Abstract
Free gemifloxacin concentrations in the interstitial space fluid of skeletal muscle and subcutaneous adipose tissue were measured by means of in vivo microdialysis to characterize the ability of gemifloxacin to penetrate human soft tissues. Twelve healthy volunteers received a single oral dose of 320 mg of gemifloxacin. The mean areas under the concentration-time curves from 0 to 10 h (AUC0-10) were significantly higher for soft tissue than for unbound gemifloxacin in plasma (P < 0.05). The ratios of the mean AUC0-10 for tissue to the AUC0-10 for free gemifloxacin in plasma were 1.7 ± 0.7 (mean ± standard deviation) for skeletal muscle and 2.4 ± 1.0 for adipose tissue. The AUC0-24 ratios for free gemifloxacin in tissues to the MIC at which 90% of frequently isolated bacteria are inhibited were close to or higher than 100 h. Therefore, based on pharmacokinetic and pharmacodynamic calculations, we conclude that gemifloxacin might be a useful therapeutic option for the treatment of soft tissue infections.
Gemifloxacin, one of the recently developed fluoroquinolones (gemifloxacin mesylate, Factive, and SB-265805), exerts a strong in vitro antibacterial activity against a broad spectrum of pathogens (3, 18). The adverse reaction profile is similar to that of older members of this class (3, 24). Previous studies have shown that gemifloxacin displays a favorable plasma pharmacokinetic (PK) profile allowing a once-daily dosing regimen (1, 2).
Gemifloxacin was approved by the U.S. Food and Drug Administration in April 2003 for infections of the upper respiratory tract (24), but initially the manufacturer did not seek to receive approval for the therapy of soft tissue infections (STIs). Regulatory authorities such as the Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products advocate that the concentration of an antibiotic at the target site needs to be determined before the approval of drugs is extended to new indications (9, 10). This determination allows for the assessment of the bacterial growth inhibition and killing potential of an antimicrobial agent at the relevant site of drug action. It is generally accepted that for most infections, this site is represented by the interstitial space fluid (ISF) of the tissue (23), and the in vivo microdialysis technique is capable of sampling interstitial fluid at the target site (8, 20).
In the present study, we used microdialysis to investigate the potential of gemifloxacin to penetrate skeletal muscle and subcutaneous adipose tissue in healthy volunteers. Thus, we clarified whether gemifloxacin might be an appropriate therapeutic option for the treatment of STIs. For this purpose, the PK of gemifloxacin was measured in the ISF of soft tissues and compared to the PK of this drug in plasma.
MATERIALS AND METHODS
The study took place at the Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria. The study protocol was approved by the local Ethics Committee and was performed in accordance with the Declaration of Helsinki (1964) in the revised version of 1996 (24a), the Guidelines of the International Conference on Harmonization (14a), the Guideline for Good Clinical Practice (8a), and the Austrian drug law. All volunteers were given a detailed description of the study, and their written consent was obtained prior to enrollment in the study.
Healthy volunteers.
A total of 12 healthy male volunteers were included in the present study (age, 29 ± 6 years [mean ± standard deviation]; height, 184 ± 7 cm; weight, 79 ± 7 kg). All volunteers passed a comprehensive medical examination, comprising medical history, 12-lead electrocardiogram, complete blood count, urinalysis, urine drug screen, clinical blood chemistry, blood coagulation tests, HBs antigen test, and human immunodeficiency virus antibody test. Subjects were excluded if they had taken any prescribed medication or over-the-counter drugs within a period of 2 weeks prior to the study.
Microdialysis.
The principles of microdialysis have been described previously (8, 19). In brief, microdialysis is based on the sampling of analytes from the ISF by means of a semipermeable membrane at the tip of a microdialysis probe. The probe was constantly perfused with a physiological solution (perfusate) at a flow rate of 1.5 μl/min. Once the probe is implanted in the tissue, substances present in the interstitial fluid at a certain concentration (Ctissue) diffuse into the probe, resulting in a concentration in the perfusion medium (Cdialysate). For most analytes, equilibrium between interstitial tissue fluid and the perfusion medium is incomplete; therefore, Ctissue is greater than Cdialysate. The factor by which the concentrations are interrelated is termed “relative recovery. ” For calibration of the microdialysis probes, in vivo recovery was assessed in each experiment by the retrodialysis method by use of gemifloxacin (6).
Study protocol.
