Abstract
A modified fixed-ratio isobologram method for studying the in vitro interactions between antiplasmodial drugs is described. This method was used to examine the interactions between atovaquone, proguanil, and dihydroartemisinin. The interaction between atovaquone and proguanil was synergistic against atovaquone-sensitive strains K1 and T996; however, there was a loss of synergy against atovaquone-resistant strain NGATV01 isolated after Malarone (the combination of atovaquone and proguanil) treatment failure. While the interaction between atovaquone and dihydroartemisinin was indifferent against isolate NGATV01, the interaction displayed indifference tending toward antagonism against the atovaquone-sensitive strains tested. The relevance of in vitro interactions to in vivo treatment is discussed.
Combination drug regimens for the treatment of cancer, AIDS, and tuberculosis often achieve a therapeutic efficacy greater than that achieved with monotherapy. Other benefits may include decreased toxicity, the delay or prevention of drug resistance development, and the favorable effects of synergistic drug interactions. Antimalarial drug resistance is becoming a major public health disaster in many areas of the tropical world (31). In the search for effective combination regimens, Malarone (the combination of atovaquone and proguanil), LapDap (the combination of chlorproguanil and dapsone), and Coartem (the combination of artemether and lumefantrine) have recently become available or are in development. Other combination therapies involving artemisinin derivatives such as artesunate-mefloquine have many benefits and are thought to be very effective against drug resistance (31). It is hoped that combination chemotherapy will delay the onset of resistance to new agents and reduce the effects of resistance to existing agents (24, 30).
In vitro interactions between antiplasmodials, as represented in isobolograms, provide an essential background for clinical studies. However, they do not necessarily determine the efficacy of a combination in the host, since this also depends on pharmacokinetic characteristics. Synergism, indifference (addition), and antagonism are the expected outcomes of drug-drug interactions. The antimalarial Malarone (GlaxoSmithKline) is a recently introduced combination regimen for the treatment (18, 19) and prophylaxis (14, 23) of falciparum malaria. It has been previously reported that the synergistic interaction between the components of Malarone, atovaquone (ATV) and proguanil (PG), is reduced to an indifferent interaction in ATV-resistant Plasmodium yoelii parasites (25). However, this interaction has been demonstrated to remain synergistic in ATV-resistant isolate C2B of P. falciparum derived from a clinical trial of ATV alone, although the parasites showed a 95-fold decrease in sensitivity to ATV compared to that of the pretreatment isolate (2). Despite recent reports of Malarone treatment failures (7, 8), no studies have investigated the in vitro interaction between these two drugs against isolates from patients with Malarone clinical treatment failures. In the present study we investigated the interaction between ATV and PG or dihydroartemisinin (DHA), the active metabolite of artesunate, against a recently isolated ATV-resistant strain, NGATV01, from a patient who failed such treatment (8) using a newly developed fixed-ratio procedure for the in vitro assessment of the interaction of two drugs against P. falciparum.
MATERIALS AND METHODS
Parasite cultivation.
Malaria parasites were continuously cultured (29) and maintained with type A-positive erythrocytes suspended in complete culture medium (pH 7.3), which consisted of filter-sterilized RPMI 1640 solution supplemented with 0.01 M gentamicin, 2 g of sodium bicarbonate (BDH, Dorset, United Kingdom) per liter, and 10% pooled type AB-positive human serum (The National Blood Service, Bristol and Colindale, United Kingdom). Incubation was at 37°C under a gas phase of 3% O2, 4% CO2, and 93% N2 (BOC Gases, Manchester, United Kingdom). The levels of parasitemia in the cultures were kept at between 2 and 10%, with 5% hematocrit.
Drug solution preparation.
