Abstract
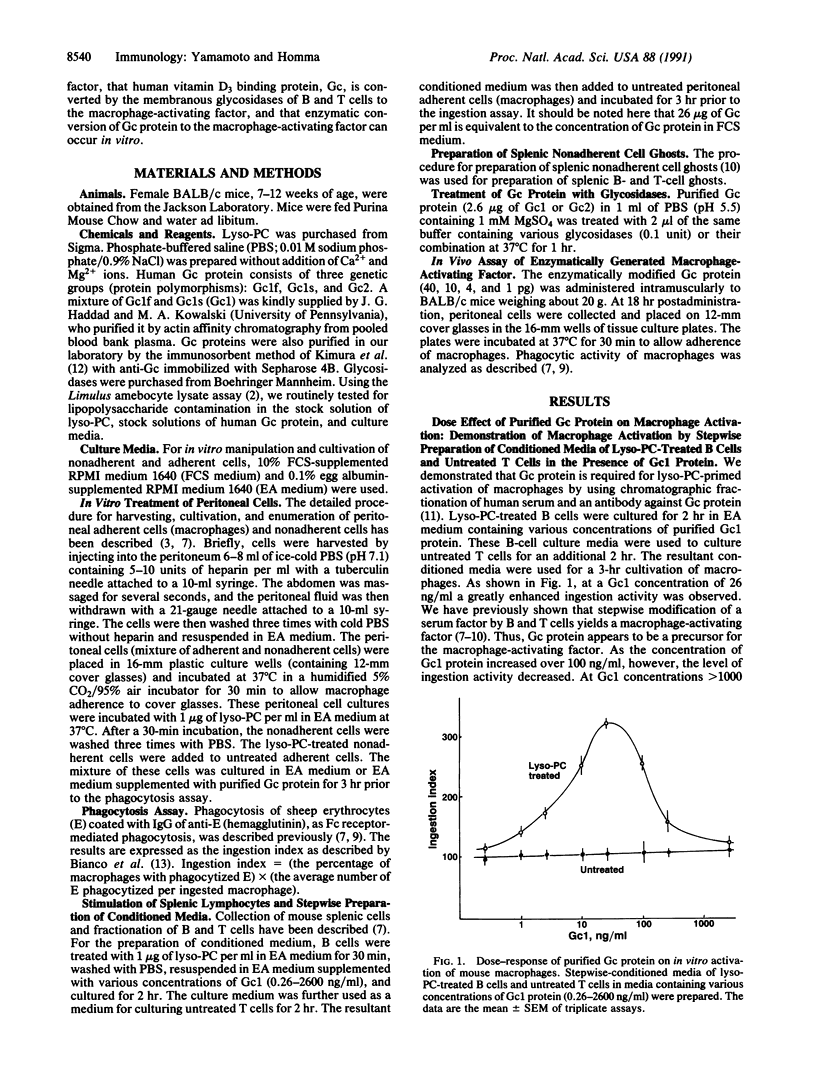
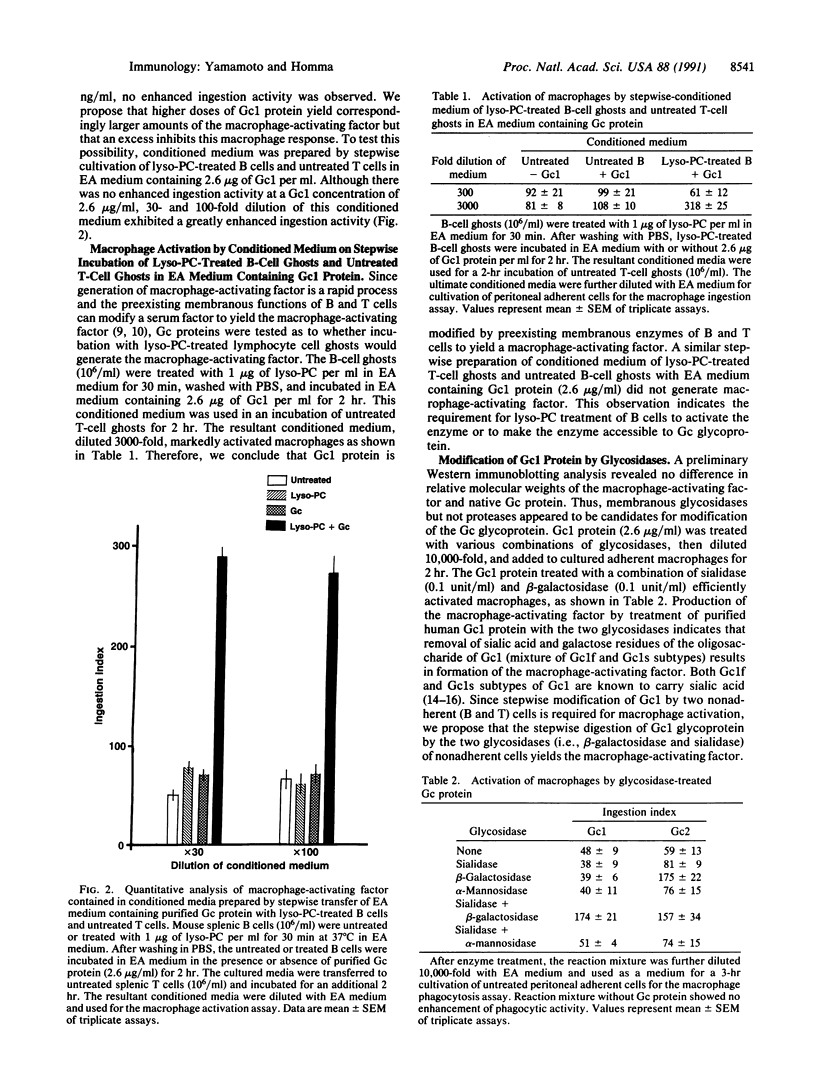
A brief (30 min) treatment of mouse peritoneal cells (mixture of nonadherent lymphocytes and adherent macrophages) with 1-20 micrograms of lysophosphatidylcholine (lyso-PC) per ml in serum-supplemented RPMI medium 1640, followed by a 3-hr cultivation of the adherent cells alone, results in a greatly enhanced Fc receptor-mediated phagocytic activity of macrophages. This rapid process of macrophage activation was found to require a serum factor, the vitamin D3 binding protein (the human protein is known as group-specific component; Gc). Efficient activation of macrophages was achieved by using medium containing purified human Gc protein. Analysis of intercellular signal transmission among nonadherent (B and T) cells revealed that lyso-PC-treated B cells modify Gc protein to yield a proactivating factor, which can be converted by T cells to the macrophage-activating factor. This rapid generation process of the macrophage-activating factor was also demonstrated by stepwise incubation of Gc protein with lyso-PC-treated B-cell ghosts and untreated T-cell ghosts, suggesting that Gc protein is modified by preexisting membranous enzymes to yield the macrophage-activating factor. Incubation of Gc protein with a mixture of beta-galactosidase and sialidase efficiently generated the macrophage-activating factor. Stepwise incubation of Gc protein with B- or T-cell ghosts and sialidase or beta-galactosidase revealed that Gc protein is modified by beta-galactosidase of B cells and sialidase of T cells to yield the macrophage-activating factor. Administration to mice of a minute amount (4-10 pg per mouse) of in vitro, enzymatically generated macrophage-activating factor resulted in a greatly enhanced (3- to 7-fold) ingestion activity of macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIDS and the Gc protein. Lancet. 1987 Jun 13;1(8546):1377–1378. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppenhaver D. H., Sollenne N. P., Bowman B. H. Post-translational heterogeneity of the human vitamin D-binding protein (group-specific component). Arch Biochem Biophys. 1983 Oct 1;226(1):218–223. doi: 10.1016/0003-9861(83)90287-4. [DOI] [PubMed] [Google Scholar]
- Homma S., Millman I., Yamamoto N. A serum factor for macrophage activation after in vitro dodecylglycerol treatment of mouse lymphocytes. Immunol Cell Biol. 1990 Apr;68(Pt 2):137–142. doi: 10.1038/icb.1990.19. [DOI] [PubMed] [Google Scholar]
- Homma S., Yamamoto N. Activation process of macrophages after in vitro treatment of mouse lymphocytes with dodecylglycerol. Clin Exp Immunol. 1990 Feb;79(2):307–313. doi: 10.1111/j.1365-2249.1990.tb05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. V., Morris H. P., Bailey J. M. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972 Jul;32(7):1533–1538. [PubMed] [Google Scholar]
