Abstract
Albaconazole is an experimental triazole derivative with potent and broad-spectrum antifungal activity and a remarkably long half-life in dogs, monkeys, and humans. In the present work, we investigated the in vivo activity of this compound against two strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi, the causative agent of Chagas' disease, using dogs as hosts. The T. cruzi strains used in the study were previously characterized (murine model) as susceptible (strain Berenice-78) and partially resistant (strain Y) to the drugs currently in clinical use, nifurtimox and benznidazole. Our results demonstrated that albaconazole is very effective in suppressing the proliferation of the parasite and preventing the death of infected animals. Furthermore, the parasitological, PCR, serological, and proliferative assay results indicated parasitological cure indices of 25 and 100% among animals inoculated with T. cruzi strain Y when they were treated with albaconazole at 1.5 mg/kg of body weight/day for 60 and 90 days, respectively. On the other hand, although albaconazole given at 1.5 mg/kg/day was very effective in suppressing the proliferation of the parasite in animals infected with the Berenice-78 T. cruzi strain, no parasitological cure was observed among them, even when a longer treatment period (150 doses) was used. In conclusion, our results demonstrate that albaconazole has trypanocidal activity in vivo and is capable of inducing radical parasitological cure, although natural resistance to this compound was also indicated. Furthermore, the compound can be used in long-term treatment schemes (60 to 150 days) with minimal toxicity and thus represents a potentially useful candidate for the treatment of human Chagas' disease.
Chagas' disease is endemic from Mexico to Argentina, where it is estimated that 16 million to 18 million people are infected with its causative agent, Trypanosoma (Schizotrypanum) cruzi, and where 40 million remain at risk, emphasizing the need to sustain and extend control strategies (38). Although great progress in the control of the vector and in the prevention of transmission of the disease by blood transfusion has been made, the specific treatment to be used for infected individuals remains unsolved.
Specific chemotherapy with benznidazole (Rochagan and Rodanil; Roche, Rio de Janeiro, Brazil) or nifurtimox (Lampit; Bayer) has been recommended for the treatment of acute and congenital infections (20). Basic studies have determined the molecular basis of the anti-T. cruzi activity. Nifurtimox acts via the reduction of the nitro group to unstable nitroanion radicals, which in turn react to produce highly toxic reduced oxygen metabolites (superoxide anion and hydrogen peroxide) (9). Benznidazole seems to act by a different mechanism (reductive stress), which involves covalent modification of macromolecules by nitroreduction intermediates (9). However, clinical trials with nifurtimox, benznidazole, and allopurinol have shown that these compounds have very low or no activity in preventing the development of chronic Chagas' disease; moreover, the drugs have significant side effects, including anorexia, vomiting, peripheral polyneuropathy, and allergic dermopathy (6, 21, 26). The discovery of new, active, nontoxic compounds would probably expand the number of patients who could receive treatment, including those patients in whom clinical manifestations are absent or in whom symptoms can be disclosed only by more elaborate medical procedures. Although benznidazole and nifurtimox are still the drugs most frequently used for the etiological treatment of Chagas' disease patients, they are far from the ideal medicines for the treatment of this condition. The most important requirements for the treatment of Chagas' disease are a capacity to induce parasitological cure in both patients with acute cases and patients with chronic cases, oral activity in a single or few doses, affordability, a lack of significant side effects, ability to be administered in ambulatory treatment settings with minimal monitoring, and a low probability of the development of parasite resistance (4).
The search for new compounds with activities against T. cruzi and with low toxicities and increased efficacies during the chronic phase continues. Ergosterol biosynthesis inhibitors (EBIs) are the most advanced and the most frequently used compounds for the treatment of fungal infections (34). Like many fungi, T. cruzi has a strict requirement for specific endogenous sterols for cell viability and growth and is extremely susceptible to sterol biosynthesis inhibitors in vitro and in vivo (28). Recently, it has been shown that some of these compounds, such as D0870 (Astra-Zeneca Pharmaceuticals), posaconazole (SCH 56592; Schering-Plough Research Institute), and TAK-187 (Takeda Chemical Company), are capable of inducing parasitological cure in murine models of both acute and chronic Chagas' disease with no toxic side effects to the hosts (17, 18, 19, 28, 31, 32, 33).
