Abstract
We studied the MICs of triazoles against 15 Candida glabrata clinical isolates by the NCCLS M27-A2, Sensititre YeastOne, and Etest methods by using media at pHs 6.0, 7.0, and 7.4. Thirteen isolates were less susceptible to triazoles at pH 6.0 and more susceptible to triazoles at pH 7.4 compared to pH 7.0.
The standardized NCCLS M27-A2 method has become an important tool to characterize the susceptibility profile of triazoles against Candida glabrata and is currently performed at pH 7.0 (6). Previous studies have demonstrated that triazole MICs are inversely correlated to the pH (3, 4, 5). We have demonstrated that an alkaline pH affects triazole activity against C. glabrata and Candida tropicalis but not that against Candida albicans, Candida parapsilosis, or Candida krusei (M. P. Pai and R. C. Mercier, Abstr. Annu. Meet. Am. Coll. Clin. Pharm., abstr. 157, 2002). The present study was performed to evaluate triazole MICs under the influence of two clinically relevant pH values, 6.0 and 7.4, to mimic the pHs of urine and blood, respectively. In addition, this study compared the influence of these clinically relevant pH values to that of the standard pH of 7.0 used by the NCCLS M27-A2, Sensititre YeastOne (SYO), and Etest methods.
(This work was previously presented in part [Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 121, 2003].)
Fifteen unique clinical C. glabrata bloodstream isolates obtained from TriCore Reference Laboratories (Albuquerque, N.Mex.) and two quality control isolates, ATCC 6258 (C. krusei) and ATCC 22019 (C. parapsilosis), were tested. Fluconazole and itraconazole powder were obtained through Sigma Chemicals (St. Louis, Mo.). Pfizer, Inc. (New York, N.Y.) donated the voriconazole powder used in this study. Drug stock solutions (100×) were prepared as outlined by the NCCLS M27-A2 document and stored frozen at −80°C until plate preparation. Etest strips of fluconazole, itraconazole, and voriconazole were purchased through AB Biodisk North America (Piscataway, N.J.). Alamar Blue 100× was purchased through Trek Diagnostics Inc. (Cleveland, Ohio) to perform susceptibility testing by the SYO method. Isolates were tested against all antifungal agents with 0.075 M 3-(N-morpholino)propanesulfonic acid (MOPS)-buffered RPMI 1640 medium supplemented with dextrose to 20 g/liter and adjusted to pHs 7.0, 6.0, and 7.4. Microplates containing appropriate drug dilutions were sealed, stored frozen at −80°C, and thawed prior to each experiment. MICs were interpreted in duplicate at 24 and 48 h after incubation at 35°C both visually and by the use of a spectrophotometer reading at 490 nm as previously described (7).
Triazole MIC determinations were also performed in duplicate by the SYO method that included incorporation of Alamar Blue 100× to a final concentration of 1% (vol/vol) per well. MICs were interpreted as the lowest antifungal concentration that corresponded to the first purple or blue well after 24 h of incubation at 35°C (1). The Etest procedure included preparation of agar plates with MOPS-buffered RPMI 1640 medium-2% dextrose adjusted to the specified pH, Bacto Agar (1.5 g/dl), and previously described experimental procedures (2). Etest triazole results include a wide array of elliptical profiles and trailing growth that required interpretation based on Etest photographically illustrated technical guideline 4 (AB Biodisk NA, Piscataway, N.J.).
