Abstract
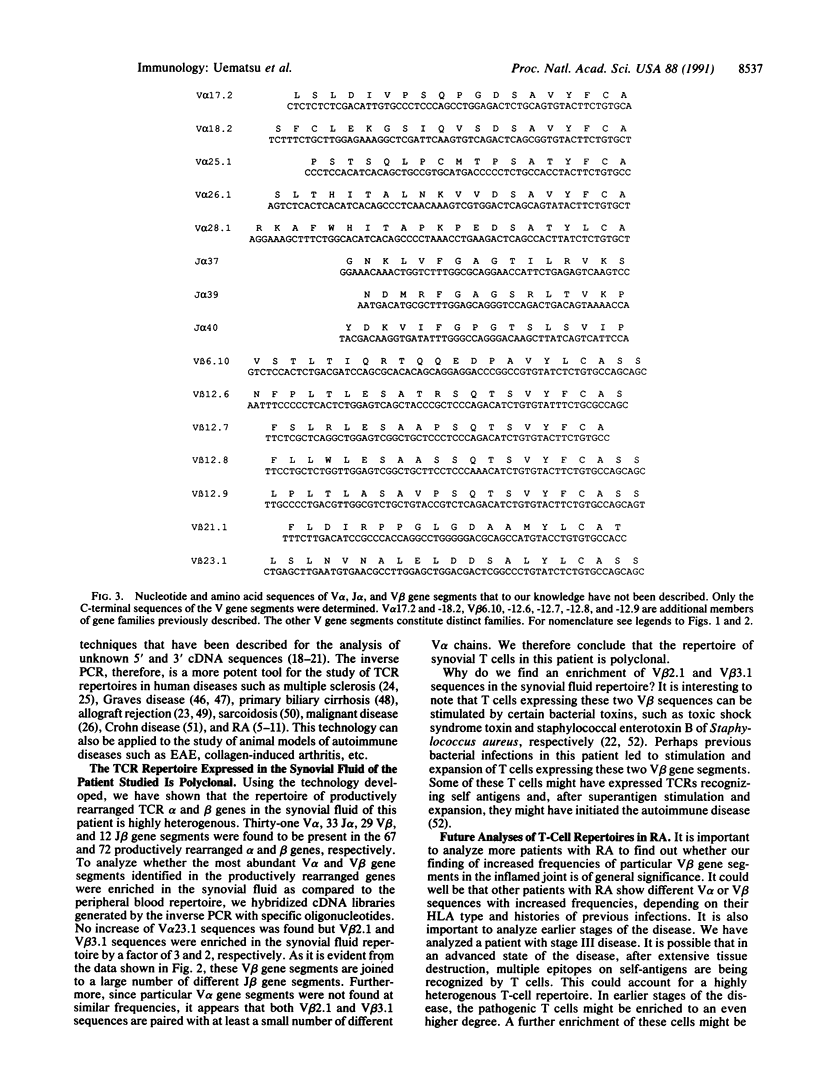
We have analyzed the T-cell-receptor repertoire expressed in the synovial fluid of a patient with rheumatoid arthritis by using an inverse polymerase chain reaction. Total RNA was isolated from Ficoll-purified mononuclear cells and converted into circularized double-stranded cDNA. Specific amplification of alpha- and beta-chain variable regions (V alpha and V beta) was achieved with inverted alpha- and beta-chain constant region (C alpha and C beta) primer pairs, and the amplification products were cloned into phage vectors. A total of 78 alpha and 76 beta clones were sequenced, and 67 and 72 productively rearranged alpha and beta genes were identified, respectively. Thirty-one V alpha, 33 alpha-chain joining region (J alpha), 29 V beta, and 12 beta-chain joining region (J beta) gene segments were found in the productively rearranged clones, indicating that the T-cell repertoire expressed in the synovial fluid of this RA patient is highly heterogenous and polyclonal. Comparison of peripheral blood and synovial fluid repertoires showed that the most abundant V beta sequences, V beta 2.1 and V beta 3.1, were enriched in the inflamed joint by a factor of 2 to 3. It is possible that T cells expressing these V beta gene segments, which recognize bacterial superantigens, play a role in the disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Baer R., Lefranc M. P., Minowada J., Forster A., Stinson M. A., Rabbitts T. H. Organization of the T-cell receptor alpha-chain gene and rearrangement in human T-cell leukaemias. Mol Biol Med. 1986 Jun;3(3):265–277. [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Bragado R., Lauzurica P., López D., López de Castro J. A. T cell receptor V beta gene usage in a human alloreactive response. Shared structural features among HLA-B27-specific T cell clones. J Exp Med. 1990 Apr 1;171(4):1189–1204. doi: 10.1084/jem.171.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Allard S., Londei M., Savill C., Boylston A., Carrel S., Maini R. N., Feldmann M. Heterogeneity of T cell receptor idiotypes in rheumatoid arthritis. Clin Exp Immunol. 1988 Sep;73(3):417–423. [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Demaine A. G., Ratanachaiyavong S., Pope R., Ewins D., Millward B. A., McGregor A. M. Thyroglobulin antibodies in Graves' disease are associated with T-cell receptor beta chain and major histocompatibility complex loci. Clin Exp Immunol. 1989 Jul;77(1):21–24. [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby A. D., Sinclair A. K., Osborne-Lawrence S. L., Zeldes W., Kan L., Fox D. A. Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6206–6210. doi: 10.1073/pnas.86.16.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradini L., Roman-Roman S., Azocar J., Michalaki H., Triebel F., Hercend T. Studies on the human T cell receptor alpha/beta variable region genes. II. Identification of four additional V beta subfamilies. Eur J Immunol. 1991 Apr;21(4):935–942. doi: 10.1002/eji.1830210412. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi P., Davi F., d'Auriol L., Bories J. C., Dausset J., Bensussan A. Use of a variable alpha region to create a functional T-cell receptor delta chain. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5634–5638. doi: 10.1073/pnas.85.15.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S., Brenner M. B., Krangel M. S. Identification of putative human T cell receptor delta complementary DNA clones. Science. 1987 Oct 30;238(4827):678–682. doi: 10.1126/science.3499667. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989 Nov 3;246(4930):668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Minden M., Klock R., Poplonski L., Zalcberg J., Takadera T., Mak T. W. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Dec;31(12):1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Kimura N., Toyonaga B., Yoshikai Y., Du R. P., Mak T. W. Sequences and repertoire of the human T cell receptor alpha and beta chain variable region genes in thymocytes. Eur J Immunol. 1987 Mar;17(3):375–383. doi: 10.1002/eji.1830170312. [DOI] [PubMed] [Google Scholar]