Blood and microdialysate samples were collected at defined time points or time intervals ranging from 15 min to 2 h. Two commercially available microdialysis probes (CMA 60; CMA, Solna, Sweden) with molecular weight cutoffs of 20,000 were inserted into the subcutaneous adipose tissue and a skeletal muscle of the thigh without prior anesthesia (17). The microdialysis system was connected to a precision pump (CMA 100 perfusion pump) and perfused with Ringer's solution at a flow rate of 1.5 μl/min. The volume of the microdialysates available for the determination of gemifloxacin concentrations ranged from 22 to 180 μl, depending on the sampling interval. After a 30-min baseline sampling period and in vivo calibration of the probe, 320 mg of gemifloxacin was administered to the subjects as a single oral dose with 200 ml of water. Sampling of microdialysates and plasma was continued at defined time intervals for up to 10 h postdosing. The volume of blood collected at each sampling point was 3 ml. All samples were stored at −20°C until analysis.
Bioanalysis.
Frozen plasma and microdialysate samples were transferred to Quintiles Scotland Limited, Heriot-Watt University Research Park, Riccarton, Edinburgh, United Kingdom. Drug analysis was performed under the management of the Drug Analysis Department, Drug Metabolism and Pharmacokinetics, Smith Kline Beecham, Greenford, United Kingdom. Specimens were assayed by liquid chromatography-tandem mass spectrometry analysis. Prior to quantitative determinations of gemifloxacin in plasma samples, a protein precipitation extraction procedure was carried out. The lower limit of quantification was 0.01 μg/ml for both dialysate and plasma samples. The accuracy and precision ranged from 96.3 to 100.4% and 5.1 to 8.2%, respectively, for microdialysates and from 93.4 to 99.4% and 4.2 to 6.4%, respectively, for plasma samples.
Calculations and data analysis.
Each microdialysis probe was calibrated in vivo. The absolute concentrations in the ISF (in milligrams per liter) were calculated by the following formula: C = 100 × (Cdialysate/mean recovery value).
PK analysis was performed using a commercially available computer software program (Kinetica, version 3.0; Innaphase Sarl, Paris, France), and PK parameters were calculated by noncompartmental approaches. Area under the concentration-time curve (AUC) values for plasma and the interstitium were calculated from nonfitted data by using the linear trapezoidal rule. The following main PK parameters were determined: AUC from 0 to 10 h (AUC0-10), AUC from 0 to 24 h (AUC0-24), maximum concentration (Cmax), and the time to Cmax (Tmax). The half-life for the terminal slope (t1/2β) was calculated by the equation t1/2β = ln(2)/kel. The total drug clearance (CL) and the distribution volume (V) were determined for plasma by use of standard formulae as follows: V(F) = dose/(AUCtotal × kel) and CL(F) = dose/AUCtotal, where F is bioavailability.
Statistical analysis.
Wilcoxon matched-pairs tests were performed for statistical comparison of PK parameters between different compartments. All PK data are presented as means ± standard deviations. A two-sided P value of <0.05 was considered significant.
RESULTS
Tolerability.
Gemifloxacin was well tolerated by 11 of the 12 volunteers. One subject suffered from a macular skin rash after oral administration of the study drug. After antiallergic treatment, the adverse effect resolved quickly. The microdialysis procedure was well tolerated by all participants.
Microdialysis.
The mean in vivo recovery values were 34.2% ± 13.8% for muscle and 21.6% ± 7.8% for adipose tissue.
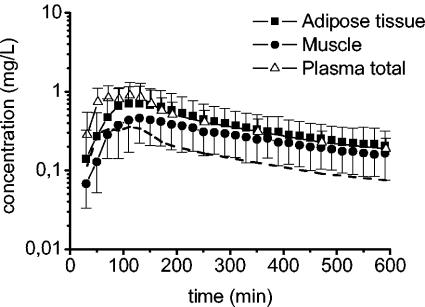
Figure 1 displays the mean time versus concentration profiles of gemifloxacin for plasma, muscle, and adipose tissue. The total oral dose of 320 mg of gemifloxacin was corrected for its oral bioavailability of 71% (GlaxoSmithKline, unpublished data). Correcting the total dose for oral bioavailability did not exert any effect on tissue penetration properties of this drug but affected values of CL and V. These PK parameters, however, were not the subject of the present study. Relevant PK data are provided in Table 1. Based on a plasma protein binding value of 0.6 (GlaxoSmithKline, unpublished data), we calculated the concentrations of unbound gemifloxacin in plasma. The concentrations of gemifloxacin in ISF of muscle tissue are uniformly lower than the total concentrations in plasma. In contrast, estimated concentrations of free gemifloxacin in plasma were consistently lower than concentrations in adipose tissue and skeletal muscle (Fig. 1). This finding is reflected by the AUC0-10 for free plasma (AUC0-10 free plasma), which was significantly lower than the AUC0-10 for adipose tissue (AUC0-10 tissue) (P = 0.004) and skeletal muscle (P = 0.02), and the mean ratios of the AUC0-10 tissue to the AUC0-10 free plasma of 1.7 for muscle and 2.4 for adipose tissue (Table 1).