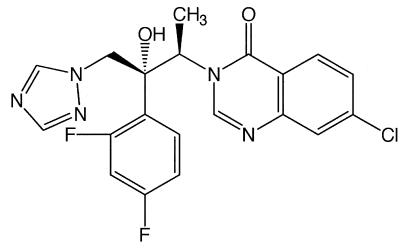
More recently, it has been shown that the new triazole derivative albaconazole (UR-9825; Uriach & Company, Barcelona, Spain) (Fig. 1) displays in vitro activities against T. cruzi comparable to those of the most potent EBIs tested against this organism, and detailed biochemical studies indicated that its mechanism of action is due to a specific interference of the parasite's sterol C-14α-demethylase (29). However, in vivo efficacy against T. cruzi requires both potent intrinsic antiparasitic activities and special pharmacokinetic properties (long terminal half-lives and large volumes of distribution) (28, 33). Thus, the extremely short terminal half-life of albaconazole in mice (<0.5 h) prevented us from testing the compound in murine models (3). On the other hand, its long half-life in dogs and cynomolgus monkeys (51 and 24 h, respectively) suggested that this compound could have significant activity against T. cruzi in theses animal models (3). A dog model for experimental chemotherapy studies of acute and chronic Chagas' disease has recently been described (14). In those studies benznidazole treatment of dogs infected with T. cruzi strains that were naturally susceptible, partially resistant, or resistant to this drug led to results similar to those reported from studies with human patients in the acute and the chronic phases of the disease.
FIG. 1.
Chemical structure of albaconazole (UR-9825).
In the present study we investigated the in vivo activities of albaconazole in dogs infected with T. cruzi strains susceptible and partially resistant to benznidazole (12, 35).
MATERIALS AND METHODS
T. cruzi strains.
The T. cruzi strains used in this study were T. cruzi Y, which is partially resistant to benznidazole, and T. cruzi II Berenice-78, which is susceptible to benznidazole (12, 15, 24, 35).
Experimental animals and infection.
Forty-four 3-month-old mongrel dogs of both sexes from the kennel of the Federal University of Ouro Preto, Minas Gerais, Brazil, were used in this study. All procedures and experimental protocols were conducted in accordance with the COBEA (Brazilian School of Animal Experimentation) and behavior instructions for the use of animals in research. Animals were fed a commercial chow and were given water ad libitum. Before the study, the animals were treated with antihelminthic drugs and immunized against infectious diseases. The animals were inoculated with 2,000 blood trypomastigotes of T. cruzi Berenice-78 (24 dogs) or Y (20 dogs) per kg of body weight by the intraperitoneal route. Twelve animals infected with the Berenice-78 strain were treated with albaconazole for 60, 90, or 150 days. Eight animals infected with the Y strain were treated with albaconazole for 60 or 90 days. Four dogs were used for each treatment period. For each strain of T. cruzi studied, two dogs were treated with benznidazole and two untreated dogs were used as controls.
Drugs.
Albaconazole (UR-9825), (1R,2R)-7-chloro-3[2-(2,4-difluoropeny)2-hydroxyl-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]quinazilin-4(3H)-one (Fig. 1), was kindly provided by Xavier Bartrolí (Uriach & Cia). Benznidazole, 2-nitroimidazole-(N-benzil-2-nitro-1-imidazolacetamide), is a product of Roche.
Treatment scheme.
Oral treatment started 12 to 22 days postinfection, immediately after the appearance of parasitemia, as detected by the examination of fresh blood. Groups of eight animals each were infected with strain Y or Berenice-78 and treated orally with 1.5 mg of albaconazole per kg of body weight daily for a total of 60 doses, and eight animals were treated for a total of 90 doses. Four animals inoculated with strain Berenice-78 received 150 doses. As positive controls, 12 animals received 7 mg of benznidazole per kg of body weight administered in two daily doses for 60 days (120 doses), while 12 untreated dogs (which received only the drug vehicle) were used as negative controls.
Evaluation of parasitological cures.
To verify the occurrence of parasitological cure, we ran a battery of five independent tests, including examination of fresh blood, hemoculture, PCR assay, a serological enzyme-linked immunosorbent assay (ELISA), and a proliferative assay.
(i) Examination of fresh blood.
The parasitemia of the animals was examined from day 10 of infection until the parasites could no longer be detected by collecting fresh blood from the marginal ear vein. The mortality rate was expressed as the cumulative percentage of dead animals.
(ii) Hemoculture.
The hemoculture technique was performed at 1 and 6 months posttreatment with blood from the treated and controls animals (7). Hemocultures were examined monthly for up to 120 days for detection of parasites.
(iii) PCR assay.
