Abstract
Hypocrellins A and B were evaluated for in vitro antimicrobial and antileishmanial activities. Hypocrellin A exhibited promising activity against Candida albicans and moderate activity against Staphylococcus aureus, methicillin-resistant S. aureus, Pseudomonas aeruginosa, and Mycobacterium intracellulare. Hypocrellin B showed weak antimicrobial activities. Hypocrellin A exhibited potent antileishmanial activity, while hypocrellin B was only moderately active. These results of promising antifungal and antileishmanial activity of hypocrellin A may be useful for further structure-activity relationship and in vivo studies.
Antifungal drugs, such as amphotericin B, ketoconazole (and other azoles), and griseofulvin, have been widely used in the treatment of patients with various fungal infections. However, their clinical use is limited, due either to lack of efficacy or their toxicity and resistance (8, 17). Therefore, there is a need for new antifungal agents that are more effective and less toxic. For leishmanial infections, only a few drugs, which are highly toxic, are available (4), and their use has further been compromised due to development of drug resistance. Thus, there is a continuous interest in developing new antileishmanial compounds with different modes of action and low toxicities to satisfy clinical use.
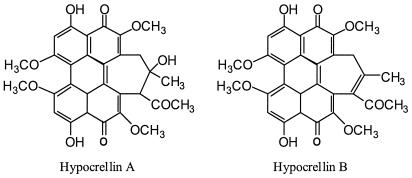
Hypocrellins A and B (Fig. 1) are two main pigments isolated from the parasitic fungus Hypocrella bambusae (Berk. et Broome) Sacc., which grows abundantly in the northwest region of Yunnan Province, People's Republic of China, and the southeastern region of Xizang (Tibet), an autonomous region of the People's Republic of China. These pigments have a long history of use as traditional medicinal agents and were commonly used to treat rheumatoid arthritis, gastric diseases (20), and skin diseases related to fungal infections (18, 19). Previous studies showed that hypocrellins exhibited photodynamic anticancer (2, 5, 12, 21) and antiviral (9, 10) activities. These activities were related to their ability to generate active oxygen (1O2, 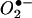 , and
, and 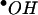 ) (1, 16) and inhibit protein kinase C activity (6). However, no antifungal or antileishmanial activity has been reported. In this study, hypocrellins A and B were evaluated for activities against a panel of fungi and bacteria and for activity against Leishmania donovani, the causative agent of visceral leishmaniasis.
) (1, 16) and inhibit protein kinase C activity (6). However, no antifungal or antileishmanial activity has been reported. In this study, hypocrellins A and B were evaluated for activities against a panel of fungi and bacteria and for activity against Leishmania donovani, the causative agent of visceral leishmaniasis.
FIG. 1.
Structures of hypocrellin A and hypocrellin B.
Hypocrellins A and B were isolated from H. bambusae as described previously (3) at the Experimental Center of Yunnan University, Yunnan, People's Republic of China. Purity was determined to be 99.2%. Samples were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in all assays was less than 0.2%, which has no effect on the tested organisms.
Activity against a panel of microorganisms, including Candida albicans, Cryptococcus neoformans, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, and Mycobacterium intracellulare, was evaluated in vitro. All organisms were obtained from the American Type Culture Collection (Manassas, Va.). Susceptibility testing was performed using a modified version of the NCCLS methods (13, 14, 15) for all organisms except for M. intracellulare, for which the modified Alamar blue procedure described by Franzblau et al. (7) was followed. Samples (dissolved in DMSO) were serially diluted by using 0.9% saline and transferred in duplicate to 96-well microplates. Microbial inocula were prepared after comparison of the absorbance (at 630 nm) of cell suspensions to the 0.5 McFarland standard and dilution of the suspensions in broth (Sabouraud dextrose and cation-adjusted Mueller-Hinton broth [Difco] for the fungi and bacteria, respectively, and 5% Alamar blue [BioSource International] in Middlebrook 7H9 broth with oleic acid-albumin-dextrose-catalase enrichment for M. intracellulare) to afford recommended inoculum sizes. Microbial inocula were added to the samples to achieve a final volume of 200 μl and final sample concentrations starting with 100 μg/ml. Growth, solvent, and medium controls were included on each test plate. The plates were read at either 630 nm or excitation and emission wavelengths of 544 and 590 nm (for M. intracellulare) prior to and after incubation. Percent growth was calculated and plotted with the concentration tested to afford the concentration that inhibits 50% of growth (IC50). The lowest concentrations that kill 100% of organisms, the minimum bactericidal concentration (MBC) and the minimum fungicidal concentration (MFC), were determined by removing 5 μl from each clear well, transferring it to agar, and incubating it until growth was seen.
