Abstract
The collectin pentraxin 3 (PTX3) is an essential component of host resistance to pulmonary aspergillosis. Here we examined the protective effects of administration of PTX3 alone or together with deoxycholate amphotericin B (Fungizone) or liposomal amphotericin B (AmBisome) against invasive aspergillosis in a murine model of allogeneic bone marrow transplantation. PTX3, alone or in combination with the polyenes, was given intranasally or parenterally either before, in concomitance with, or after the intranasal infection with Aspergillus fumigatus conidia. Mice were monitored for resistance to infection and parameters of innate and adaptive T-helper immunity. The results showed the following: (i) complete resistance to infection and reinfection was observed in mice treated with PTX3 alone; (ii) the protective effect of PTX3 was similar or superior to that observed with liposomal amphotericin B or deoxycholate amphotericin B, respectively; (iii) protection was associated with accelerated recovery of lung phagocytic cells and T-helper-1 lymphocytes and concomitant decrease of inflammatory pathology; and (iv) PTX3 potentiated the therapeutic efficacy of suboptimal doses of either antimycotic drug. Together, these data suggest the potential therapeutic use of PTX3 either alone or as an adjunctive therapy in A. fumigatus infections.
Invasive aspergillosis (IA) is the leading cause of both nosocomial pneumonia and death in allogeneic bone marrow (BM) transplantation, with an estimated infection rate ranging between 8 to 15% and an associated mortality rate of approximately 90% (16, 29, 36, 47). Despite advances in early diagnosis and new antifungal agents (30), the majority of cases of IA remain undiagnosed and untreated at death (16). The most important risk factor for IA has historically been neutropenia (27). However, modifications in the chemotherapeutic preparative regimens and the transplanted grafts have resulted in a significant shortening of the period of neutropenia. Multiple studies have documented that aspergillosis now typically occurs late after BM transplantation, in concomitance with the occurrence of graft-versus-host disease (29). These findings, together with the occurrence in nonneutropenic patients (17), attest to the importance of specific defects in both innate and adaptive immune effector mechanisms in the pathogenesis of the disease (13, 20, 22, 39, 44). In particular, the role of Th lymphocytes in providing a critical secondary defense against the fungus has recently been appreciated (8-10, 12, 14, 23, 26). Because IA is extremely rare in immunocompetent individuals, therapy aimed at strengthening the host immune response offers a promising new approach in the treatment of this infection.
A complex, multifaceted innate immune system has evolved to protect the lung. The optimal defensive strategy in the lung would include not only preemptive control of microbial proliferation and immediate clearance but also the execution of a finely tuned inflammatory response, one that is sufficient to contain the infection without inducing harmful degrees of alveolar exudation and alveolar infiltration. Components of the surfactant lining layer have recently received increasing attention as primary immunomodulators in the alveolar spaces (28, 31, 42). Pentraxins (PTX) are a superfamily of proteins conserved during evolution from Limulus polyphemus to humans, usually characterized by a pentameric structure (21). PTX3 is prototypic of long pentraxin consisting of an N-terminal portion coupled to a C-terminal pentraxin domain, the latter related to short PTX (7). PTX3 is rapidly produced and released by diverse cell types, in particular by mononuclear phagocytes, endothelial cells, and dendritic cells (DCs), in response to primary inflammatory cytokines in vitro and in vivo (11, 18, 38). Increased circulating levels of this protein have been detected in different infectious and inflammatory conditions (37, 19, 34, 41). It binds selected microbial agents (e.g., conidia of Aspergillus fumigatus and Pseudomonas aeruginosa) and activates several effector pathways to oppose pathogen infectivity (20). Analysis of gene-targeted mice has revealed that PTX3 is a unique pattern recognition receptor which plays a nonredundant role in resistance against selected pathogens (20). The susceptibility of PTX3-deficient mice to A. fumigatus was associated with failure to mount an adaptive type I immune response that could be restored by the exogenous supply of PTX3 (20).
There has been a recent surge in the development of newer antifungals to treat IA, including entirely new classes of drugs with novel targets (45), creating hope for treatment and increasing the permutations of new potential combination therapies (45). On the basis of treatment of other infectious diseases (4), combination therapy seems logical. In the present study, we assessed the therapeutic efficacy of PTX3, alone or combined with antifungals such as amphotericin B or AmBisome, in a murine model of BM-transplanted mice that replicates the immunodeficiency seen in BM transplantation. Mice were subjected to different treatment schedules and assessed for resistance to IA and parameters of innate and adaptive Th immunity. The results showed that PTX3 induced complete resistance to infection and reinfection, activated protective type 1 responses with minimum pathology, and greatly increased the therapeutic efficacy of either drug when given in combination.
MATERIALS AND METHODS
Animals.
Female, 8- to 10-week-old, inbred BALB/c and C3H/HeJ mice were obtained from Charles River Breeding Laboratories (Calco, Italy). Mice were bred under specific-pathogen-free conditions at the breeding facilities of the University of Perugia, Perugia, Italy. BM-transplanted mice were kept in small sterile cages (five animals in each cage) and fed with sterile food and water. Procedures involving animals and their care were conducted in conformity with national and international laws and policies. All in vivo studies were done in compliance with National and Perugia University Animal Care and Use Committee guidelines.
BM transplantation model.