Ten milliliters of blood from each animal was collected at months 1 and 6 posttreatment. The samples were immediately mixed with an equal volume of 6 M guanidine HCl-0.2 M EDTA solution, maintained at room temperature for 2 weeks, and boiled for 15 min to break the minicircles (2, 5). Three DNA extractions were performed as described by Wincker et al. (37), but 40 μg of glycogen (Boehringer Mannheim) was used to precipitate the DNA. The PCR conditions were the same as those described by Gomes et al. (13) except that 20 pmol of primers S35 (5′-AAATAATGTACGGG(T/G)GAGATGCATGA-3′) and S36 (5′-GGGTTCGATTGGGGTTGGTGT-3′) was used (1, 13, 37). Briefly, 2 μl of blood DNA template was added to 10 mM Tris-HCl (pH 9.0); 75 mM KCl; 3.5 mM MgCl2; 0.1% Triton X-100; 0.2 mM each dATP, dCTP, dGTP, and dTTP (Sigma Chemical Co.); 1.0 U of Taq DNA polymerase (Promega); and water in a 20-μl reaction volume. The reaction mixtures were overlaid with 30 μl of mineral oil and subjected to 35 amplification cycles in a Research Programmable Thermal Controller (MiniCycler). The temperature profile was 95°C for 5 min for denaturation and two cycles of annealing at 30°C for 2 min, followed by 33 cycles with the annealing temperature increased to 40°C and a final extension at 72°C for 5 min. Five microliters of each of the PCR products was analyzed by electrophoresis on a 6% polyacrylamide gel and visualized by silver staining (22).
(iv) Serological profile.
Serum samples were collected from the blood of all dogs before and monthly after the inoculation for 6 months posttreatment. Serum samples were stored at −20°C, and ELISAs were performed as described by Voller et al. (36). Sera were tested by using the T. cruzi Y strain, obtained from an acellular culture in liver infusion tryptose medium, as the antigen and peroxidase-conjugated goat anti-dog immunoglobulin G (Sigma Chemical Co.). The cutoff was determined by using the mean absorbance for 10 uninfected animals plus 2 standard deviations.
(v) PBMC proliferative assay.
Blood samples were collected from all dogs before and monthly after the inoculation for 6 months posttreatment. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation and were washed three times with RPMI (GIBCO, Grand Island, N.Y.). A total of 3.5 × 106 cells were stimulated with 10 μl of parasite antigens (108 trypomastigotes/ml) derived from trypomastigotes or with 25 μl of the mitogen concanavalin A (80 μg/ml) in a final volume of 200 μl. The preparation was then incubated at 37°C in a 5% CO2 atmosphere for 3 days (stimulus with concanavalin A) and 5 days (stimulus with trypomastigote antigen). The cultures were pulsed with 0.2 μCi of [3H]thymidine per well for the last 16 h of culture and processed for scintillation counting. The proliferation data were calculated as the mean counts per minute of incorporated [3H]thymidine for duplicate cultures, and the results are expressed as the experimental counts per minute divided by the control (unstimulated) counts per minute (stimulation index [SI]).
Statistical analysis.
The mean patent period and the maximum level of parasitemia posttreatment were compared by analysis of variance by using square root transformation of the data (the Tukey test) (25). Regression analysis was used to compare antibody levels and SIs before, during, and after treatment; regression lines were compared by analysis of covariance (25). The association between antibody levels and the SI was tested by using the Pearson correlation. In all cases, differences were considered statistically significant when the P value was less than 0.05.
RESULTS
Albaconazole was well tolerated by the dogs, and no side effects were observed during the study with a dose of 1.5 mg/kg/day administered for 60 or 90 days. However, when the dogs were treated for longer periods (150 days), a loss of weight and gastrointestinal disturbances were observed in one of four treated animals after 120 days of treatment (data not shown). During the necropsy of this animal, edema and small hemorrhages were observed along the intestinal tract. The other three animals treated for 150 days did not show any collateral effects.
The in vivo trypanocidal effects of albaconazole are shown in Table 1. The parasitemia was suppressed between the first and second day posttreatment in all treated animals. Similar results were observed for animals treated with benznidazole, the drug most frequently used for the treatment of human Chagas' disease. All control (untreated) animals showed significantly higher parasitemia levels (P < 0.005) and patent periods (P < 0.001) than treated animals (Table 1). A survival rate of 100% was obtained with all therapeutic schemes used; in contrast, untreated controls inoculated with the Y strain showed 50% mortality.
TABLE 1.
Trypanocidal effects of albaconazole and benznidazole, administered in different therapeutic schemes, on biological parameters for dogs infected with Berenice- 78 and Y T. cruzi strains
| Group | Therapeutic scheme (T. cruzi strain/drug/treatment time [days]) | No. of animals surviving/total no. tested | Prepatent period (days)a | No. of trypomastigotes in bloodb before treatmenta | Patent period (days [highest level of parasitemiab]) after treatmenta |
|---|---|---|---|---|---|
| 1 | Berenice-78/albaconazole/60 | 4/4 | 21 ± 0.82 | 6,000 ± 2,708 | 1.5 ± 1.29c (3,750 ± 2,500)c |
| 2 | Berenice-78/albaconazole/90 | 4/4 | 18.75 ± 1.89 | 5,000 ± 3,535 | 0.75 ± 0.96c (2,500 ± 2,886)c |
| 3 | Berenice-78/albaconazole/150 | 3/4d | 22.75 ± 5.5 | 4,500 ± 577 | 0.5 ± 0.58c (1,750 ± 2,362)c |
| 4 | Berenice-78/benznidazole | 6/6 | 20.6 ± 1.51 | 5,250 ± 3,574 | 0.33 ± 0.5c (1,250 ± 2,091)c |
| 5 | Berenice-78/untreated control | 6/6 | 22.5 ± 0.76 | 9.0 ± 2.59e (14,333 ± 5,887)e | |
| 6 | Y/albaconazole/60 | 4/4 | 12.25 ± 0.5 | 3,500 ± 1,732 | 0.5 ± 0.6c (1,000 ± 11,540)c |
| 7 | Y/albaconazole/90 | 4/4 | 13 ± 1.15 | 5,000 ± 0 | 0.25 ± 0.5c (1,250 ± 2,500)c |
| 8 | Y/benznidazole/60 | 6/6 | 12.75 ± 1.5 | 5,000 ± 0 | 0.75 ± 0.96c (1,750 ± 2,362)c |
| 9 | Y/untreated control | 3/6 | 11.67 ± 1.8 | 11.2 ± 3.27e (23,000 ± 9,082)e |
Data represent means ± standard deviations.