- Kimura H., Saheki S., Yoshida K., Ohsawa M. Characterization of purified group-specific components (Gc 1 and Gc 2 proteins) from human plasma. Nihon Hoigaku Zasshi. 1985 Apr;39(2):113–123. [PubMed] [Google Scholar]
- Landolfi N. F., Leone J., Womack J. E., Cook R. G. Activation of T lymphocytes results in an increase in H-2-encoded neuraminidase. Immunogenetics. 1985;22(2):159–167. doi: 10.1007/BF00563513. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Activation of peritoneal macrophages by lysophosphatidylcholine. Biochim Biophys Acta. 1985 Mar 29;839(1):9–15. doi: 10.1016/0304-4165(85)90175-8. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Contribution of lysophosphatidylcholine-treated nonadherent cells to mechanism of macrophage activation. Proc Soc Exp Biol Med. 1990 Feb;193(2):118–124. doi: 10.3181/00379727-193-43011. [DOI] [PubMed] [Google Scholar]
- Potier M., Lu Shun Yan D., Womack J. E. Neuraminidase deficiency in the mouse. FEBS Lett. 1979 Dec 15;108(2):345–348. doi: 10.1016/0014-5793(79)80560-8. [DOI] [PubMed] [Google Scholar]
- Snyder F., Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969 Jan;29(1):251–257. [PubMed] [Google Scholar]
- Svasti J., Bowman B. H. Human group-specific component. Changes in electrophoretic mobility resulting from vitamin D binding and from neuraminidase digestion. J Biol Chem. 1978 Jun 25;253(12):4188–4194. [PubMed] [Google Scholar]
- Taira S., Nariuchi H. Possible role of neuraminidase in activated T cells in the recognition of allogeneic Ia. J Immunol. 1988 Jul 15;141(2):440–446. [PubMed] [Google Scholar]
- Viau M., Constans J., Debray H., Montreuil J. Isolation and characterization of the O-glycan chain of the human vitamin-D binding protein. Biochem Biophys Res Commun. 1983 Nov 30;117(1):324–331. doi: 10.1016/0006-291x(83)91579-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Homma S., Millman I. Identification of the serum factor required for in vitro activation of macrophages. Role of vitamin D3-binding protein (group specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J Immunol. 1991 Jul 1;147(1):273–280. [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z. Activation of mouse peritoneal macrophages by lysophospholipids and ether derivatives of neutral lipids and phospholipids. Cancer Res. 1987 Apr 15;47(8):2008–2013. [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z., Sery T. W., Pieringer R. A. Activation of macrophages by ether analogues of lysophospholipids. Cancer Immunol Immunother. 1987;25(3):185–192. doi: 10.1007/BF00199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., St Claire D. A., Jr, Homma S., Ngwenya B. Z. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988 Nov 1;48(21):6044–6049. [PubMed] [Google Scholar]