BM cells from donor BALB/c mice were prepared by differential agglutination with soybean agglutinin. T-cell-depleted cells (containing less than 1% contaminating T cells on fluorescence-activated cell sorting (FACS) analysis) were injected intravenously at a concentration of ≥4 × 106/ml into recipient C3H/HeJ mice exposed to a lethal dose of 9 Gy (33). Without BM transplantation, mice died within 14 days. According to previous studies (33), more than 95% of the mice survived, showing a stable, donor-type hematopoietic chimerism, as revealed by donor type major histocompatibility complex class I antigen expression on cells from spleens.
Microorganism, culture conditions, and infection.
An A. fumigatus strain was obtained from a fatal case of pulmonary aspergillosis at the Infectious Diseases Institute of the University of Perugia (13). For infection, mice were lightly anesthetized by inhaled diethyl ether before instillation of a suspension of 2 × 107 conidia/20 μl of saline, which was applied slowly through the nostrils with a micropipette with a sterile disposable tip. This procedure was repeated for three consecutive days. For reinfection, mice surviving the primary intranasal (i.n.) infection were challenged intravenously with 5 × 105 Aspergillus conidia. Fungal loads in lungs, brains, and kidneys of infected mice were quantified by serial plating on Sabouraud dextrose agar, and the results (means ± standard errors) were expressed as numbers of CFU/organs. For histological analysis, lungs were excised and immediately fixed in formalin. Sections (3 to 4 μm) of paraffin-embedded tissues were stained with the periodic acid-Schiff procedure (13, 20).
Treatments.
PTX3 (SIGMA-Tau, Pomezia, Rome, Italy) was obtained under endotoxin-free conditions by immunoaffinity of culture supernatants of CHO cells transfected with PTX3 (20). PTX3, deoxycholate amphotericin B (D-AMB) (Fungizone; Bristol-Myers Squibb, Sermoneta, Italy) and liposomal amphotericin B (L-AMB) (AmBisome; GILEAD, Milan, Italy) were diluted at the desired concentrations in sterile saline (PTX3) or a 5% glucose-water solution. Treatment schedules were as follows: different doses of PTX3, amphotericin B, or L-AMB, alone or in combination, were administered intraperitoneally or i.n. (PTX3 only) for 5 days before Aspergillus infection (prophylactic treatment) in concomitance with the infection and continuing for 5 days later or for 5 days after the last injection of conidia (therapeutic treatment). In the case of concomitant i.n. administration, PTX3 and conidia were given separately. Controls received the diluent alone or sterile saline.
Flow cytometry.
Cell surface phenotype was assessed by reacting samples with fluorescein isothiocyanate-conjugated rat antimouse antibodies from PharMingen (San Diego, Calif.). Before labeling, FcR blocking was performed by incubating cells with 5% normal serum. Unrelated isotype-matched antibodies were used as the control. Analysis was performed on a FACScan instrument (Becton Dickinson, Mountain View, Calif.). The data obtained were evaluated as percentages of positive cells. Histograms are representative of one out of four independent experiments.
Quantitation of cytokine transcripts by real-time reverse transcription-PCR.
Total RNA (5 μg, extracted from splenic CD4+ T cells using the RNeasy Mini kit; QIAGEN S.P.A., Milan, Italy), was reverse transcribed with Sensiscript reverse transcriptase (QIAGEN) according to the manufacturer's directions. PCR primers were obtained from Applied Biosystems (Foster City, Calif.). Samples were subjected to 40 cycles of amplification at 95°C for 15 seconds followed by 60°C for 1 min using an ABI PRISM 7000 sequence detection system (Applied Biosystems). PCR amplification of the housekeeping eukaryotic 18S rRNA gene was performed for each sample to control for sample loading and allow normalization between samples as per the manufacturer's instructions (Applied Biosystems). Water controls were included to ensure specificity. Each sample was examined for integrity by electrophoretic analysis of the amplification plot. The eukaryotic 18S rRNA-normalized data were expressed as relative cytokine mRNA (ΔΔCt) in experimental groups compared to that of naive mice (10).
Cytokine and spot enzyme-linked immunosorbent (ELISPOT) assays.
The levels of cytokines in the bronchoalveolar lavage fluids and culture supernatants of splenocytes stimulated with heat-inactivated Aspergillus (9, 10) were determined by ELISA (R&D Systems, Inc., Space Import-Export srl, Milan, Italy). The detection limits (pg/ml) of the assays were <16 for interleukin 12 (IL-12) p70, <32 for tumor necrosis factor alpha (TNF-α), <10 for gamma interferon (IFN-γ), and <3 for IL-4 and IL-10. For enumeration of cytokine-producing CD4+ T cells, an ELISPOT assay was used on purified splenic CD4+ T cells (9, 10). Results are expressed as the mean number of cytokine-producing cells (± standard error) per 105 cells, calculated by using replicates of serial twofold dilutions of cells.
Statistical analysis.
The log-rank test was used for paired data analysis of the Kaplan-Meier survival curves. Student's t test or analysis of variance and Bonferroni's test were used to determine the statistical significance of differences in organ clearance and in vitro assays, as indicated in the figure legends. Significance was defined as a P value of <0.05. In vivo groups consisted of four to six animals. The data reported were pooled from three to five experiments, unless otherwise specified.
RESULTS
Administration of PTX3 cures BM-transplanted mice from primary A. fumigatus infection and reinfection.