Number of blood trypomastigotes/0.1 ml of blood.
Significant differences from the results for the groups labeled with footnote e.
One animal was killed because of treatment side effects.
Significant differences from the results for the groups labeled with footnote c.
Parasitological cure was verified by five independent criteria: fresh blood examination, hemoculture, PCR, the presence of anti-T. cruzi antibodies, detection by ELISA, and a decreased SI in PBMC proliferation assays.
Among the dogs infected with the Y strain, at least one of two tests, hemoculture and PCR, were positive for 75% of the animals that received albaconazole at 1.5 mg/kg orally in one daily dose for 60 days, while 100% of the animals treated for 90 days had negative results by both tests and were considered parasitologically cured (Table 2). Similar results were observed for animals treated with 7 mg of benznidazole per kg, administered orally in two daily doses for 60 days (120 doses) (Table 2).
TABLE 2.
Parasitological and molecular tests of blood of dogs infected with the Berenice-78 and Y T. cruzi strains and treated with albaconazole or benznidazole
| Group | Therapeutic scheme (T. cruzi strain/drug/treatment time [days]) | No. of animals hemoculture positivea/total no. tested
|
No. of animals PCR positivea/total no. tested
|
No. of animals with positive test results posttreatment/total no. of animals tested (%) | ||
|---|---|---|---|---|---|---|
| 1 mob | 6 mob | 1 mo | 6 mo | |||
| 1 | Berenice-78/albaconazole/60 | 1/4 | 1/4 | 4/4 | 4/4 | 4/4 (100) |
| 2 | Berenice-78/albaconazole/90 | 2/4 | 1/4 | 4/4 | 4/4 | 4/4 (100) |
| 3 | Berenice-78/albaconazole/150 | 2/3 | 1/3 | 3/3 | 3/3 | 3/3 (100) |
| 4 | Berenice-78/benznidazole/60 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 (0) |
| 5 | Berenice-78/untreated control | 3/6 | 3/6 | 6/6 | 6/6 | 6/6 (100) |
| 6 | Y/albaconazole/60 | 0/4 | 1/4 | 3/4 | 3/4 | 3/4 (75) |
| 7 | Y/albaconazole/90 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 (0) |
| 8 | Y/benznidazole/60 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 (0) |
| 9 | Y/untreated control | 2/3 | 1/3 | 3/3 | 3/3 | 3/3 (100) |
Positive indicates positive by a at least one test.
Month after treatment.
Although albaconazole given at 1.5 mg/kg/day was very effective in suppressing the proliferation of the Berenice-78 T. cruzi strain in all treatment schemes (Table 1), no parasitological cures were observed among animals infected with this organism, even after 150 days of treatment (Table 2). In contrast, animals infected with this strain and treated with benznidazole showed persistently negative hemoculture and PCR results (Table 2).
Comparative analysis of specific T. cruzi antibodies levels and trypomastigote-induced antigen PBMC proliferation were performed. For these analyses the experimental animals were classified into five groups on the basis of the results of the parasitological and PCR evaluations: group 1, untreated controls; group 2, dogs treated with albaconazole with positive parasitological and PCR test results; group 3, dogs treated with albaconazole with negative parasitological and PCR test results; group 4, dogs treated with benznidazole with a negative parasitological test result; and group 5, uninfected control dogs (Fig. 2 to 4).
FIG. 2.