FIG. 1.
Time-concentration profiles for total and free gemifloxacin in plasma (n = 12), skeletal muscle (n = 12), and subcutaneous adipose tissue (n = 11) following administration of a single oral dose of 320 mg. The dotted line represents the calculated free gemifloxacin time-concentration profile in plasma.
TABLE 1.
Pharmacokinetic parameters for plasma, adipose tissue, and skeletal muscle following oral administration of 320 mg of gemifloxacin
| Compartment (no. of samples) | AUC0-10 for tissue/AUC0-10 for free plasma | AUC0-10 (mg · h/liter) | AUC0-24 (mg · h/liter) | Cmax (mg/liter) | Tmax (h) | t1/2β (h) | CL(F) (liters/h) | V(F) (liters) |
|---|---|---|---|---|---|---|---|---|
| Plasma, total (12) | 4.0 ± 1.2 | 5.3 ± 1.6 | 1.2 ± 0.4 | 1.3 ± 0.6 | 5.0 ± 0.8 | 49 ± 22 | 334 ± 118 | |
| Plasma, free (12) | 1.6 ± 0.5 | 2.2 ± 0.6 | 0.5 ± 0.2 | 1.3 ± 0.6 | 5.0 ± 0.8 | NDa | ND | |
| Muscle (12) | 1.7 ± 0.7 | 2.6 ± 1.2b | 4.0 ± 1.9 | 0.5 ± 0.2 | 2.1 ± 0.4 | 5.7 ± 1.6 | ND | ND |
| Adipose tissue (11) | 2.4 ± 1.0 | 3.7 ± 2.1b | 5.4 ± 3.0 | 0.8 ± 0.5 | 1.8 ± 0.4 | 5.4 ± 1.1 | ND | ND |
ND, not determined.
P < 0.05 compared to free plasma value.
DISCUSSION
Bacterial drug resistance against antibiotics increases worldwide and accelerates the search for novel antimicrobial agents. Gemifloxacin is a very promising antibiotic that has proven to be effective in vitro as well as in experimental infection models in animals (1). In contrast to most other quinolones exclusively inhibiting topoisomerase II, gemifloxacin is expected to delay the development of new resistances by targeting bacterial topoisomerases II and IV (13). In previous PK studies, it was shown that maximum concentrations of gemifloxacin in plasma exceed the MIC at which 90% of the most clinically relevant pathogens are inhibited (12, 24). However, the conclusion that a concentration of an antibiotic in plasma exceeding the MIC for a pathogen reflects a strong therapeutic potential is true only for drugs that combat pathogens directly in the bloodstream. In most cases, the infection is located in the peripheral compartment. Therefore, the use of plasma drug levels is questionable for the prediction of antimicrobial and clinical efficacy. This is underlined by the fact that drug PK differs markedly between plasma and various tissues (15, 16). Moreover, huge differences in tissue PK profiles for the same class of antimicrobials have been reported for different sampling methods, such as skin blister (21), tissue biopsy (5), and fibrin clots (4).
In the present study, we used the microdialysis technique to assess in vivo concentrations of free gemifloxacin in soft tissues and investigated its ability to penetrate soft tissues.