Activity of the compounds against a culture of L. donovani promastigotes was tested in vitro. The promastigotes were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (Gibco Chemical Co.) at 26°C. A 3-day-old culture was diluted to 5 × 105 promastigotes/ml. Drug dilutions (50 to 3.1 μg/ml) were prepared directly in cell suspension in 96-well plates. Plates were incubated at 26°C for 48 h, and growth of leishmanial promastigotes was determined by the Alamar blue assay (11). Standard fluorescence was measured by a Fluostar Galaxy plate reader (BMG LabTechnologies) at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Pentamidine and amphotericin B were used as the control drugs. Percent growth was calculated and plotted with the concentration tested for computing the IC50s and IC90s.
The activities of hypocrellins A and B against two opportunistic infection pathogens, C. albicans and Cryptococcus neoformans, were evaluated. Their IC50s, MICs, and MFCs are summarized in Table 1. Hypocrellin A exhibited significant activity against C. albicans, with an IC50 of 0.65 ± 0.14 μg/ml, a MIC of 1.41 ± 0.22 μg/ml, and an MFC of 1.41 ± 0.22 μg/ml. Hypocrellin B showed weak activity against C. albicans. Both hypocrellin A and hypocrellin B were not active against C. neoformans.
TABLE 1.
Antifungal activities of hypocrellins A and Ba
| Species | Hypocrellin A
|
Hypocrellin B
|
Amphotericin B
|
Fluconazole
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | MIC | MFC | IC50 | MIC | MFC | IC50 | MIC | MFC | IC50 | MICd | MFC | |
| C. albicans | 0.65 ± 0.14 | 1.41 ± 0.22 | 1.41 ± 0.22 | 5.00 ± 1.41 | 22.0 ± 3.5 | NAb | 0.14 ± 0.08 | 0.47 ± 0.22 | 1.56 ± 1.33 | 0.28 ± 0.1 | 1.25 ± 1.56 | NA |
| Cryptococcus neoformans | NA | NA | NA | NA | NA | NA | 0.30 ± 0.07 | 0.94 ± 0.44 | 1.88 ± 0.88 | NTc | NT | NT |
| Candida glabrata | NA | NA | NA | NA | NA | NA | 0.15 ± 0.07 | 0.31 ± 0.00 | 0.63 ± 0.00 | 25.0 ± 7.1 | 80.0 ± 25.0 | NA |
| Candida parapsilosis | 1.00 ± 0.0 | 100.0 ± 0.0 | NA | 70.0 ± 0.0 | NA | NA | 0.33 ± 0.11 | 0.94 ± 0.44 | 2.50 ± 0.00 | 1.50 ± 0.0 | 5.60 ± 0.88 | NA |
| Candida tropicalis | 85.0 ± 0.0 | NA | NA | NA | NA | NA | 0.20 ± 0.14 | 0.47 ± 0.23 | 0.94 ± 0.44 | 1.00 ± 0.0 | 2.50 ± 3.13 | NA |
| Candida krusei | NA | NA | NA | NA | NA | NA | 0.35 ± 0.07 | 2.81 ± 3.09 | 3.13 ± 2.65 | 10.0 ± 0.0 | 22.5 ± 3.5 | NA |
Data shown are the means ± standard deviations (SDs), in micrograms per milliliter, of results from triplicate experiments.
NA, not active at the highest test concentration, 100 μg/ml.
NT, not tested.
The MIC for fluconazole is the concentration that allows no more than 20% growth.
Further, to explore the antifungal activities of hypocrellins A and B, Candida species other than C. albicans (listed in Table 1) which are known to contribute to opportunistic fungal infections were also evaluated. However, hypocrellins A and B were active against none of these species except Candida parapsilosis. Hypocrellin A showed moderate activity against S. aureus, MRSA, P. aeruginosa, and M. intracellulare, with IC50s of 3 to 10 μg/ml. It did not show any bactericidal activity. Hypocrellin B showed mild activity against M. intracellulare but was not active against other organisms tested (Table 2).