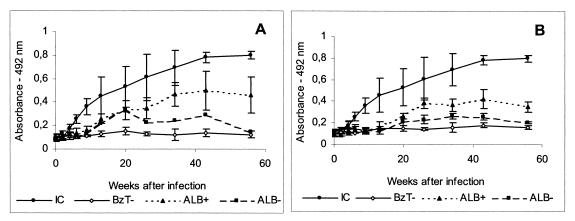
T. cruzi-specific immunoglobulin G antibodies in the sera of dogs in the infected control group and dogs treated with 1.5 mg of albaconazole (UR-9825) per kg of body weight for 60 (A) or 90 (B) days or with 7 mg of benznidazole per kg divided into two daily doses for 60 days. IC, infected nontreated controls; BzT−, dogs treated with benznidazole with negative hemoculture and PCR test results; ALB+, dogs treated with albaconazole with positive hemoculture and PCR test results; ALB−, dogs treated with albaconazole with negative hemoculture and PCR test results. Data represent means ± standard deviations.
FIG. 4.
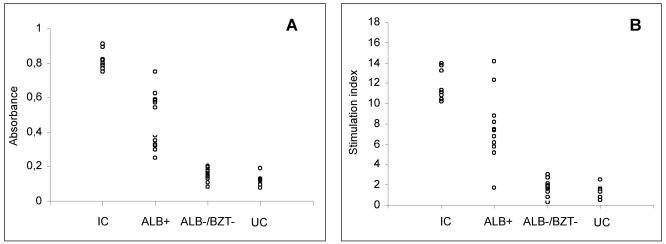
Comparative analysis between the immunological profiles determined by ELISA (A) and the proliferative assay (B) 6 months posttreatment with blood from dogs infected with the Y or the Berenice-78 T. cruzi strain for the infected control group and dogs treated with 1.5 mg of albaconazole (UR-9825) per kg of body weight or 7 mg of benznidazole per kg divided into two daily doses for 60 days. IC, infected nontreated controls; ALB+, dogs treated with albaconazole with positive hemoculture and PCR test results; ALB−, dogs treated with albaconazole with negative hemoculture and PCR test results; BZT−, dogs treated with benznidazole with negative hemoculture and PCR test results; UC, uninfected controls. The symbols represent individual observations for each group.
Regardless of the chemotherapeutic regimen used, treated animals had antibody levels significantly less (groups 2, 3, and 4) than those of the untreated control group (group 1). The disappearance of circulating antibodies (seroconversion) 6 months after the end of treatment was observed in one of four and four of four animals that were inoculated with the Y T. cruzi strain and that received 1.5 mg of albaconazole for 60 and 90 days, respectively. On the other hand, no seroconversion was observed in animals infected with the Berenice-78 strain (Fig. 2). All animals treated with benznidazole (group 4) had negative serology results at least up to 6 months posttreatment (Fig. 2A and B).
The effect of specific treatment against T. cruzi on trypomastigote-induced PBMC proliferation was also evaluated. The SIs of PBMCs from treated and untreated animals were similar until 8 weeks postinoculation. After that, the SIs were consistently lower in the animals in the treated groups (groups 2, 3, and 4) than in the control animals (group 1). Among the treated animals, the SIs were similar during the treatment period, regardless of the result of the treatment. After the end of treatment, the majority of animals with negative hemoculture and PCR test results had SIs of about 2, while those with both positive test results had SIs of about 5 (Fig. 3A and B). Similar results were observed in relation to the specific antibody levels obtained by ELISA (Fig. 2A and B). Comparative analysis of the results of these two assays showed a 75% correlation (Fig. 4).
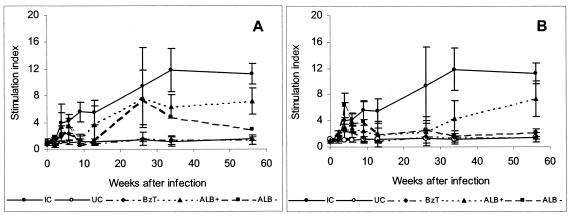
FIG. 3.
In vitro stimulation of PBMCs with trypomastigote antigen before, during, and 6 months after treatment from dogs infected with the Y and the Berenice-78 T. cruzi strains for the control group and dogs treated with 1.5 mg of albaconazole per kg of body weight for 60 (A) and 90 (B) days or 7 mg of benznidazole per kg divided into two daily doses for 60 days. IC, infected nontreated controls; UC, uninfected controls; BzT−, dogs treated with benznidazole with negative hemoculture and PCR test results; ALB+, dogs treated with albaconazole with positive hemoculture and PCR test results; ALB−, treated with albaconazole with negative hemoculture and PCR test results. Data represent means ± standard deviations.
DISCUSSION
Although there have been recent advances in vector control in the American Southern Cone countries, we are still challenged by the same critical problems, including the treatment of chronic cases of Chagas' disease and the fact that vectorial transmission of the disease continues in several countries where the disease is endemic, such as Bolivia and Mexico, where the incidence of infection in some regions is very significant (8, 23). Furthermore, the transmission of Chagas' disease by blood transfusion represents a significant threat in areas where the disease is endemic and countries where the disease is not endemic, due to active migratory activities.