- Klein M. H., Concannon P., Everett M., Kim L. D., Hunkapiller T., Hood L. Diversity and structure of human T-cell receptor alpha-chain variable region genes. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6884–6888. doi: 10.1073/pnas.84.19.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H., Imai K., Nakayama F., Sado T., Moriwaki K., Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5248–5252. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L., Willard H. F., Smith C. I., Hammarström L., Fischer P., Sherrington P., Lucas G., Thompson P. W., Baer R., Rabbitts T. H. Human T-cell tumours containing chromosome 14 inversion or translocation with breakpoints proximal to immunoglobulin joining regions at 14q32. EMBO J. 1987 Aug;6(8):2273–2280. doi: 10.1002/j.1460-2075.1987.tb02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli M. C., Finn O. J. T cell receptor beta-chain selection in human allograft rejection. J Immunol. 1989 Jan 1;142(1):81–86. [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Moebius U., Manns M., Hess G., Kober G., Meyer zum Büschenfelde K. H., Meuer S. C. T cell receptor gene rearrangements of T lymphocytes infiltrating the liver in chronic active hepatitis B and primary biliary cirrhosis (PBC): oligoclonality of PBC-derived T cell clones. Eur J Immunol. 1990 Apr;20(4):889–896. doi: 10.1002/eji.1830200426. [DOI] [PubMed] [Google Scholar]
- Moller D. R., Konishi K., Kirby M., Balbi B., Crystal R. G. Bias toward use of a specific T cell receptor beta-chain variable region in a subgroup of individuals with sarcoidosis. J Clin Invest. 1988 Oct;82(4):1183–1191. doi: 10.1172/JCI113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Oksenberg J. R., Rao N. A., Steinman L. Predominant expression of T cell receptor V alpha 7 in tumor-infiltrating lymphocytes of uveal melanoma. Science. 1990 Aug 10;249(4969):672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Gottlieb A., Bussel J. B., Friedman S. M., Chiorazzi N., Li Y., Szabo P., Farid N. R., Robinson M. A. T cell antigen receptors in autoimmunity. J Immunol. 1988 Sep 15;141(6):1963–1969. [PubMed] [Google Scholar]
- Posnett D. N., Schmelkin I., Burton D. A., August A., McGrath H., Mayer L. F. T cell antigen receptor V gene usage. Increases in V beta 8+ T cells in Crohn's disease. J Clin Invest. 1990 Jun;85(6):1770–1776. doi: 10.1172/JCI114634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Potts W., Wakeland E. K., Kappler J., Marrack P. Surprisingly uneven distribution of the T cell receptor V beta repertoire in wild mice. J Exp Med. 1990 Jan 1;171(1):49–62. doi: 10.1084/jem.171.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Roman S., Ferradini L., Azocar J., Genevée C., Hercend T., Triebel F. Studies on the human T cell receptor alpha/beta variable region genes. I. Identification of 7 additional V alpha subfamilies and 14 J alpha gene segments. Eur J Immunol. 1991 Apr;21(4):927–933. doi: 10.1002/eji.1830210411. [DOI] [PubMed] [Google Scholar]
- Savill C. M., Delves P. J., Kioussis D., Walker P., Lydyard P. M., Colaco B., Shipley M., Roitt I. M. A minority of patients with rheumatoid arthritis show a dominant rearrangement of T-cell receptor beta chain genes in synovial lymphocytes. Scand J Immunol. 1987 Jun;25(6):629–635. doi: 10.1111/j.1365-3083.1987.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Shiroky J. B., Yocum D. E., Wilder R. L., Klippel J. H. Experimental basis of innovative therapies of rheumatoid arthritis. Concepts Immunopathol. 1989;7:106–144. [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah S., Harrison S. C. A method for multiple sequence alignment with gaps. J Mol Biol. 1989 Oct 20;209(4):539–548. doi: 10.1016/0022-2836(89)90592-5. [DOI] [PubMed] [Google Scholar]
- Teng W. P., Cohen S. B., Posnett D. N., Weetman A. P. T cell receptor phenotypes in autoimmune thyroid disease. J Endocrinol Invest. 1990 Apr;13(4):339–342. doi: 10.1007/BF03349575. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Wilson R. K., Lai E., Concannon P., Barth R. K., Hood L. E. Structure, organization and polymorphism of murine and human T-cell receptor alpha and beta chain gene families. Immunol Rev. 1988 Jan;101:149–172. doi: 10.1111/j.1600-065x.1988.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zauderer M., Natarajan K. Imprint of thymic selection on autoreactive repertoires. Immunol Rev. 1990 Aug;116:159–170. doi: 10.1111/j.1600-065x.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]