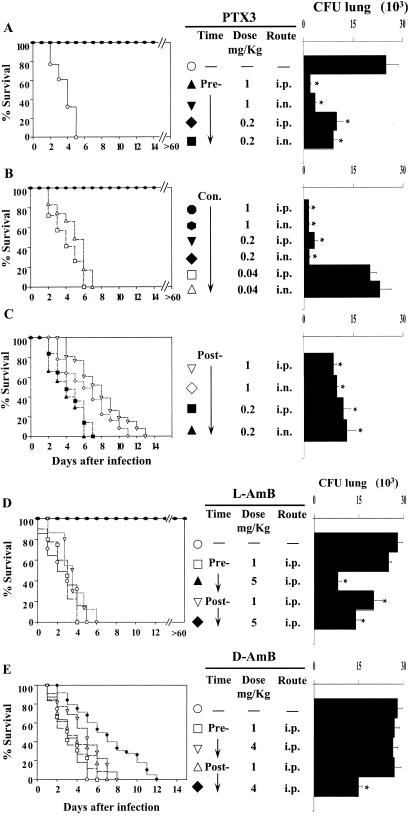
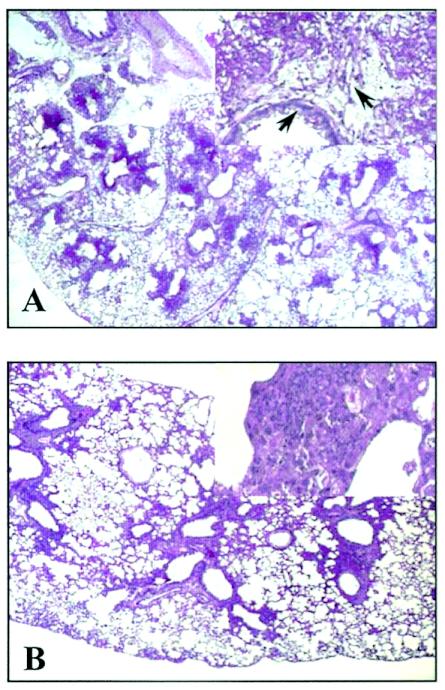
To assess the effects of PTX3 in BM-transplanted mice highly susceptible to IA (15), mice were subjected to treatments with different doses of PTX3 given i.n. or intraperitoneally, before, in concomitance with, or after the infection. Doses were selected on the bases of preliminary experiments showing that PTX3 levels (at the dose of 2 mg/kg of body weight given i.n.) were elevated in the bronchoalveolar lavage fluids for at least 24 h (between 70 and 25 ng/ml from 2 to 24 h) and were superior to those found in mice with IA a day after the infection (between 2 and 15 ng/ml) (20; also unpublished data). PTX3 was also measurable in the serum (from 22 to 50 ng/ml at 2 and 24 h after infection, respectively) and was in the range previously found to occur in patients with aspergillosis (20). The parameters of survival and fungal load in the lung were then recorded and comparatively analyzed with those obtained in mice treated with different doses of L-AMB or D-AMB. The results showed that PTX3, at doses of 1 and 0.2 mg/kg given prophylactically, induced complete resistance to IA (Fig. 1A), as revealed by the increased survival (>60 days) of 100% treated mice and the significantly (P < 0.05, treated versus untreated mice) reduced fungal burden in the lung, particularly in mice receiving the higher dose (Fig. 1A) and in the brain (data not shown). High-level resistance was also obtained in mice receiving PTX3 in concomitance with the infection (Fig. 1B). However, a dose-dependent effect was observed here, since the protective efficacy of PTX3 was lost at the dose of 0.04 mg/kg. PTX3 administered after the infection significantly (P < 0.05; 1-mg/kg PTX3 treatment versus no treatment) increased the survival of mice at the higher dose only, although at both doses it reduced significantly the fungal burden in the lung and at the higher dose in the brain (Fig. 1C). No differences were observed between the two routes of administration. Similar results were observed in mice treated with 5 mg of L-AMB/kg, since all the mice survived the infection upon treatment before or after infection (Fig. 1D). D-AMB did not afford the same level of protection, since increased survival and decreased fungal burden were observed only at the highest tolerated dose (4 mg/kg) given after the infection (Fig. 1E). We also assessed the susceptibility of cured PTX3-treated mice to Aspergillus reinfection and found that treatment with PTX3 also significantly increased resistance to reinfection, as revealed by decreased fungal growth in the kidneys of reinfected mice (data not shown). PTX3 also ameliorated lung pathology. Lung sections from infected mice showed the presence of numerous Aspergillus hyphae infiltrating the lung parenchyma, with severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment (Fig. 2A). These features were not observed in PTX3-treated mice, whose lungs were characterized by healing infiltrates of inflammatory poly- and mononuclear cells with no evidence of fungal growth and bronchial wall destruction (Fig. 2B). These data point to the therapeutic efficacy of PTX3 in BM transplantation settings where antifungals are known to have reduced activity (16, 30).
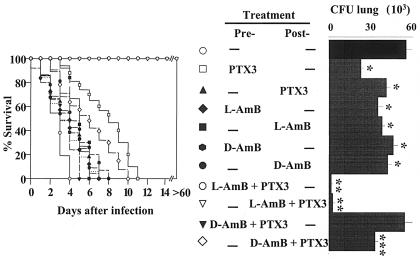
FIG. 1.
Effect of PTX3, L-AMB, or D-AMB administration in mice with invasive aspergillosis. Lethally irradiated C3H/HeJ mices were infused with ≥2 × 106 T-cell-depleted allogeneic bone marrow cells from BALB/c mice a week before intranasal infection with 2 × 107 Aspergillus conidia. Mice were treated with PTX3 or the polyenes at the doses and routes indicated for 5 days before (Pre-) or after (Post-) the infection or in concomitance with the infection and continuing for 5 days later (Con.). Resistance to infection was assessed in terms of percent survival and fungal growth (CFU) in the lungs and brains, determined at the time of death for mice dying earlier or 6 days after infection. Each group consisted of six animals. Bars indicate the standard errors. (−, untreated mice). The asterisk indicates a P value of <0.05 (treated versus untreated mice). i.n., intranasal; i.p., intraperitoneal.