At present, only two compounds, benznidazole and nifurtimox, are used for the etiological treatment of Chagas' disease. The results obtained with both drugs varied according to the phase of Chagas' disease, the period of treatment, the dose, and the geographical origin of the patient. Furthermore, toxic side effects are common, leading in some cases to the discontinuation of treatment (11, 12, 13). This situation reflects the lack of interest of the pharmaceutical industry in the screening and development of new drugs for Chagas' disease.
Recent developments in the study of the basic biochemistry of T. cruzi have allowed the identification of novel targets for chemotherapy. The recognition of an essential requirement for specific endogenous sterols in fungi opened the possibility of interference in this pathway, leading to the development of several drugs for the treatment of different types of fungal infections. Since the main sterol of T. cruzi is ergosterol, an intensive investigation on the potential trypanocidal effects of specific EBIs has been performed, and it has been demonstrated that some of these compounds exhibit curative rather than suppressive activities in murine models of acute and chronic Chagas' disease (28, 33).
Recently, Urbina et al. (29) demonstrated the high in vitro anti-T. cruzi activity of the new triazole derivative albaconazole (UR-9825), a specific inhibitor of sterol C14α-demethylase. The minimal concentration of albaconazole required to induce epimastigote lysis was 30 nM, which is 33 times lower than that of ketoconazole, D0870, or itraconazole and which is comparable to that of posaconazole, the most potent EBI known against this parasite. Furthermore, against the clinically relevant intracellular amastigote form, the minimal concentration required to eliminate the parasite was just 10 nM (16, 17, 29, 30, 31, 32). These data suggest that albaconazole could be a good candidate for in vivo chemotherapeutic studies.
The dog is almost ideal as an experimental model for these studies for two important reasons: the first is the fact that the results for experimentally infected dogs treated with benznidazole were similar to those observed for human patients in terms of therapeutic effectiveness and cure rates in both the acute and the chronic phases of the disease (14). The second is that, in this experimental animal, albaconazole has a long terminal half-life (51 h) and a large volume of distribution, which, together with the high level of intrinsic anti-T. cruzi activity, are of crucial importance for curative activity in vivo (3, 28).
Evaluation of the in vivo activity of this compound in a stringent dog model of acute disease indicated that it has a potent trypanocidal activity. Our results demonstrated that the compound is very effective in suppressing the proliferation of the parasite and preventing death in infected animals. Furthermore, the parasitological, PCR, serological, and proliferative assay results indicated parasitological cure indices of 25 and 100% among animals that were inoculated with the Y strain and that received albaconazole at 1.5 mg/kg/day for 60 and 90 days, respectively (Table 2). Similar results were obtained with another experimental triazole, TAK-187, in a murine model, in which the prolongation of treatment indeed led to higher levels of parasitological cure (33). It was also found in the present study that all dogs infected with the T. cruzi Y strain and treated with benznidazole were parasitologically cured. However, the number of doses and the total amount of drug required to induce parasitological cure were significantly smaller for albaconazole than for benznidazole (90 and 120 doses, respectively, and 135 and 420 mg/kg, respectively).
Interestingly, albaconazole was unable to induce parasitological cure in dogs infected with the Berenice-78 strain, even with a prolonged treatment of 90 or 150 days. These findings are in contrast to the fact that this strain is 100% susceptible to benznidazole, as shown in this study (Table 2) and previous studies (14, 35), and point to the existence of different mechanisms of natural resistance to these two drugs. Several investigators (12, 27) have demonstrated the natural resistance of T. cruzi populations to benznidazole and nifurtimox. Our results agree with those obtained by Molina et al. (18, 19), who demonstrated that many T. cruzi strains which are naturally resistant to nifurtimox and benznidazole are responsive to EBIs such as D0870 and posaconazole.
Even though no parasitological cure could be demonstrated among animals infected with the Berenice-78 T. cruzi strain, our results demonstrate marked differences in the serological and the parasite-specific T-cell responsiveness between the controls and all treated animals. Evaluation of the PBMC proliferation kinetics revealed clearly different cell reactivity profiles between the treated and the cured animals, the treated and the not cured animals, and the untreated controls (Fig. 3). All animals treated and cured exhibited normalization of cell reactivity posttreatment. On the other hand, cell reactivity at an intermediate level was detected among the animals treated but not cured. A similar profile was observed in relation to the specific antibody levels determined by standard serological tests (Fig. 2 and 4). When the hypothesis that the parasite triggers a chain of immune alterations is considered, these results are consistent with a marked reduction in the parasite load. Our results agree with those obtained by Dutra et al. (10), who demonstrated that parasitological cure can be correlated with the normalization of cell reactivity.
In conclusion, our results indicate that albaconazole has trypanocidal activity in vivo and is capable of inducing radical parasitological cure in the dog model of acute infection, although natural resistance to this compound was also demonstrated. Furthermore, the compound can be used in long-term treatment schemes (60 to 150 days) with minimal toxicity and thus represents a potentially useful candidate for the treatment of human Chagas' disease.