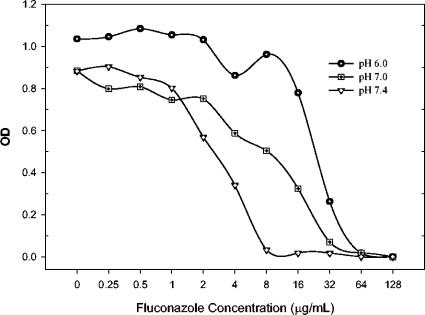
Tables 1, 2, and 3 include the specific MICs generated by the NCCLS M27-A2 (48 h), SYO (24 h), and Etest (48 h) methods at the three pH values used against fluconazole, itraconazole, and voriconazole, respectively. In general, the MICs of 2 of the 15 C. glabrata isolates and both ATCC strains were unaffected by the alterations in pH. These two clinical isolates included a highly susceptible strain (Z-zAN) and a highly resistant strain (Z-zAS) that demonstrated essentially identical MIC profiles at the three pH values and by all of the susceptibility test methods used. The two quality control American Type Culture Collection strains were not affected by pH. The MICs were more easily interpretable at pH 6.0 compared to either pH 7.0 or 7.4. The 48-h MICs generated by the NCCLS M27-A2 method with a spectrophotometric endpoint was identical to the visual endpoint for >90% of the isolates tested at all three pH values. Figure 1 illustrates the optical density profile of a C. glabrata isolate tested by the NCCLS M27-A2 method. The graph demonstrates the higher MIC but sharper and correspondingly easier to visually interpret endpoint seen at pH 6.0. Assessment of MICs by the Etest method demonstrated reduction in triazole MICs with incremental pH increases (Fig. 2).
TABLE 1.
Comparison of fluconazole MICs against C. glabrata isolates by the NCCLS M27-A2 (48 h), Sensititre YeastOne (24 h), and Etest (48 h) methods at different pHs
| Isolate | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 6.0
|
pH 7.0
|
pH 7.4
|
|||||||
| NCCLS | SYO | ETa | NCCLS | SYO | ET | NCCLS | SYO | ET | |
| 2 | 32 | 32 | 48 | 16 | 16 | 16 | 1 | 4 | 8 |
| J | 16 | 32 | 96 | 8 | 32 | 64 | 1 | 4 | 4 |
| P | 16 | 64 | >256 | 8 | 64 | 32 | 2 | 8 | 16 |
| Q | 32 | 64 | >256 | 8 | 64 | 64 | 1 | 8 | 16 |
| R | 32 | 64 | >256 | 8 | 64 | >256 | 2 | 8 | 16 |
| Z-M | 32 | 64 | 192 | 16 | 64 | 48 | 16 | 8 | 48 |
| Z-V | 32 | 64 | 128 | 16 | 32 | 48 | 16 | 8 | 16 |
| Z-Y | 32 | 64 | >256 | 16 | 64 | 64 | 8 | 8 | 8 |
| Z-Z | 32 | 64 | >256 | 16 | 64 | 64 | 8 | 8 | 32 |
| Z-zAA | 64 | 64 | 256 | 32 | 64 | 32 | 4 | 4 | 16 |
| Z-zAC | 64 | 64 | >256 | 64 | 128 | >256 | 16 | 4 | 48 |
| Z-zAD | 32 | 64 | >256 | 32 | 32 | 48 | 16 | 4 | 12 |
| Z-zAF | 32 | 32 | 64 | 4 | 16 | 32 | 4 | 4 | 8 |
| Z-zAN | 0.5 | 2 | 0.25 | 0.5 | 1 | 0.25 | 0.5 | <0.25 | 0.25 |
| Z-zAS | >128 | >128 | >256 | >128 | 128 | >256 | >128 | 64 | >256 |
ET, Etest.
TABLE 2.