TABLE 2.
Antibacterial activities of hypocrellins A and Ba
| Drug |
S. aureus
|
MRSA
|
P. aeruginosa
|
M. intracellulare
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | MIC | MBC | IC50 | MIC | MBC | IC50 | MIC | MBC | IC50 | MIC | MBC | |
| Hypocrellin A | 3.00 ± 1.41 | NAb | NA | 7.0 ± 2.8 | NA | NA | 10.0 ± 2.5 | NA | NA | 2.5 ± 1.4 | 22.5 ± 3.5 | NA |
| Hypocrellin B | NA | NA | NA | NA | NA | NA | NA | NA | NA | 22.5 ± 3.5 | NA | NA |
| Ciprofloxacin | 0.15 ± 0.07 | 0.63 ± 0.0 | 0.79 ± 0.22 | 0.10 ± 0.0 | 0.63 ± 0.0 | NA | 0.04 ± 0.01 | 0.16 ± 0.0 | 2.5 ± 0.0 | 0.23 ± 0.04 | 0.63 ± 0.0 | NA |
Data shown are the means ± SDs (in micrograms per milliliter) of results from triplicate experiments.
NA, not active at highest test concentration, 100 μg/ml.
A similar pattern of activity was observed when hypocrellins A and B were evaluated for antileishmanial activity. Hypocrellin A exhibited potent antileishmanial activity, with an IC50 of 0.27 ± 0.03 μg/ml and an IC90 of 0.71 ± 0.15 μg/ml, while hypocrellin B was moderately active, with an IC50 of 12.7 ± 2.1 μg/ml and an IC90 of 36.9 ± 6.9 μg/ml (Table 3). It was interesting that the antileishmanial activity of hypocrellin A (IC50) was three- and sixfold more potent than that of amphotericin B and pentamidine, respectively.
TABLE 3.
Activity of hypocrellins A and B against Leishmania donovani promastigote culturea
| Drug | IC50 (μg/ml) | IC90 (μg/ml) |
|---|---|---|
| Hypocrellin A | 0.27 ± 0.03 | 0.71 ± 0.15 |
| Hypocrellin B | 12.7 ± 3.1 | 36.9 ± 6.9 |
| Pentamidine | 1.58 ± 0.27 | 3.17 ± 0.79 |
| Amphotericin B | 0.79 ± 0.17 | 1.69 ± 0.39 |
Data shown are the means ± SDs of results from triplicate experiments.
The results presented indicate the promising activity of hypocrellin A against C. albicans, with a fungicidal effect. However, hypocrellin A was not active against Cryptococcus neoformans. This result indicates that hypocrellin A had selective growth-inhibitory and fungicidal activities against C. albicans. Hypocrellin B was also active against C. albicans and M. intracellulare, but the activity was relatively weak compared to that of hypocrellin A. Previous reports have indicated that hypocrellins, mainly hypocrellin A, were used for treatment of skin diseases related to fungal infections, such as white lesions of vulva, vitiligo, psoriasis, tinea capitis, and lichen amyloidosis (18, 19). This investigation provides significant experimental evidence for development of hypocrellin A as a potential antifungal agent. It is worth noting that hypocrellin A is not active against Cryptococcus neoformans, which indicates that it might have a mechanism for fungicidal effect different from that of amphotericin B, which is active against both C. albicans and Cryptococcus neoformans.
The antileishmanial activity of hypocrellin A was more potent than that of pentamidine and amphotericin B, which are currently used for the treatment of leishmaniasis. However, the toxicities of these drugs have limited their clinical use. Hypocrellin A may have an advantage over these drugs.
The results reported herein thus indicate promising antifungal and antileishmanial actions of hypocrellin A. Further evaluation of in vivo antifungal and antileishmanial activities in an animal model is needed.
Acknowledgments
This investigation was supported by the United States Department of Agriculture, Agricultural Research Service specific cooperative agreement number 58-6408-2-0009. B.L.T. is also supported by Centers for Disease Control and Prevention cooperative agreements USO/CCV 418839 and UR3/CCU 418652.
