]
- 14.Stich, R. W., Y. Rikihisa, S. A. Ewing, G. R. Needham, D. L. Grover, and S. Jittapalapong. 2002. Detection of Ehrlichia canis in canine carrier blood and in individual experimentally infected ticks with a p30-based PCR assay. J. Clin. Microbiol. 40:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen, B., Y. Rikihisa, J. M. Mott, R. Greene, H. Y. Kim, N. Zhi, G. C. Couto, A. Unver, and R. Bartsch. 1997. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 35:1852-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waner, T., S. Harrus, D. J. Weiss, H. Bark, and A. Keysary. 1995. Demonstration of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet. Immunol. Immunopathol. 48:177-182. [DOI] [PubMed] [Google Scholar]