Comparison of itraconazole MICs against C. glabrata isolates by the NCCLS M27-A2 (48 h), Sensititre YeastOne (24 h), and Etest (48 h) methods at different pHs
| Isolate | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 6.0
|
pH 7.0
|
pH 7.4
|
|||||||
| NCCLS | SYO | ETa | NCCLS | SYO | ET | NCCLS | SYO | ET | |
| 2 | 1 | 2 | >32 | 2 | 4 | 6 | 0.125 | 1 | 0.75 |
| J | 1 | >4 | >32 | 1 | 4 | 8 | 0.125 | 2 | 0.75 |
| P | 1 | >4 | >32 | 2 | >4 | 4 | 0.125 | >4 | 3 |
| Q | 2 | >4 | >32 | 1 | >4 | 4 | 0.125 | >4 | 2 |
| R | 2 | >4 | >32 | 1 | >4 | 4 | 0.125 | >4 | 2 |
| Z-M | 4 | >4 | >32 | 0.5 | >4 | >32 | 0.5 | >4 | 4 |
| Z-V | 4 | >4 | >32 | 4 | >4 | 8 | 1 | >4 | 2 |
| Z-Y | 4 | >4 | >32 | 4 | >4 | 16 | 0.5 | >4 | 1 |
| Z-Z | 4 | >4 | >32 | 4 | >4 | 8 | 0.5 | >4 | 4 |
| Z-zAA | 4 | >4 | >32 | 4 | 4 | 16 | 0.5 | 2 | 1.5 |
| Z-zAC | 4 | >4 | >32 | 1 | 4 | >32 | 1 | 0.5 | >32 |
| Z-zAD | 1 | 2 | >32 | 1 | 2 | 32 | 1 | 0.5 | 8 |
| Z-zAF | 2 | 1 | >32 | 1 | 1 | 6 | 0.5 | 0.5 | 2 |
| Z-zAN | 0.06 | 0.125 | 0.016 | 0.125 | 0.25 | 0.016 | 0.06 | 0.125 | 0.016 |
| Z-zAS | >4 | >4 | >32 | >4 | 4 | >32 | >4 | >4 | >32 |
ET, Etest.
TABLE 3.
Comparison of voriconazole MICs against C. glabrata isolates by the NCCLS M27-A2 (48 h), Sensititre YeastOne (24 h), and Etest (48 h) methods at different pHs
| Isolate | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 6.0
|
pH 7.0
|
pH 7.4
|
|||||||
| NCCLS | SYO | ETa | NCCLS | SYO | ET | NCCLS | SYO | ET | |
| 2 | 0.25 | 0.5 | 0.75 | 0.25 | 0.25 | 0.5 | 0.03 | 0.03 | 0.25 |
| J | 0.5 | 2 | 1 | 0.125 | 0.25 | 0.75 | 0.03 | 0.125 | 0.094 |
| P | 0.5 | 2 | 1.5 | 0.125 | 0.25 | 1 | 0.06 | 0.125 | 0.38 |
| Q | 1 | 2 | 2 | 0.25 | 0.5 | 0.75 | 0.06 | 0.25 | 0.5 |
| R | 1 | 2 | 1.5 | 0.25 | 0.5 | 0.75 | 0.06 | 0.25 | 0.38 |
| Z-M | 2 | 2 | 1.5 | 1 | 0.5 | 1 | 0.5 | 0.125 | 0.25 |
| Z-V | 2 | 2 | 1 | 2 | 0.5 | 0.75 | 0.5 | 0.25 | 0.38 |
| Z-Y | 2 | 2 | 1.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | 0.38 |
| Z-Z | 2 | 2 | 2 | 1 | 0.5 | 1 | 0.25 | 0.25 | 0.38 |
| Z-zAA | 2 | 2 | 1.5 | 1 | 0.25 | 1 | 0.25 | 0.125 | 0.19 |
| Z-zAC | 4 | 4 | 4 | 2 | 0.25 | 2 | 1 | 0.125 | 1.5 |
| Z-zAD | 1 | 1 | 1.5 | 0.5 | 0.125 | 0.38 | 0.5 | 0.06 | 0.19 |
| Z-zAF | 2 | 1 | 1 | 0.5 | 0.25 | 0.38 | 0.25 | 0.125 | 0.094 |
| Z-zAN | 0.015 | 0.06 | 0.006 | 0.015 | 0.015 | 0.008 | 0.015 | 0.008 | 0.006 |
| Z-zAS | >4 | >4 | >32 | 4 | 4 | 16 | 4 | 2 | 4 |
ET, Etest.
FIG. 1.
Optical density (OD) of microwells at 48 h of fluconazole exposure at the specified pH values against C. glabrata (isolate R).