Acknowledgments
This work was supported by grants from Uriach & Cia, the Universidade Federal de Ouro Preto (UFOP), and the UNDP/World Bank/World Health Organization Programme for Research and Training in Tropical Diseases (grant 990201). J.A.U. is an International Research Scholar of the Howard Hughes Medical Institute (grant 55000620).
We thank Xavier Bartroli for kindly providing the albaconazole used for this study.
REFERENCES
- 1.Ávila, H. A., A. M. Gonçalves, N. C. Nehme, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Mol. Biochem. Parasitol. 42:175-188. [DOI] [PubMed] [Google Scholar]
- 2.Ávila, H. A., D. S. Sigman, L. M. Cohen, R. C. Millikan, and L. Simpson. 1991. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolation from whole blood lysates: diagnosis of chronic Chagas' disease. Mol. Biochem. Parasitol. 40:211-222. [DOI] [PubMed] [Google Scholar]
- 3.Bartrolí, J., E. Turmo, E. E. Algueró, M. L. Bomcompte, L. Vericat, J. Conte, J. Ramis, M. Merlos, J. Garcia-Rafanell, and J. Forn. 1998. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 41:1869-1882. [DOI] [PubMed] [Google Scholar]
- 4.Brener, Z. 1984. Recent advances in the chemoterapy of Chagas disease. Mem. Inst. Oswaldo Cruz 79:149-155. [Google Scholar]
- 5.Britto, C., M. A. Cardoso, P. Wincker, and C. M. A. Morel. 1993. Simple protocol for cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR) based diagnosis of chronic Chagas' disease. Mem. Inst. Oswaldo Cruz 88:171-172. [DOI] [PubMed] [Google Scholar]
- 6.Cançado, J. R. 1980. Forma aguda da doença de Chagas no Brasil. Rev. Assoc. Med. Bras. 26:285-288. [PubMed] [Google Scholar]
- 7.Chiari, E., J. C. P. Dias, M. Lana, and C. A. Chiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 8.Dias, J. C. P., A. C. Silveira, and C. J. Schofield. 2002. The impact of Chagas disease control in Latin America. Mem. Inst. Oswaldo Cruz 97:603-612. [DOI] [PubMed] [Google Scholar]
- 9.Docampo, R. 1990. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem. Biol. Interact. 73:1-27. [DOI] [PubMed] [Google Scholar]
- 10.Dutra, W. O., Z. M. Luz, J. R. Cançado, M. E. Pereira, R. M. Brigido-Nunes, L. M. C. Galvão, D. G. Colley, Z. Brener, G. Gazzinelli, and J. F. Carvalho-Parra. 1996. Influence of parasite presence on the immunologic profile of peripheral blood mononuclear cells from chagasic patients after specific drug therapy. Parasite Immunol. 18:579-585. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira, H. O. 1990. Tratamento da forma indeterminada da doença de Chagas com nifurtimox e benznidazol. Rev. Soc. Bras. Med. Trop. 23:209-211. [DOI] [PubMed] [Google Scholar]
- 12.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 13.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. J. Pena, L. M. C. Galvão, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 14.Guedes, P. M. M., V. M. Veloso, W. L. Tafuri, L. M. C. Galvão, C. M. Carneiro, M. Lana, E. Chiari, K. A Soares, and M. T. Bahia. 2002. The dogs as model for chemoterapy of the Chagas disease. Acta Trop. 84:9-17. [DOI] [PubMed] [Google Scholar]
- 15.Lana, M., and C. A. Chiari. 1986. Caracterização biológica comparativa das cepas Berenece-78 de Trypanosoma cruzi, isoladas da mesma paciente em diferentes períodos. Mem. Inst. Oswaldo Cruz 81:247-253. [DOI] [PubMed] [Google Scholar]
- 16.Lazardi, K., J. A. Urbina, and W. de Souza. 1991. Ultrastructural alterations induced by ICI-195739, a bis-triazole derivative with strong antiproliferative action against Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 35:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liendo, A., K. Lazardi, and J. A. Urbina. 1998. In-vitro antiproliferative effects and mechanism of action of the bis-triazole D0870 and its S(−) enantiomer against Trypanosoma cruzi. J. Antimicrob. Chemother. 41:197-205. [DOI] [PubMed] [Google Scholar]
- 18.Molina, J. T., Z. Brener, J. A. Urbina, and A. J. Romanha. 2000. Activity of TAK-187 triazole on mice infected with Trypanosoma cruzi stains differently susceptible to benznidazole. Mem. Inst. Oswaldo Cruz 95:304-308. [Google Scholar]
- 19.Molina, J. T., O. A. Martins-Filho, Z. Brener, A. J. Romanha, D. Loebenberg, and J. A. Urbina. 2000. Activities of the triazole derivative SCH 56592 (posoconazole) against drug-resistant strains of the protozoan parasite Trypanosoma cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob. Agents Chemother. 44:150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassi, A., and A. O. Luquetti. 1992. Therapy of Chagas disease, p. 237-247. In S. Wendel, Z. Brener, E. Camargo, and A. Rassi, (ed.), Chagas diseases (American trypanosomiasis): its impacts on transfusion and clinical medicine. ISBT, São Paulo, Brazil.