FIG. 2.
PTX3 reduces lung pathology in mice with invasive aspergillosis. Periodic acid-Schiff-stained sections were prepared from lungs of bone marrow-transplanted mice infected with Aspergillus conidia either untreated (A) or treated (B) with 1 mg of PTX3/kg intraperitoneally from the day of the infection and continuing for an additional 5 days. Numerous Aspergillus hyphae (arrows) infiltrating the lung parenchyma, with extensive parenchymal destruction, severe signs of bronchial wall damage, and necrosis and scarce inflammatory cell recruitment are observed in the lungs of untreated mice (at 3 days after infection), as opposed to what is observed for PTX3-treated mice, whose lungs were characterized by healing infiltrates of inflammatory cells with no evidence of parenchymal destruction and fungal growth (at 6 days after infection). Magnification, ×100 in panels A and B; ×400 in the insets.
PTX3 accelerates myeloid and Th1 cell recovery in mice with IA.
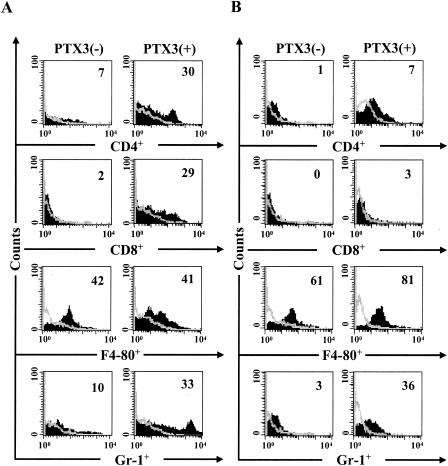
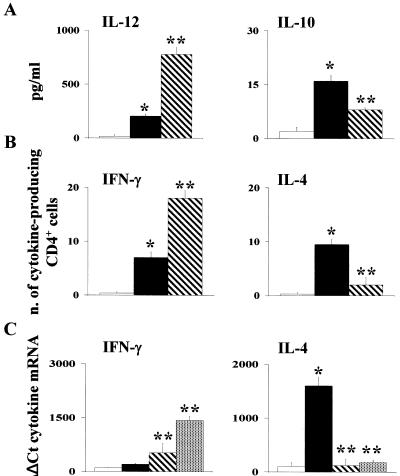
In mice with IA, resistance to infection correlates with the activation of IFN-γ-producing Th1 cells (12, 13). To evaluate whether PTX3 would activate Th1-cell reactivity in BM-transplanted mice with IA, we assessed cell recovery by FACS analysis together with the pattern of local cytokine production and antifungal activity of effector phagocytes. Total and differential counts of blood leukocytes indicated that the absolute number of circulating neutrophils significantly increased after PTX3 treatment (data not shown). However, since neutrophil levels in blood do not predict susceptibility to aspergillosis (5), a cytofluorimetric analysis was performed on cells from lungs and spleens. The numbers of CD4+ cells, CD8+ cells, and Gr-1+ neutrophils were significantly increased in the lungs of mice upon treatment with PTX3 (Fig. 3A). Recovery of neutrophils and, partially, of CD4+ T cells was also observed in the spleens. No differences were observed in the numbers of lung or splenic F4-80+ cells with and without treatment with PTX3 (Fig. 3B). PTX3 alone did not modify cell recovery in uninfected mice (data not shown). Recovered cells and lymphocytes were functionally active, as indicated by the production of proinflammatory (IL-12) and anti-inflammatory (IL-10) cytokines in lung homogenates, the frequency of Th1 (IFN-γ) or Th2 (IL-4) cytokine-producing CD4+ T splenocytes, and pattern of cytokine gene expression. Figure 4A shows that treatment with PTX3 greatly increased (about fourfold) the production of IL-12, while the production of IL-10 was only cut by half (compared to results for untreated control), a finding suggesting that PTX3 exerts a fine control over the inflammatory process at the site of the infection. Moreover, PTX3 treatment increased the frequency of Th1 and decreased that of IL-4-producing CD4+ cells in the spleens (Fig. 4B), a finding corroborated by the assessment of the levels of specific cytokine mRNA expression by quantitative PCR. Figure 4C shows that both prophylactic and therapeutic treatment with PTX3 significantly increased the IFN-γ expression and reduced that of IL-4. On assessing the level of antifungal activity of effector phagocytes, it was found that the conidiocidal activity of effector phagocytes was higher in PTX3-treated mice than in untreated mice (data not shown), a finding consistent with previous data showing the positive effect of PTX3 on both the phagocytosis and killing activity of effector mononuclear phagocytes (20). Because in vitro studies have ruled out a direct killing activity of PTX3 on the fungus (data not shown), these data qualify PTX3 as a new agent with potent immunomodulatory activity in both innate and adaptive antifungal immunity.
FIG. 3.
PTX3 accelerates cell recovery in mice with invasive aspergillosis. Bone marrow-transplanted mice, generated as detailed in the legend to Fig. 1, were infected intranasally with 2 × 107 Aspergillus conidia and treated (+) with 1 mg of PTX3/kg intraperitoneally from the day of infection and continuing for an additional 5 days or left untreated (−). The numbers refer to the percentages of positive cells, as assessed by FACS analysis, in the lung (A) and spleen (B) 3 or 6 days after infection (untreated or PTX3-treated mice, respectively).