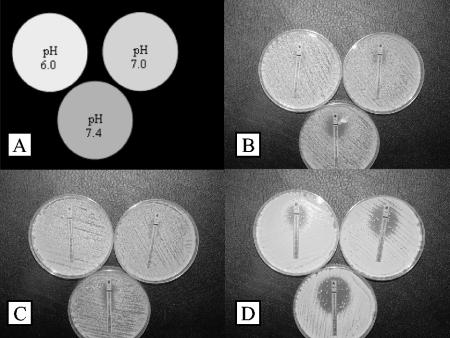
FIG. 2.
Forty-eight-hour triazole Etest results of C. glabrata (isolate Z-zAD). Panels: A, key; B, fluconazole; C, itraconazole; D, voriconazole.
The 24-h MICs were within 2 doubling dilutions of the 48-h MICs for 93 to 100% of the isolates at pHs 6.0, 7.0, and 7.4. Reduction of the pH to 6.0 was associated with significantly higher MICs (2 doubling dilutions higher) of fluconazole by both the NCCLS M27-A2 and Etest methods (P < 0.001) but not by the SYO method (P > 0.05). Conversely, the MICs at pH 7.4 were significantly lower than those at pH 7.0 by approximately 2 to 3 dilutions for all three susceptibility-testing methods (P < 0.01). More importantly, the number of isolates classified as fluconazole susceptible (≤8 μg/ml) was different: 6.6% at pH 6.0, 40% at pH 7.0, and 66% at pH 7.4. Itraconazole MICs at pH 7.4 were significantly lower than those at pH 7.0 by all three susceptibility-testing methods (P < 0.02). However, the MICs at pH 6.0 were not significantly higher than those at pH 7.0. Most of the isolates were resistant to itraconazole at pH 7.0, but 38% of them were susceptible (≤0.125 μg/ml) and 38% were dose-dependently susceptible (0.25 to 0.5 μg/ml) at pH 7.4 by the NCCLS M27-A2 method. Reduction of the pH to 6.0 was associated with significantly higher voriconazole MICs (2 doubling dilutions higher) by both the NCCLS M27-A2 and SYO methods (P < 0.001) but not by the Etest method (P > 0.05). These MICs at pH 7.4 were significantly lower than those at pH 7.0 by approximately 2 dilutions by all three susceptibility-testing methods (P < 0.001). The percentages of isolates classified as voriconazole susceptible (≤1 μg/ml) were different: 47% at pH 6.0, 80% at pH 7.0, and 93% at pH 7.4.
As demonstrated in the present study, the NCCLS M27-A2 method with media buffered to pH 7.0 did not consistently predict the activity of triazoles against C. glabrata at urine and blood pH values. The susceptibility profiles of fluconazole, itraconazole, and voriconazole were markedly improved at pH 7.4 compared to those at pH 7.0 when C. glabrata was tested. The two commercially available susceptibility-testing methods produced similar results. Further investigations assessing the clinical utility of antifungal susceptibility testing should take these findings into consideration.
REFERENCES
- 1.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C. Knapp, N. Holliday, and S. B. Killian. 2004. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 42:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Favel A., A. Michel-Nguyen, A. Datry, S. Challier, F. Leclerc, C. Chastin, K. Fallague, and P. Regli. 2004. Susceptibility of clinical isolates of Candida lusitaniae to five systemic antifungal agents. J. Antimicrob. Chemother. 53:526-529. [DOI] [PubMed] [Google Scholar]
- 3.Gadea, I., M. Cuenca, M. I. Gegúndez, J. Zapardiel, M. L. Valero, and F. Soriano. 1997. Effect of pH and buffer system on the in-vitro activity of five antifungals against yeasts. J. Antimicrob. Chemother. 39:453-459. [DOI] [PubMed] [Google Scholar]
- 4.García, M. T., M. T. Llorente, F. Mínguez, and J. Prieto. 2000. Influence of pH and concentration on the postantifungal effect and on the effects of sub-MIC concentrations of 4 antifungal agents on previously treated Candida spp. Scand. J. Infect. Dis. 32:669-673. [DOI] [PubMed] [Google Scholar]
- 5.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]