- 21.Rassi, A., V. Amato Neto, A. F. De Siqueira, F. Ferriolli Filho, V. S. Amato, and A. Rassi, Jr. 1999. Protective effects of benznidazole against parasite reactivation in patients chronically infected with Trypanosoma cruzi and treated with corticoids for associated diseases. Rev. Soc. Bras. Med. Trop. 32:475-482. [DOI] [PubMed] [Google Scholar]
- 22.Santos, F. R., S. D. J. Pena, and J. T. Epplen. 1993. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum. Genet. 90:655-656. [DOI] [PubMed] [Google Scholar]
- 23.Schofield, C. J., and J. C. P. Dias. 1999. The Southern Cone Initiative against Chagas disease. Adv. Parasitol. 42:1-27. [DOI] [PubMed] [Google Scholar]
- 24.Silva, L. H. P., and V. Nussenzweig. 1953. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin. Biol. 20:191-203. [Google Scholar]
- 25.Snedecor, G. W., and W. G. Cochran. 1989. Statistical methods, 8th ed. Iowa State University Press, Ames.
- 26.Suasnábar, F., E. Arias, and M. Streiger. 2000. Evolutive behaviour towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev. Inst. Med. Trop. São Paulo 42:99-109. [DOI] [PubMed] [Google Scholar]
- 27.Toledo, M. J., M. T. Bahia, C. M. Carneiro, O. A. Martins-Filho, M. Tibayrenc, C. Barnabé, W. L. Tafuri, and M. Lana. 2003. Chemotherapy with benznidazole or itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob. Agents Chemother. 47:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbina, J. A. 2002. Chemotherapy of Chagas disease. Curr. Pharm. Design 8:287-295. [DOI] [PubMed] [Google Scholar]
- 29.Urbina, J. A., R. Lira, G. Visbal, and J. Bartroli. 2000. In vitro antiproliferative effects and mechanism of action of the new triazole derivative UR-9825 against the protozoan parasite Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 44:2498-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbina, J. A., E. Marchan, K. Lazardi., G. Visbal, R. Apitz-Castro, and F. Gil. 1993. Inhibition of phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruzi by ajoene, an antiplatelet compound isolated from garlic. Biochem. Pharmacol. 45:2381-2387. [DOI] [PubMed] [Google Scholar]
- 31.Urbina, J. A., G. Payares, L. M. Contreras, A. Liendo, C. Sanoja, J. Molina, M. M. Piras, R. Piras, N. Perez, P. Wincker, and D. Loebenberg. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbina, J. A., G. Payares, J. Molina, C. Sanoja, A. Liendo, K. Lazardi, M. M. Piras, N. Perez, P. Wincker, and J. F. Ryley. 1996. Cure of short-and long-term experimental Chagas disease using D0870. Science 273:969-971. [DOI] [PubMed] [Google Scholar]
- 33.Urbina, J. A., G. Payares, C. Sanoja, J. Molina, R. Lira, Z. Brener, and A. J. Romanha. 2003. Parasitological cure of acute and chronic experimental Chagas disease using a long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int. J. Antimicrob. Agents 21:39-48. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Bossche, H., and P. Marichal. 1992. Azole antifungals mode of action, p. 25-40. In H. Yamaguchi, G. S. Kobayashi, and H. Takahashi (ed.), Recent progress in antifungal chemotherapy. Marcel Dekker, Inc., New York, N.Y.
- 35.Veloso, V. M., C. M. Carneiro, M. J. O. Toledo, M. Lana, E. Chiari, W. L. Tafuri, and M. T. Bahia. 2001. Variation in susceptibility to benznidazole in isolates derived from Trypanosoma cruzi parental strains. Mem. Inst. Oswaldo Cruz 96:105-111. [DOI] [PubMed] [Google Scholar]
- 36.Voller, A., D. E. Bidwell, and A. Bartlett. 1976. Enzyme immunoassays in diagnostic medicine. Theory and pratice. Bull. W. H. O. 53:55-65. [PMC free article] [PubMed] [Google Scholar]
- 37.Wincker, P., C. Britto, J. B. Pereira, M. A. Cardoso, W. Oelemann, and C. M. Morel. 1994. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg. 51:771-777. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 2002. Control of Chagas disease. Second Report of the WHO Expert Committee. Technical Report Series, no. 905. World Health Organization, Geneva, Switzerland.