FIG. 4.
PTX3 induces functional recovery of Th1 cells. Bone marrow-transplanted mice, generated as detailed in the legend to Fig. 1, were infected intranasally with 2 × 107 Aspergillus conidia and treated with 1 mg of PTX3/kg intraperitoneally either before (▧) or from the day of the infection and continuing for additional 5 days (░⃞). At 3 days after the infection, the levels of IL-12 p70 and IL-10 were determined by specific ELISAs in the bronchoalveolar lavage fluids (A), the numbers of CD4+ T splenocytes producing cytokines were enumerated by ELISPOT assays (B), and the cytokine gene expression on CD4+ splenocytes was determined by real-time PCR. Bars indicate the standard errors. *, P < 0.05, infected versus uninfected mice; **, P < 0.05, PTX3-treated versus untreated mice. (□, uninfected mice; ▪, infected mice).
PTX3 increases therapeutic efficacy of polyenes.
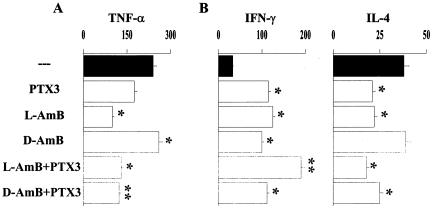
All the above findings prompted us to evaluate whether the immunomodulatory activity of PTX3 could be exploited to increase the therapeutic efficacy of L-AMB or D-AMB, since these agents are known to work synergistically with antifungal effector phagocytes (40). For this purpose, BM-transplanted mice received PTX3 alone or together with polyenes at suboptimal doses at which none of the agents achieved the maximum therapeutic effect. Combined and single treatments were done either before or after infection. Mice were monitored for survival, fungal growth, and cytokine production. It was found that each single agent given alone, while significantly reducing the fungal growth in the lung, did not significantly modify the survival of mice, except for PTX3 given alone before infection. However, combination therapy with PTX3 and L-AMB, either before or after infection, cured the mice from infection, as judged by the increased survival (>60 days) and reduced fungal growth. The combined administration of PTX3 and D-AMB significantly increased resistance over that with D-AMB alone when given after infection (Fig. 5). Analysis of cytokine production in lung homogenates and in culture supernatants of antigen-stimulated splenocytes revealed that PTX3 greatly reduced TNF-α production in the lungs of mice receiving D-AMB compared to the levels observed upon treatment with the drug alone; the level of TNF-α production in response to L-AMB was lower than that induced upon treatment with D-AMB and was not modified by the combined treatment with PTX3 (Fig. 6A). The production of IFN-γ by splenocytes was significantly increased over that in untreated mice after each single treatment and further increased in mice treated with PTX3 and L-AMB; in contrast, the production of IL-4 was greatly decreased upon treatment with PTX3 and/or L-AMB and slightly upon the combined treatment with PTX3 and D-AMB (Fig. 6B). Therefore, PTX3 appears to work synergistically with L-AMB, more than with D-AMB, to decrease the lung inflammatory response and to promote antifungal Th1 reactivity.
FIG. 5.
PTX3 increases the therapeutic efficacy of L-AMB and D-AMB. Bone marrow-transplanted mice, generated as detailed in the legend to Fig. 1, were infected intranasally with 2 × 107 Aspergillus conidia and treated intraperitoneally with each single agent alone or in combination, either before (Pre-) or after (Post-) infection. The dosages were as follows: 0.04 and 0.2 mg of PTX3/kg before or after the infection, respectively; 1 mg of L-AMB and 2 mg of D-AMB/kg. Resistance to infection was assessed in terms of percent survival and fungal growth (CFU) in the lung, determined at the time of death for mice dying earlier or 6 days after the infection. Bars indicate the standard errors. (−, untreated mice). *, P < 0.05, treated versus untreated mice; **, P < 0.001, combined treatment with PTX3 plus L-AMB versus each single treatment alone; ***, P < 0.05, combined treatment with PTX3 plus D-AMB versus D-AMB alone.
FIG. 6.
PTX3 decreases TNF-α production and increases the ratio of Th1/Th2 cytokines in mice treated with polyenes. Bone marrow-transplanted mice, generated as detailed in the legend to Fig. 1, were infected intranasally with 2 × 107 Aspergillus conidia and treated intraperitoneally with each single agent alone or in combination after the infection. The dosages were as detailed in the legend to Fig. 6. The levels (in picograms/milliliter) of TNF-α were determined in the bronchoalveolar lavage fluids 3 days after the infection (A), and the levels (in picograms/milliliter) of IFN-γ and IL-4 were determined in culture supernatants of antigen-activated splenocytes (B) at the time of death for mice dying earlier or 6 days after the infection. Bars indicate the standard errors. (−, untreated mice). *, P < 0.05, treated versus untreated mice; **, P < 0.05, combined treatment with PTX3 plus D-AMB versus D-AMB alone; ***, P < 0.05, combined treatment with PTX3 plus L-AMB versus each single treatment alone.
DISCUSSION
This study shows that PTX3, either alone or in combination with antifungal agents, induced a curative response with minimum pathology in mice with IA. PTX3 was effective when given prophylactically, either locally or systemically, and did not show direct activity on fungal cells. Therefore, the beneficial effect of PTX3 appears to rely on its ability to activate protective Th1-dependent resistance.