The central finding of our study is that gemifloxacin penetrates skeletal muscle and subcutaneous adipose tissues well (Fig. 1). The results derived from our study are comparable to the data reported by Gee et al. (12) regarding cantharidine-induced skin blister fluid and ISF concentrations of gemifloxacin. Nevertheless, it should be kept in mind that blister fluid measurements generate only limited information on target site concentrations of drugs in tissues because of various problems, such as drug binding to blister proteins, varying blister size, and accumulation of gemifloxacin in polymorphonuclear cells migrating into the inflammatory fluids (11, 16). In addition, note that in the present study, the Cmaxs of gemifloxacin in plasma were only about 50% of those measured by Gee et al. (12). This might be related to the different methods used for the determination of gemifloxacin concentrations (microbiological methods versus liquid chromatography-tandem mass spectrometry), because other confounders such as different dosages, fasting conditions, and noncomparable study populations were not detected between the two studies. If tissue penetration of gemifloxacin is calculated by means of the ratios of the AUC0-10 tissue to the AUC0-10 free plasma, substantial differences can be detected between the two studies.
Based on the microdialysis data, the analysis of the time-concentration profiles (Fig. 1) reveals that free gemifloxacin concentrations in muscle and subcutaneous adipose tissue are persistently higher than concentrations of unbound gemifloxacin in plasma over the entire study period. This is clearly reflected by the high ratios of the AUC0-10 tissue to the AUC0-10 free plasma (Table 1) and represents a novel finding that was not found in previous studies with other members of the class of fluoroquinolones (7, 20). Even though this novel finding is not yet fully understood and requires further exploration and validation, there is evidence that tissue penetration by antibiotics depends on several key characteristics and chief among those are chemical and physical properties of the drug, including lipophilicity or hydrophilicity, the molecular weight, and plasma protein binding. Molecular weights are comparable between fluoroquinolones, but substantial differences with respect to hydro- and lipophilicity may be detected. The plasma protein binding of gemifloxacin has been demonstrated to be independent from the drug concentration and to range from 60 to 70% in healthy subjects (24) but was not measured in each individual in the present study.
One potential explanation for our observation is related to the ability of fluoroquinolones to concentrate intracellularly in human cells (11, 22, 25). This assumption is confirmed by the higher V of gemifloxacin (Table 1) than of other quinolones. Indeed, it is generally accepted that after penetrating the ISF, gemifloxacin accumulates intracellularly to a large extent and then is slowly released thereafter. As a result of the high lipophilicity of gemifloxacin, this phenomenon is more pronounced in the cells of subcutaneous adipose tissue than in skeletal muscle tissue and therefore leads to higher concentrations of gemifloxacin in the ISF of adipose tissue. This drug depot effect can be amplified by the lower blood perfusion rate of healthy adipose tissue than of muscle tissue resulting in lower CL rates of gemifloxacin from the ISF.
Since gemifloxacin, like all fluoroquinolones, belongs to the class of concentration-dependent antibiotics, the ratio of the AUC0-24 to the MIC90 is the most relevant PK or pharmacodynamic (PD) parameter to determine antibacterial effects (14) and to predict clinical efficacy (24). For pathogens commonly causing STIs, such as Streptococcus pyogenes and Staphylococcus aureus, the calculated ratios of AUC0-24/MIC90 for muscle and adipose tissue reach or exceed the generally accepted breakpoint for efficient dosing of 100 h (14). Moreover, it needs to be pointed out that breakpoint values are generally based on total drug concentrations, whereas free concentrations of gemifloxacin in tissues and plasma were used for PK and PD calculations in the present study.
In conclusion, based on PK and PD calculations, it is tempting to speculate that gemifloxacin will effectively combat bacteria in the extracellular space fluid in soft tissues. However, tissue PK data should also be derived from patients with STIs, because tissue penetration might be significantly altered by infection.
Acknowledgments
This work was supported by a research grant from GlaxoSmithKline.