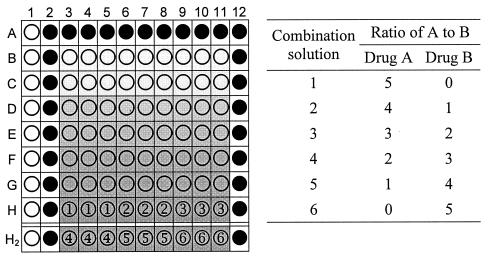
Stock solutions of the drugs were prepared at 10 mg/ml in dimethyl sulfoxide, which, once diluted, had no effect on parasite growth at a final concentration of <0.05%. On the day of the experiment dilutions were prepared from stock solutions with serum-free medium. Dose-response assays were first carried out to obtain the 50% inhibitory concentration (IC50s) of the individual drugs, drug A and drug B. For the combination assay, drug dilutions were made to allow the IC50 of the individual drugs to fall at about the fourth twofold serial dilution. The dilutions of each of the two drugs were then prepared in fixed ratios (Fig. 1). For example, solutions one to six for ATV-PG in assays with T996 and K1 were prepared at 10:0, 8:5,000, 6:10,000, 4:15,000, 2:20,000, and 0:25,000, respectively (concentration ratios of ATV to PG in nanograms per milliliter, with the first and last solutions being each drug alone). Drug dilutions were prepared in sterile flat-bottom 96-well microtiter plates (Techno Plastic Products AG, Trasadingen, Switzerland) across rows from a row of wells containing twice the desired final concentration (Fig. 1, row H).
FIG. 1.
Layout of a combination experiment on a 96-well plate with the concentration ratios of drug A to drug B prepared as six solutions. When the plates were prepared as described in the text, clear wells serve as an RBC control (no drug and no parasites; 100% growth inhibition), black wells serve as a parasite control (no drug; 0% growth inhibition), and wells labeled 1 to 6 serve as drug wells for six drug combination solutions, in triplicate, with the wells in row H holding the highest drug concentration. Both 96-well plates are prepared similarly, with row H2 representing solutions 4 to 6 in the second 96-well plate.
Validating the fixed-ratio method.
To validate the indifferent effect, the activities of artemisinin and DHA in combination against chloroquine-resistant strain 7G8 and chloroquine-sensitive strain FC27 were assayed. Verapamil combined with chloroquine (21) and quinine combined with chloroquine (28) were assayed against chloroquine-resistant strain K1 to validate synergism and antagonism, respectively.
Plate preparation for drug combination assay.
A final 1% parasitemia at 1% hematocrit was used in a total volume of 200 μl of the drug, blood, and medium mixture per well. The controls, drug-free unparasitized erythrocytes (RBC) and parasitized erythrocytes (pRBC), were similarly prepared. The combination assay was carried out in triplicate with the RBC blank control in column 1, rows A to H, of a 96-well flat-bottom microtiter plate (Fig. 1). The outer wells in row A, columns 2 to 11, and columns 2 and 12, rows A to H, contained pRBC controls, although column 12 was not used for the IC50 calculations. Two hundred microliters of the top concentration of each drug or drug combination was placed, in triplicate, in row H, columns 3 to 11. One hundred microliters of complete medium was added to all wells except those in row H, columns 3 to 11. The combination solutions were serially diluted with a multichannel pipette from row H to row B, with 100 μl transferred each time after a thorough mixing and discarding of the last 100 μl from the wells in row B. The wells in column 1, rows A to H, received 100 μl of RBC suspension, and the remaining wells received 100 μl of a pRBC suspension. Two plates were used to test the six drug solutions (Fig. 1, row H2). The plates were stacked in a sterile modular chamber, gassed for 3 min with the gas mixture mentioned above, and incubated at 37°C for 20 to 24 h. Incubation was briefly halted to allow dosing of each test well with 10 μl of [3H]hypoxanthine (Amersham, Little Chalfont, United Kingdom) to a final concentration of 0.2 μCi/well (5). Then the plates were regassed and incubated for another 24 h, after which they were stored at −80°C until harvested. Each combination experiment was carried out at least twice.
IC50 or IC90 determination and isobologram construction.