PTX3 activates at least two effector pathways to oppose pathogen infectivity: the classic pathway of complement activation by binding C1q (35) and the promotion of phagocytosis by interacting with an as yet unidentified cellular receptor(s) (20). It is likely that the prompt handling of conidia by resident mononuclear cells may serve to limit fungal infectivity while allowing the recovery of myeloid and lymphoid cells into the lung. This will be consistent with the ability of PTX3 to bind murine and human alveolar macrophages and to promote the phagocytosis and conidiocidal activities of these cells (20). However, PTX3 also activates DCs for IL-12 production and costimulatory antigen expression in response to Aspergillus conidia (20). Therefore, the rapid triggering of PTX3 production in DCs via members of the Toll-like receptor (TLR) family (18) may imply a direct role of PTX3 in the amplification of innate resistance and orientation of adaptive immunity.
From clinical experience, it is known that BM recovery and increasing numbers of leukocytes in blood are crucial factors in the outcome of treatment (16). PTX3 accelerated recovery of myeloid and lymphoid cells; however, no signs of inflammatory pathology were observed at the site of infection. This is particularly intriguing, since it is known that neutrophils play a key role in determining the type of pathology associated with pulmonary aspergillosis in different clinical settings (3). The production of IL-12 was increased and that of IL-10 was decreased in the lungs of infected mice treated with PTX3, a finding indicating the occurrence of an inflammatory response. However, TNF-α production was not increased upon PTX3 treatment, thus suggesting that PTX3 may act as a fine regulator of the balance between proinflammatory and anti-inflammatory stimuli. In this regard, it is worth mentioning that not only is the production of PTX3 dependent on selected TLR activation (18) but PTX3 itself may modulate TLR functioning on phagocytic cells (data not shown). Whatever the mechanism, this may account for the unique effect of PTX3 on effector phagocytes, which includes promotion of the phagocytosis and killing machinery (20) without signs of a dysregulated inflammatory response (this study). Moreover, the study also indicates that the balance between proinflammatory and anti-inflammatory cytokines, such as TNF-α and IL-10, more than the actual level of cytokine being produced, may determine the optimal protective immunity to the fungus. This may explain, therefore, the observed increased resistance to the infection despite decreased local production of TNF-α.
There is a need for therapeutic advances against aspergillosis, despite a recent expansion in the armamentarium of newer antifungals (45, 46). Treatment with amphotericin B, one first drug of choice, is limited by the dose-related nephrotoxicity of the drug, which precludes full-dose therapy in patients who have undergone BM transplantation (24). Several lipid-based formulations of amphotericin B have been developed to reduce the toxicity associated with conventional D-AMB (25), including L-AMB (1, 2). We found that L-AMB showed activity superior to that of D-AMB in BM-transplanted mice with IA. Both prophylactic and therapeutic daily treatments with 5 mg of L-AMB/kg cured the mice of infection and reduced the fungal burden in the lungs. A previous study had shown that prophylactic more than therapeutic administration of 5 mg of L-AMB/kg was effective against IA in BM-transplanted mice (6). Differences in the treatment schedule, including the timing of BM graft after irradiation, may account for the discrepancy observed with the results of the present study. With D-AMB, we observed only a modestly increased resistance to infection at the highest tolerated doses (i.e., 4 mg/kg) given after infection. However, in mice with chemotherapy-induced neutropenia, both drugs exerted a comparable level of antifungal resistance as revealed by the complete resistance to the infection observed after treatment with 5 or 4 mg of L-AMB or D-AMB/kg, respectively, beginning the day of the infection and continuing daily for 5 to 7 days (data not shown). Therefore, it appears that the toxicity of D-AMB is increased in BM transplantation.
It has been postulated that the signs of toxicity of D-AMB, including fever and chills, are the result of production of proinflammatory cytokines, such as TNF-α, by innate immune cells through a TLR-dependent mechanism (43). We found here that production of TNF-α was higher in D-AMB-treated mice than in L-AMB-treated mice but, interestingly, was decreased upon the combined treatment with PTX3. However, the cooperative activity of PTX3 with the antifungals may rely on an effect that goes beyond the balancing between proinflammatory an anti-inflammatory cytokine production. In this regard, it is known that the efficacy of the antifungal chemotherapy depends on host immune reactivity (32) and that the different amphotericin B formulations exert additive antifungal activity in combination with effector phagocytes against A. fumigatus (40). PTX3 also was reported to enhance the phagocytosis and killing activity of effector phagocytes against Aspergillus conidia (20). It is likely, therefore, that a stimulatory activity for effector phagocytes may underlay the synergistic activity of PTX3 and the antifungals in BM-transplanted mice with IA. Whatever the mechanism, the results of the present study suggest the feasibility of combination therapy with antifungals and immunomodulatory substances, such as PTX3, aimed at restoring the optimal innate and adaptive immune resistance to A. fumigatus in BM transplantation.
Acknowledgments
This work was supported by the National Research Project on AIDS, contract no. 50D.27, and project no. 1AF/F, both from the ISS, Rome, Italy.
We thank Lara Bellocchio for superb editorial assistance and Paolo Mosci for the histology.