REFERENCES
- 1.Allen, A., E. Bygate, S. Oliver, M. Johnson, C. Ward, A.-J. Cheon, Y. S. Choo, and I.-C. Kim. 2000. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob. Agents Chemother. 44:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, A., E. Bygate, M. Vousden, S. Oliver, M. Johnson, C. Ward, A.-J. Cheon, Y. S. Choo, and I.-C. Kim. 2001. Multiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteers. Antimicrob. Agents Chemother. 45:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, P. 2000. Quinolone generations: natural history or natural selection? J. Antimicrob. Chemother. 46(Suppl. T1):17-24. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron, M. G. 1989. The pharmacokinetics and tissue penetration of the fluoroquinolones. Clin. Invest. Med. 12:20-27. [PubMed] [Google Scholar]
- 5.Birmingham, M. C., R. Guarino, A. Heller, J. H. Wilton, A. Shah, L. Hejmanowski, D. E. Nix, and J. J. Schentag. 1999. Ciprofloxacin concentrations in lung tissue following a single 400 mg intravenous dose. J. Antimicrob. Chemother. 43(Suppl. A):43-48. [DOI] [PubMed] [Google Scholar]
- 6.Bouw, M. R., and M. Hammarlund-Udenaes. 1998. Methodological aspects of the use of a calibrator in in vivo microdialysis—further development of the retrodialysis method. Pharm. Res. 15:1673-1679. [DOI] [PubMed] [Google Scholar]
- 7.Brunner, M., U. Hollenstein, S. Delacher, D. Jäger, R. Schmid, E. Lackner, A. Georgopoulos, H. G. Eichler, and M. Müller. 1999. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 43:1307-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmquist, W. F., and R. J. Sawchuk. 1997. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 14:267-288. [DOI] [PubMed] [Google Scholar]
- 8a.The European Agency for the Evaluation of Medicinal Products. 1996. Guideline for good clinical practice. [Online.] http://www.emea.eu.int/pdfs/human/ich/013595en.pdf.
- 9.The European Agency for the Evaluation of Medicinal Products. 2000. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. [Online.] http://www.emea.eu.int/pdfs/human/ewp/265599en.pdf.
- 10.Food and Drug Administration. 1998. Guidance for industry: developing antimicrobial drugs — general considerations for clinical trials. [Online.] http://www.fda.gov/cder/guidance/2580dft.pdf.
- 11.García, I., A. Pascual, S. Ballesta, P. Joyanes, and E. J. Perea. 2000. Intracellular penetration and activity of gemifloxacin in human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 44:3193-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee, T., J. M. Andrews, J. P. Ashby, G. Marshall, and R. Wise. 2001. Pharmacokinetics and tissue penetration of gemifloxacin following a single oral dose. J. Antimicrob. Chemother. 47:431-434. [DOI] [PubMed] [Google Scholar]
- 13.Hooper, D. C. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyatt, J. M., P. S. McKinnon, G. S. Zimmer, and J. J. Schentag. 1995. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin. Pharmacokinet. 28:143-160. [DOI] [PubMed] [Google Scholar]
- 14a.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2004. ICH guidelines. [Online.] http://www.ich.org/UrlGrpServer.jser?@_ID=276&@_TEMPLATE=254 [DOI] [PMC free article] [PubMed]
- 15.Joukhadar, C., H. Derendorf, and M. Müller. 2001. Microdialysis. A novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211-219. [DOI] [PubMed] [Google Scholar]
- 16.Joukhadar, C., and M. Müller. Microdialysis: current applications to clinical pharmacokinetic studies and its role in the future. Clin. Pharmacokinet., in press. [DOI] [PubMed]
- 17.Joukhadar, C., H. Stass, U. Müller-Zellenberg, E. Lackner, F. Kovar, E. Minar, and M. Müller. 2003. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob. Agents Chemother. 47:3099-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandell, L. 2000. Gemifloxacin: survival of the fittest. J. Antimicrob. Chemother. 46(Suppl. T1):33-37. [DOI] [PubMed] [Google Scholar]
- 19.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller, M., H. Stass, M. Brunner, J. G. Moller, E. Lackner, and H. G. Eichler. 1999. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob. Agents Chemother. 43:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namour, F., E. Sultan, M. H. Pascual, and B. Lenfant. 2002. Penetration of telithromycin (HMR 3647), a new ketolide antimicrobial, into inflammatory blister fluid following oral administration. J. Antimicrob. Chemother. 49:1035-1038. [DOI] [PubMed] [Google Scholar]
- 22.Pascual, A., I. García, S. Ballesta, and E. J. Perea. 1999. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 43:12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan, D. M. 1993. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl. D):1-16. [DOI] [PubMed] [Google Scholar]
- 24.Saravolatz, L. D., and J. Leggett. 2003. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones. Clin. Infect. Dis. 37:1210-1215. [DOI] [PubMed] [Google Scholar]
- 24a.World Medical Association. 1996. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. [Online.] http://www.wma.net/e/policy/pdf/17c.pdf. [PubMed]
- 25.Yang, Q., R. J. Nakkula, and J. D. Walters. 2002. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J. Dent. Res. 81:836-840. [DOI] [PMC free article] [PubMed] [Google Scholar]