Plates were removed from cold storage, thawed at room temperature, and harvested onto Wallac printed glass fiber filter mats (Perkin-Elmer, Beaconsfield, United Kingdom) with a 96-well cell harvester (Tomtec, Hamden, Conn.). The filter mats were dried for an hour at 55°C. Each filter mat was placed in a sample bag containing 4.5 ml of Wallac Betaplate liquid scintillation fluid (Perkin-Elmer), and the bag was heat sealed. The amount of radioactivity (in counts per minute) of the [3H]hypoxanthine incorporated into the parasitized material trapped in the mat relative to the amount incorporated by the untreated controls was determined with a Wallac 1450 Microbeta scintillation β-counter (Perkin-Elmer). Data were transferred to Excel software (Microsoft Inc.), and the IC50s along with the standard error of the mean were calculated with the XLfit software (ID Business Solutions Ltd., Guildford, United Kingdom) Excel add-on in the nonlinear dose-response curve mode. Two IC50s for each of the four combination curves were calculated separately by using the known concentration ratios of both drugs A and B, the fractional inhibitory concentration of drug A (FICA) and FICB were calculated for each point, and isobolograms were plotted. The unpaired Student's t test of SigmaPlot 2000 software (SPSS Inc., Chicago, Ill.) was used to compare the mean FICs for two parasite strains and to calculate 95% confidence intervals. In order to calculate the FIC90s, the dose-response curves were plotted with the same data used to plot the FIC50s. The IC90s obtained were then used to calculate the mean FICs, as described above.
RESULTS
In vitro dose-response assay.
The mean IC50s for nine P. falciparum strains examined for their sensitivities to ATV, PG, and DHA are presented in Table 1. The ATV resistance status of isolate NGATV01 was confirmed, and ATV was shown to be an extremely potent antimalarial, although there was about an eightfold range of ATV IC50s for the strains. There was a large difference between the PG IC50s for the five strains tested. The PG IC50 for NGATV01 was about fourfold lower than those for T996 and K1. However, this value was not significantly lower than the PG IC50 for RSA11, a line sensitive to ATV.
TABLE 1.
In vitro sensitivities of isolate NGATV01 and other strains to antimalarial drugs ATV, PG, and DHAa
| Chloroquine sensitivity and strain | Mean IC50 ± SEM (nmol/liter)
|
||
|---|---|---|---|
| ATV | DHA | PG | |
| Sensitive | |||
| NGATV01 | 2,987.26 ± 116.13 | 3.83 ± 0.27 | 8,430.97 ± 552.76 |
| T996 | 1.37 ± 0.03 | 3.46 ± 0.12 | 37,956.66 ± 3,610.06 |
| D10 | 1.06 ± 0.12 | 3.76 ± 0.36 | 17,628.33 ± 516.90 |
| 3D7 | 0.84 ± 0.08 | 3.55 ± 0.71 | NDb |
| FC27 | 0.66 ± 0.09 | 2.24 ± 0.66 | ND |
| Resistant | |||
| K1 | 2.41 ± 0.51 | 1.67 ± 0.43 | 34,266.72 ± 2,893.64 |
| RSA11 | 1.24 ± 0.08 | 1.98 ± 0.16 | 11,463.34 ± 1,871.39 |
| 7G8 | 4.53 ± 0.03 | 2.30 ± 0.14 | ND |
| B303 | 0.56 ± 0.13 | 1.16 ± 0.06 | ND |
The drug assay was performed at 1% parasitemia and 1% hematocrit. The experiment was repeated at least twice, in triplicate.
ND, not determined.
Fixed-ratio method validation.
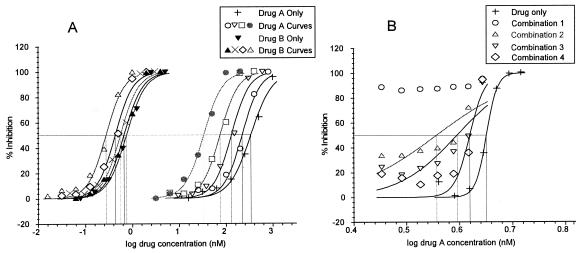
For the combination assays, the top concentrations of the six solutions were prepared to allow the IC50 of the individual drug to fall around the midpoint in a twofold serial dilution (wells in row E or D in Fig. 1), and six dose-response curves were created. Of these resulting six dose-response curves, two represented the two drugs alone (solutions 1 and 6) and the remaining four each had a component relating to each of the two drugs in the combination. Although there are only four combination curves, one can construct eight dose-response curves using the same percent inhibition data, but over two different concentration ranges relating to each of the two drugs combined. Ultimately, two sets of five dose-response curves are obtained: four curves relating to the drugs combined and one curve relating to the drug alone (Fig. 2A). The IC50s (or IC90s) can then be calculated and used to construct an isobologram. The fixed-ratio validation results were consistent with published results obtained by other methods (data not shown). The procedure was easy to follow, and the mean FIC50s and FIC90s of a particular combination were consistent between experiments.