REFERENCES
- 1.Adler-Moore, J., and R. T. Profitt. 1993. Development, characterization, efficacy, and mode of action of AmBisome, a unilamellar formulation of amphotericin B. J. Liposome Res. 3:429-450. [Google Scholar]
- 2.Adler-Moore, J., and R. T. Proffitt. 2002. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 49:21-30. [DOI] [PubMed] [Google Scholar]
- 3.Babbin, B. A., J. N. Greene, R. Vega, S. Iravani, N. N. Ku, and R. L. Sandin. 2000. Pathologic manifestations of invasive pulmonary aspergillosis in cancer patients: the many faces of aspergillus. Cancer Control 7:566-571. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, J. E., W. E. Dismukes, R. J. Duma, G. Medoff, M. A. Sande, H. Gallis, J. Leonard, B. T. Fields, M. Bradshaw, H. Haywood, Z. A. McGee, T. R. Cate, C. G. Cobbs, J. F. Warner, and D. W. Alling. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N. Engl. J. Med. 301:126-131. [DOI] [PubMed] [Google Scholar]
- 5.BitMansour, A., S. M. Burns, D. Traver, K. Akashi, C. H. Contag, I. L. Weissman, and J. M. Brown. 2002. Myeloid progenitors protect against aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood 100:4660-4667. [DOI] [PubMed] [Google Scholar]
- 6.BitMansour, A., and J. M. Y. Brown. 2002. Prophylactic administration of liposomal amphotericin B is superior to treatment in a murine model of invasive aspergillosis after hematopoietic cell transplantation. J. Infect. Dis. 186:134-137. [DOI] [PubMed] [Google Scholar]
- 7.Bottazzi, B., V. Vouret-Craviari, A. Bastone, L. De Gioia, C. Matteucci, G. Peri, F. Spreafico, M. Pausa, C. D'Ettorre, E. Gianazza, A. Tagliabue, M. Salmona, F. Tedesco, M. Introna, and A. Mantovani. 1997. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J. Biol. Chem. 272:32817-32823. [DOI] [PubMed] [Google Scholar]
- 8.Bozza, S., R. Gaziano, G. B. Lipford, C. Montagnoli, A. Bacci, P. Di Francesco, V. P. Kurup, H. Wagner, and L. Romani. 2002. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 4:1281-1290. [DOI] [PubMed] [Google Scholar]
- 9.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. Di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362-1371. [DOI] [PubMed] [Google Scholar]
- 10.Bozza, S., K. Perruccio, C. Montagnoli, R. Gaziano, S. Bellocchio, E. Burchielli, G. Nkwanyuo, L. Pitzurra, A. Velardi, and L. Romani. 2003. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102:3807-3814. [DOI] [PubMed] [Google Scholar]
- 11.Breviario, F., E. M. d'Aniello, J. Golay, G. Peri, B. Bottazzi, A. Bairoch, S. Saccone, R. Marzella, V. Predazzi, M. Rocchi, G. Della Valle, E. Dejana, A. Mantovani, and M. Introna. 1992. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J. Biol. Chem. 267:22190-22197. [PubMed] [Google Scholar]
- 12.Cenci, E., S. Perito, K. H. Enssle, P. Mosci, J. P. Latge, L. Romani, and F. Bistoni. 1997. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect. Immun. 65:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenci, E., A. Mencacci, C. Fè d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 14.Cenci, E., A. Mencacci, A. Bacci, F. Bistoni, V. P. Kurup, and L. Romani. 2000. T cell vaccination in mice with invasive pulmonary aspergillosis. J. Immunol. 165:381-388. [DOI] [PubMed] [Google Scholar]
- 15.Cenci, E., A. Mencacci, A. Spreca, C. Montagnoli, A. Bacci, K. Perruccio, A. Velardi, W. Magliani, S. Conti, L. Polonelli, and L. Romani. 2002. Protection of killer antiidiotypic antibodies against early invasive aspergillosis in a murine model of allogeneic T-cell depleted bone marrow transplantation. Infect. Immun. 70:2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 17.Denning, D. W., S. E. Follansbee, M. Scolaro, S. Norris, H. Edelstein, and D. A. Stevens. 1991. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 324:654-662. [DOI] [PubMed] [Google Scholar]
- 18.Doni, A., G. Peri, M. Chieppa, P. Allavena, F. Pasqualini, L. Vago, L. Romani, C. Garlanda, and A. Mantovani. 2003. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 33:2886-2893. [DOI] [PubMed] [Google Scholar]
- 19.Fazzini, F., G. Peri, A. Doni, G. Dell'Antonio, E. Dal Cin, E. Bozzolo, F. D'Auria, L. Praderio, G. Ciboddo, M. G. Sabbadini, A. A. Manfredi, A. Mantovani, and P. R. Querini. 2001. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 44:2841-2850. [DOI] [PubMed] [Google Scholar]
- 20.Garlanda, C., E. Hirsch, S. Bozza, A. Palustri, M. De Acetis, R. Nota, A. Maccagno, F. Riva, B. Bottazzi, G. Peri, A. Doni, L. Vago, M. Botto, R. De Santis, P. Carminati, G. Siracusa, F. Altruda, A. Vecchi, L. Romani, and A. Mantovani. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182-186. [DOI] [PubMed] [Google Scholar]
- 21.Gewurz, H., X. H. Zhang, and T. F. Lint. 1995. Structure and function of the pentraxins. Curr. Opin. Immunol. 7:54-64. [DOI] [PubMed] [Google Scholar]
- 22.Grazziutti, M., D. Przepiorka, J. H. Rex, I. Braunschweig, S. Vadhan-Raj, and C. A. Savary. 2001. Dendritic cell-mediated stimulation of the in vitro lymphocyte response to Aspergillus. Bone Marrow Transplant. 27:647-652. [DOI] [PubMed] [Google Scholar]