REFERENCES
- 1.Ali, S. M., S. K. Chee, G. Y. Yuen, and M. Olivo. 2002. Hypocrellins and hypericin induced apoptosis in human tumor cells: a possible role of hydrogen peroxide. Int. J. Mol. Med. 9:461-472. [PubMed] [Google Scholar]
- 2.Cao, E. H., and L. S. Cheng. 1988. DNA single-strand breakage and its rejoining in HeLa cells caused by hypocrellin A photosensitization. Shih Yen Sheng Wu Hsueh Pao 21:79-85. [PubMed] [Google Scholar]
- 3.Chen, W. S., Y. T. Chen, X. Y. Wan, E. Friedrichs, H. Puff, and E. Breitmaier. 1981. Structure of hypocrellin and its photooxidation product peroxyhypocrellin. Liebigs Ann. Chem. 10:880-885. [Google Scholar]
- 4.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 5.Diwu, Z. 1995. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem. Photobiol. 61:529-539. [DOI] [PubMed] [Google Scholar]
- 6.Diwu, Z., J. Zimmermann, T. Meyer, and J. W. Lown. 1994. Design, synthesis and investigation of mechanisms of action of novel protein kinase C inhibitors: perylenequinonoid pigments. Biochem. Pharmacol. 47:373-385. [DOI] [PubMed] [Google Scholar]
- 7.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groll, A. H., and H. Kolve. 2004. Antifungal agents: in vitro susceptibility testing, pharmacodynamics, and prospects for combination therapy. Eur. J. Clin. Microbiol. Infect. Dis. 23:256-270. [DOI] [PubMed] [Google Scholar]
- 9.Hirayama, J., K. Ikebuchi, H. Abe, K. W. Kwon, Y. Ohnishi, M. Horiuchi, M. Shinagawa, K. Ikuta, N. Kamo, and S. Sekiguchi. 1997. Photoinactivation of virus infectivity by hypocrellin A. Photochem. Photobiol. 66:697-700. [DOI] [PubMed] [Google Scholar]
- 10.Hudson, J. B., J. Zhou, J. Chen, L. Harris, L. Yip, and G. H. Towers. 1992. Hypocrellin, from Hypocrella bambusae, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 60:253-255. [DOI] [PubMed] [Google Scholar]
- 11.Mikes, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania by using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 12.Miller, G. G., K. Brown, A. M. Ballangrud, O. Barajas, Z. Xiao, J. Tulip, J. W. Lown, J. M. Leithoff, M. J. Allalunis-Turner, R. D. Mehta, and R. B. Moore. 1997. Preclinical assessment of hypocrellin B and hypocrellin B derivatives as sensitizers for photodynamic therapy of cancer: progress update. Photochem. Photobiol. 65:714-722. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.National Committee for Clinical Laboratory Standards. 2000. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes, 2nd ed. Tentative standard M24-T2. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 16.Park, J., D. S. English, Y. Wannemuehler, S. Carpenter, and J. W. Petrich. 1998. The role of oxygen in the antiviral activity of hypericin and hypocrellin. Photochem. Photobiol. 68:593-597. [PubMed] [Google Scholar]
- 17.Walsh, T. J. 1992. Topoisomerase II inhibitors: prospects for new antifungal agents, p. 349-373. In J. Sutcliffe and N. H. Georgopapadakou (ed.), Emerging targets in antibacterial and antifungal chemotherapy. Chapman and Hall, New York, N.Y.
- 18.Wan, X. Y., and Y. T. Chen. 1981. Hypocrellin A, a new drug for photo-chemo-therapy. Kexue Tongbao 26:1040-1042. [Google Scholar]
- 19.Wang, J. B., and J. N. Bao. 1985. Clinical analysis and observation of hypocrellin photochemotherapy in the treatment of lichen amyloidosis: report of 37 cases. Acta Acad. Med. Sin. 7:349-352. [PubMed] [Google Scholar]
- 20.Yu, C., T. Huang, Z. Ding, X. Gao, and Z. Zhang (ed.). 1993. Encyclopedia of Chinese medicines, vol. 1, item 1187. Chinese Medicinal Science and Technology Press, Beijing, People's Republic of China.
- 21.Zhang, J., E. H. Cao, J. F. Li, T. C. Zhang, and W. J. Ma. 1998. Photodynamic effects of hypocrellin A on three human malignant cell lines by inducing apoptotic cell death. J. Photochem. Photobiol. B 43:106-111. [DOI] [PubMed] [Google Scholar]