FIG. 2.
(A) Typical dose-response curves produced by the fixed-ratio method. In this example an indifferent interaction is demonstrated. (B) One set of typical dose-response curves obtained by the checkerboard method. In this example, the concentration of drug A was constant and that of drug B varied.
Interaction of ATV with DHA.
The mean sums of the FICs of the interaction of ATV with DHA are shown in Table 2. The interaction between ATV and DHA was indifferent but tended toward antagonism against all the strains except strain NGATV01 by using both FIC50 and FIC90 calculations. The indifferent interaction against the ATV-resistant isolate (NGATV01) differed significantly (P < 0.05) from that seen against the remaining eight strains.
TABLE 2.
Mean FIC of the interactions between ATV and DHA or PG with 95% confidence intervals
| Chloroquine sensitivity and strain | ATV-DHA
|
Mean FIC50 (95% CI) of ATV-PGa | |
|---|---|---|---|
| Mean FIC50 (95% CI)a | Mean FIC90 (95% CI)b | ||
| Sensitive | |||
| NGATV01 | 0.96c (0.90-1.02) | 0.89c (0.76-1.02) | 0.80c (0.75-0.85) |
| T996 | 1.35 (1.18-1.52) | 1.49 (1.01-1.97) | 0.29 (0.21-0.37) |
| D10 | 1.17 (1.09-1.25) | 1.29 (1.10-1.48) | Not determined |
| 3D7 | 1.17 (1.08-1.26) | 1.23 (1.13-1.33) | Not determined |
| FC27 | 1.14 (1.04-1.24) | 1.60 (1.27-1.93) | Not determined |
| Resistant | |||
| K1 | 1.51 (1.30-1.72) | 1.42 (1.20-1.64) | 0.17 (0.13-0.21) |
| RSA11 | 1.36 (1.25-1.47) | 1.64 (1.38-1.90) | Not determined |
| 7G8 | 1.45 (1.32-1.58) | 1.43 (1.26-1.60) | Not determined |
| B303 | 1.27 (1.08-1.46) | 1.40 (1.25-1.55) | Not determined |
Mean FIC obtained with IC50 with 95% confidence intervals (CI).
Mean FIC obtained with IC90 with 95% confidence intervals (CI).
A significantly different result in comparison with the determinations for the other strains (P < 0.05).
Interaction of ATV with PG.
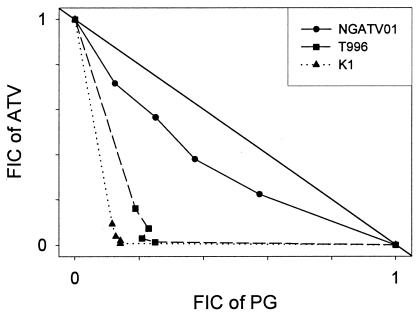
The isobolograms for the interactions between ATV and PG are presented in Fig. 3, and the mean sums of the FICs are presented in Table 2. The interaction against chloroquine-sensitive strain T996 and chloroquine-resistant strain K1 was synergistic. The drugs displayed a significantly lesser synergistic interaction against isolate NGATV01 than against isolate T996 (P = 0.011) or isolate K1 (P = 0.007).
FIG. 3.
Isobolograms showing the interaction between ATV and PG against isolate NGATV01 and against strains T996 and K1. The numbers on the axes represent normalized FICs.
DISCUSSION
In vitro interaction methods.