- 23.Hebart, H., C. Bollinger, P. Fisch, J. Sarfati, C. Meisner, M. Bauer, J. Loeffler, M. Monod, J. Latgé, and H. Einsele. 2002. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematological malignancies. Blood 100:4521-4527. [DOI] [PubMed] [Google Scholar]
- 24.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, B. de Pauw, Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer, and Global Aspergillus Study Group. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 25.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulation of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22:5133-5144. [DOI] [PubMed] [Google Scholar]
- 26.Ito, J., and J. Lyons. 2002. Vaccination of corticosteroid immunosuppressed mice against invasive pulmonary aspergillosis. J. Infect. Dis. 186:869-871. [DOI] [PubMed] [Google Scholar]
- 27.Latgè, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madan, T., U. Kishore, M. Singh, P. Strong, E. M. Hussain, K. B. M. Reid, and P. U. Sarma. 2001. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect. Immun. 69:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358-4366. [DOI] [PubMed] [Google Scholar]
- 30.Marr, K. A. 2003. New approaches to invasive fungal infections. Curr. Opin. Hematol. 10:445-450. [DOI] [PubMed] [Google Scholar]
- 31.McCormack, F. X., and J. A. Whitsett. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Investig. 109:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mencacci, A., E. Cenci, A. Bacci, F. Bistoni, and L. Romani. 2000. Host immune reactivity determines the efficacy of combination immunotherapy and antifungal chemotherapy in candidiasis. J. Infect. Dis. 181:686-694. [DOI] [PubMed] [Google Scholar]
- 33.Mencacci, A., K. Perruccio, A. Bacci, E. Cenci, R. Benedetti, M. F. Martelli, F. Bistoni, R. Coffman, A. Velardi, and L. Romani. 2001. Defective antifungal T-helper 1 (TH1) immunity in a murine model of allogeneic T-cell-depleted bone marrow transplantation and its restoration by treatment with TH2 cytokine antagonists. Blood 97:1483-1490. [DOI] [PubMed] [Google Scholar]
- 34.Muller, B., G. Peri, A. Doni, V. Torri, R. Landmann, B. Bottazzi, and A. Mantovani. 2001. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit. Care Med. 29:1404-1407. [DOI] [PubMed] [Google Scholar]
- 35.Nauta, A. J., B. Bottazzi, A. Mantovani, G. Salvatori, U. Kishore, W. J. Schwaeble, A. R. Gingras, S. Tzima, F. Vivanco, J. Egido, O. Tijsma, E. C. Hack, M. R. Daha, and A. Roos. 2003. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33:465-473. [DOI] [PubMed] [Google Scholar]
- 36.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine 79:250-260. [DOI] [PubMed] [Google Scholar]
- 37.Peri, G., M. Introna, D. Corradi, G. Iacuitti, S. Signorini, F. Avanzini, F. Pizzetti, A. P. Maggioni, T. Moccetti, M. Metra, L. D. Cas, P. Ghezzi, J. D. Sipe, G. Re, G. Olivetti, A. Mantovani, and R. Latini. 2000. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 102:636-641. [DOI] [PubMed] [Google Scholar]
- 38.Polentarutti, N., G. Picardi, A. Basile, S. Cenzuales, A. Rivolta, C. Matteucci, G. Peri, A. Mantovani, and M. Introna. 1998. Interferon-gamma inhibits expression of the long pentraxin PTX3 in human monocytes. Eur. J. Immunol. 28:496-501. [DOI] [PubMed] [Google Scholar]
- 39.Roilides, E., H. Katsifa, and T. J. Walsh. 1998. Pulmonary host defences against Aspergillus fumigatus. Res. Immunol. 149:454-465. [DOI] [PubMed] [Google Scholar]
- 40.Roilides, E., C. A. Lyman, J. Filioti, O. Akpogheneta, T. Sein, C. G. Lamaignere, R. Petraitiene, and T. J. Walsh. 2002. Amphotericin B formulations exert additive antifungal activity in combination with pulmonary alveolar macrophages and polymorphonuclear leukocytes against Aspergillus fumigatus. Antimicrob. Agents Chemother. 46:1974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolph, M. S., S. Zimmer, B. Bottazzi, C. Garlanda, A. Mantovani, and G. K. Hansson. 2002. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 22:e10-e14. [DOI] [PubMed] [Google Scholar]
- 42.Sato, M., H. Sano, D. Iwaki, K. Kudo, M. Konishi, H. Takahashi, T. Takahashi, H. Imaizumi, Y. Asai, and Y. Kuroki. 2003. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 171:417-425. [DOI] [PubMed] [Google Scholar]
- 43.Sau, K., S. S. Mambula, E. Latz, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 278:37561-37568. [DOI] [PubMed] [Google Scholar]
- 44.Scheneemann, M., and A. Schaffner. 1999. Host defence mechanism in Aspergillus fumigatus infections. Contrib. Microbiol. 2:57-68. [DOI] [PubMed] [Google Scholar]
- 45.Steinbach, W. J., and D. A. Stevens. 2003. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin. Infect. Dis. 37:S157-S187. [DOI] [PubMed] [Google Scholar]
- 46.Steinbach, W. J., D. A. Stevens, and D. W. Denning. 2003. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin. Infect. Dis. 37:S188-S224. [DOI] [PubMed] [Google Scholar]
- 47.Wingard, J. R. 1999. Fungal infections after bone marrow transplant. Biol. Blood Marrow Transplant. 5:55-68. [DOI] [PubMed] [Google Scholar]