The checkerboard method has been widely used to assess antimalarial drug interactions (2, 6, 13). Both the checkerboard and the fixed-ratio methods rely on predetermination of the IC50s of the component drugs, e.g., drug A and drug B alone. From these two values, a starting concentration of each drug is selected and solutions are prepared in various proportions of the starting concentrations to be serially diluted. In the checkerboard combination assay, the concentration of drug A is kept constant and that of drug B is varied, and vice versa. However, the fixed-ratio method uses serial dilutions of fixed ratios of both drugs; i.e., drug concentrations are varied at the same time over a predetermined concentration range (Fig. 1). The checkerboard procedure is highly reliant on accurate initial IC50s, and its utility is affected by day-to-day variations in this parameter, which may exceed the predetermined IC50, leading to a poor fit of the sigmoidal curve (e.g., Fig. 2B, combination curve 1). The points on the graph often cluster at the extremities of the axes in isobolograms showing synergy or antagonism, because the FIC of one of the drugs could tend toward 1 and that of the other could tend toward 0. The fixed-ratio method was originally developed for drug interaction studies with bacteria (12), but the principles are easily applied to P. falciparum. The method has advantages over the checkerboard method because the fixed-ratio dose-response curves depend on drug concentration ratios, each of which is calculated to range from 100 to 0% parasite inhibition, allowing a more accurate regression curve fit and IC50 calculation (Fig. 2A). The daily variations in IC50s do not dramatically affect the FIC calculations, and fewer calculation steps are also required.
Interaction of ATV with DHA or PG.
Antagonism between ATV and common antimalarials has been reported previously, including an antagonistic interaction between ATV and artesunate (2). ATV targets cytochrome b, which plays an important role in electron transport during mitochondrial respiration. It is thought that the drug, an analogue of coenzyme Q (ubiquinone), interrupts electron transport and leads to loss of the mitochondrial membrane potential (10, 26). Reports that artemisinin derivatives antagonize the actions of some antimalarials have been widely published (3, 9, 28), including a study of P. berghei and P. yoelii with a mouse model (4). Accumulation studies show that artemisinin derivatives accumulate in many parasite organelles, including the mitochondria (20), and the ultrastructural changes induced in P. falciparum by artemisinins include the early swelling of parasite mitochondria (15, 16, 20). The antimitochondrial effects of both ATV and DHA may bear some relationship to the antagonism that they exert, and it is interesting that in the present study an indifferent effect was seen against an ATV-resistant isolate with high ATV concentrations. ATV-resistant strains are reported to resist the ATV-mediated membrane potential collapse and electron transport inhibition (25). This suggests that at high concentrations ATV could have an alternative mode of action against resistant parasites compared to its mode of action at lower concentrations lethal to ATV-sensitive parasites. Using the checkerboard technique, Gupta et al. (11) reported an indifferent interaction and a synergistic interaction (obtained from IC50s and IC90s, respectively) between artemisinin and ATV in ATV-sensitive parasite lines. Although the investigators claimed an indifferent interaction, all the points on the isobologram lay above the line of indifference, which would be consistent with our results and those of Canfield et al. (2). Analysis of our results presented here, obtained by using IC90s to calculate the FIC, confirmed antagonism against all parasite lines (Table 2).
The interaction of ATV with PG against two ATV-sensitive parasite lines, T996 and K1, was synergistic, in agreement with previous reports (2, 27). PG on its own has no effect on electron transport or mitochondrial membrane potential, but it is thought to strongly enhance the ability of ATV to collapse the membrane potential, resulting in synergy (27). Canfield et al. (2) reported that the combination had a similar synergistic effect against ATV-resistant strain C2B, and this interaction was very similar to that seen against ATV-sensitive strains W2 and D6. The report of Srivastava et al. (25) on ATV-resistant P. yoelii strain AR1 shows that the addition of PG to ATV does not alter the mitochondrial membrane potential, leading those investigators to suggest that the interaction between the two drugs against this resistant strain is probably not synergistic. As our results indicate, the synergy against ATV-resistant strain NGATV01 was significantly lower than that against ATV-sensitive strains T996 and K1. P. yoelii strain AR1 has both cytb Leu271Val and Lys272Arg mutations (2), while P. falciparum NGATV01 has a Tyr268Asn mutation (8). The P. falciparum C2B strain, isolated from a patient displaying an R1-type failure to treatment with ATV alone, had ATV resistance 95 times that of the pretreatment isolate (2); but the mutations in the cytb gene were unreported. Another ATV-resistant P. falciparum isolate from a patient given ATV and pyrimethamine, TM93-C1088, with a cytb Tyr268Ser mutation, showed a high level of ATV resistance (17); but the effect of the combination of ATV and PG against the isolate was not tested. The ATV sensitivity of isolate NGATV01 in vitro was more than 2,000-fold less than that of the ATV-sensitive strains (Table 1). It is clear that the character of a mutation as well as its presence is important in determining the responses of parasite lines to the ATV-PG combination.
The rationale for combination chemotherapy for malaria has recently been reviewed (1, 22). Ideally, combination chemotherapy for malaria should take advantage of synergistic interactions, as these would enhance therapeutic efficacy and lower the risk of resistance emergence. If drugs in combination are antagonistic, the efficacies of such regimens might be compromised and the chances of resistance development and spread increase, as less effective drugs may be allowing weakly resistant clones to survive and be transmitted. It is difficult to predict in vivo drug interactions in humans on the basis of the findings of in vitro studies, although the findings from studies with animal models may be more predictive (4). Although certain drug combinations show antagonism in vitro, the effects are often not apparent in vivo. It should be pointed out that most in vitro drug interaction methodologies use IC50s to calculate the FICs; however, the IC90 or IC99 may be a more accurate indicator, as this is a better representation of the parasite population sensitive to the range of concentrations in plasma usually observed with a typical therapeutic dose (11). Although the high in vitro IC50s of PG alone in this study of drug combinations are probably irrelevant in pharmacological terms, the synergistic interaction between PG and ATV against ATV-sensitive strains is still valid, as the effect occurs at concentrations of both drugs that are achievable pharmacologically (27). The efficacy of Malarone is strongly dependent on the synergism between ATV and PG, and the inclusion of PG treatment with ATV allows ATV to effectively function at concentrations that would be suboptimal in vivo (27). Once ATV resistance is present, the potency of the combination is markedly reduced and can lead to treatment failure (8). In the example of an ATV-resistant isolate from a Malarone treatment failure described here, reduced efficacy in vivo could be predicted by reduced synergy in vitro.
Acknowledgments
We thank Geoff Butcher of Imperial College, London, United Kingdom, for originally isolating the NGATV01 parasite.
Quinton Fivelman was supported by the Association of Commonwealth Universities, Ipemida Adagu was supported by Romark Research Laboratory, and David Warhurst thanks the United Kingdom PHLS for financial support.
REFERENCES
- 1.Bloland, P. B., M. Ettling, and S. Meek. 2000. Combination therapy for malaria in Africa: hype or hope? Bull. W. H. O. 78:1378-1388. [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 3.Chawira, A. N., and D. C. Warhurst. 1987. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J. Trop. Med. Hyg. 90:1-8. [PubMed] [Google Scholar]
- 4.Chawira, A. N., D. C. Warhurst, B. L. Robinson, and W. Peters. 1987. The effect of combinations of qinghaosu (artemisinin) with standard antimalarial drugs in the suppressive treatment of malaria in mice. Trans. R. Soc. Trop. Med. Hyg. 81:554-558. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins, R., C. Canfield, J. Haynes, and J. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekong, R., and D. C. Warhurst. 1990. Synergism between arteether and mefloquine or quinine in a multidrug-resistant strain of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 84:757-758. [DOI] [PubMed] [Google Scholar]
- 7.Farnert, A., J. Lindberg, P. Gil, G. Swedberg, Y. Berqvist, M. M. Thapar, N. Lindegardh, S. Berezcky, and A. Bjorkman. 2003. Evidence of Plasmodium falciparum malaria resistant to atovaquone and proguanil hydrochloride: case reports. BMJ 326:628-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fivelman, Q. L., G. A. Butcher, I. S. Adagu, D. C. Warhurst, and G. Pasvol. 2002. Malarone treatment failure and in vitro confirmation of resistance of Plasmodium falciparum isolate from Lagos, Nigeria. Malar. J. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fivelman, Q. L., J. C. Walden, P. J. Smith, P. I. Folb, and K. I. Barnes. 1999. The effect of artesunate combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 93:429-432. [DOI] [PubMed] [Google Scholar]
- 10.Fry, M., and M. Pudney. 1992. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43:1545-1553. [DOI] [PubMed] [Google Scholar]
- 11.Gupta, S., M. M. Thapar, W. H. Wernsdorfer, and A. Bjorkman. 2002. In vitro interactions of artemisinin with atovaquone, quinine, and mefloquine against Plasmodium falciparum. Antimicrob. Agents Chemother. 46:1510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall, M. J., R. F. Middleton, and D. Westmacott. 1983. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 11:427-433. [DOI] [PubMed] [Google Scholar]
- 13.Hassan Alin, M., A. Bjorkman, and W. H. Wernsdorfer. 1999. Synergism of benflumetol and artemether in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 61:439-445. [DOI] [PubMed] [Google Scholar]
- 14.Hogh, B., P. D. Clarke, D. Camus, H. D. Nothdurft, D. Overbosch, M. Gunther, I. Joubert, K. C. Kain, D. Shaw, N. S. Roskell, and J. D. Chulay. 2000. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in non-immune travellers: a randomised, double-blind study. Lancet 356:1888-1894. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, J. B., G. Jacobs, D. S. Liang, and M. Aikawa. 1985. Qinghaosu-induced changes in the morphology of Plasmodium inui. Am. J. Trop. Med. Hyg. 34:424-428. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, S., S. Kano, and M. Suzuki. 1993. Morphologic effects of artemether on Plasmodium falciparum in Aotus trivirgatus. Am. J. Trop. Med. Hyg. 49:812-818. [DOI] [PubMed] [Google Scholar]
- 17.Korsinczky, M., N. Chen, B. Kotecka, A. Saul, K. Rieckmann, and Q. Cheng. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob. Agents Chemother. 44:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llanos-Cuentas, A., P. Campos, M. Clendenes, C. J. Canfield, and D. B. Hutchinson. 2001. Atovaquone and proguanil hydrochloride compared with chloroquine or pyrimethamine/sulfadoxine for treatment of acute Plasmodium falciparum malaria in Peru. Braz. J. Infect. Dis. 5:67-72. [DOI] [PubMed] [Google Scholar]
- 19.Looareesuwan, S., J. D. Chulay, C. J. Canfield, and D. B. Hutchinson. 1999. Malarone (atovaquone and proguanil hydrochloride): a review of its clinical development for treatment of malaria. Am. J. Trop. Med. Hyg. 60:533-541. [DOI] [PubMed] [Google Scholar]
- 20.Maeno, Y., T. Toyoshima, H. Fujioka, Y. Ito, S. R. Meshnick, A. Benakis, W. K. Milhous, and M. Aikawa. 1993. Morphologic effects of artemisinin in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 49:485-491. [DOI] [PubMed] [Google Scholar]
- 21.Martin, S. K., A. M. Oduola, and W. K. Milhous. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899-901. [DOI] [PubMed] [Google Scholar]
- 22.Nosten, F., and P. Brasseur. 2002. Combination therapy for malaria: the way forward? Drugs 62:1315-1329. [DOI] [PubMed] [Google Scholar]
- 23.Overbosch, D., H. Schilthuis, U. Bienzle, R. H. Behrens, K. C. Kain, P. D. Clarke, S. Toovey, J. Knobloch, H. D. Nothdurft, D. Shaw, N. S. Roskell, and J. D. Chulay. 2001. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin. Infect. Dis. 33:1015-1021. [DOI] [PubMed] [Google Scholar]
- 24.Peters, W. 1999. The chemotherapy of rodent malaria. LVII. Drug combinations to impede the selection of drug resistance. Part 1. Which model is appropriate? Ann. Trop. Med. Parasitol. 93:569-587. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava, I. K., J. M. Morrisey, E. Darrouzet, F. Daldal, and A. B. Vaidya. 1999. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 33:704-711. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava, I. K., H. Rottenberg, and A. B. Vaidya. 1997. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 272:3961-3966. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava, I. K., and A. B. Vaidya. 1999. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob. Agents Chemother. 43:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahel, E., P. Druilhe, and M. Gentilini. 1988. Antagonism of chloroquine with other antimalarials. Trans. R. Soc. Trop. Med. Hyg. 82:221. [DOI] [PubMed] [Google Scholar]
- 29.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 30.White, N. J. 1998. Preventing antimalarial drug resistance through combinations. Drug Res. Updates 1:3-9. [DOI] [PubMed] [Google Scholar]
- 31.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]