Abstract
Trimethoprim-sulfamethoxazole and pentamidine isethionate have been used extensively for the prophylaxis and therapy of pneumonia caused by Pneumocystis jirovecii. Problems associated with toxicity and potential emerging resistance for both therapies necessitate the development of safe and effective analogs or new treatment strategies. In the present study, a library of 36 compounds was synthesized by using the pentamidine molecule as the parent compound modified by a 1,4-piperazinediyl moiety as the central linker to restrict conformation flexibility. The compounds were evaluated for anti-Pneumocystis carinii activity in a bioluminescent ATP-driven assay. Four of the compounds were highly active, with 50% inhibitory concentration (IC50) values of <0.01 μg/ml; four had very marked activity (IC50 < 0.10 μg/ml); ten had marked activity (IC50 < 1.0 μg/ml); nine had moderate activity (IC50 < 10 μg/ml); one had slight activity (IC50 = 34.1 μg/ml); and the remaining eight did not demonstrate activity in this assay system. The high level of activity was specifically associated with an alkyl chain length of five to six carbons attached to one of the nitrogens of the bisamidinium groups. None of the highly active compounds and only one of the very marked compounds exhibited any toxicity when evaluated in three mammalian cell lines. The strategy of substitution of 1,4-piperazine-linked bisbenzamidines produced compounds with the highest level of activity observed in the ATP assay and holds great promise for the development of efficacious anti-P. carinii therapy.
Despite advances in the treatment of human immunodeficiency virus infection, Pneumocystis jirovecii pneumonia remains a leading cause of opportunistic infection and mortality in human immunodeficiency virus-infected patients. Currently available anti-Pneumocystis drugs are limited by significant problems of efficacy, toxicity, and emerging resistance (14, 21, 37, 38). No member of the genus Pneumocystis can be maintained continuously outside the mammalian lung. Thus, drug development, as well as other aspects of investigation of this organism family, has been hindered.
The effective use of pentamidine isethionate for the treatment of human Pneumocystis pneumonia was first reported in 1958 (18), and the early experience with the drug was summarized in 1967 (19). Trimethoprim-sulfamethoxazole (TMP-SMZ) later became the therapy of choice for this pneumonia due to increased efficacy and reduced toxicity (16). Despite concerted efforts focusing on modifications of the dihydrofolate reductase and dihydropteroate inhibitor portions of TMP-SMZ and the diamidine structure of pentamidine, no compound with increased anti-Pneumocystis carinii properties without toxicity has emerged as a clinical drug (11). With the potential problem of emerging resistance to the sulfa component of TMP-SMZ (1, 21, 26), the significant failure rate of prophylactic pentamidine, and its limited spectrum (17) and associated toxicity (2), it is necessary to identify new therapies or modifications of existing compounds that provide increased efficacy with no toxicity to the host.
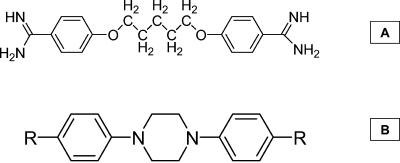
From a structural point of view, pentamidine can be considered as a bisbenzamidine derivative in which both benzamidine moieties are linked by a highly flexible pentyldioxy chain (Fig. 1A). We have been interested (12, 24, 25, 31, 34) in determining the effect of restricting the conformational flexibility of pentamidine congeners on their anti-P. carinii and antiparasitic activity. Based on these recent studies, we identified 4,4′-(1,4-piperazinediyl)bisbenzenecarboximidamide (compound 19, Table 1 as a promising lead compound. Therefore, we focused our attention on the 1,4-piperazinediyl skeleton as a rigid linker (Fig. 1B) and developed a library of piperazine-linked bisbenzamidines and related compounds for a comprehensive study on their structure-activity relationships. To further modulate the biological activity of this series of compounds, we introduced an alkyl or cycloalkyl group of variable length and size on one of the nitrogen atoms of the amidine moieties. The importance of the terminal basic amidine functions were assessed by replacing them with other nonbasic or less-basic functionalities.
FIG. 1.
Structure of pentamidine and general structure of the piperazine-linked bisbenzamidines. (A) Pentamidine has two benzamidine moieties linked by a pentyldioxy chain. (B) 1,4-piperazinediyl parent compound. Alkyl or cycloalkyl groups were introduced on one of the nitrogen atoms of the amidine moieties (R).
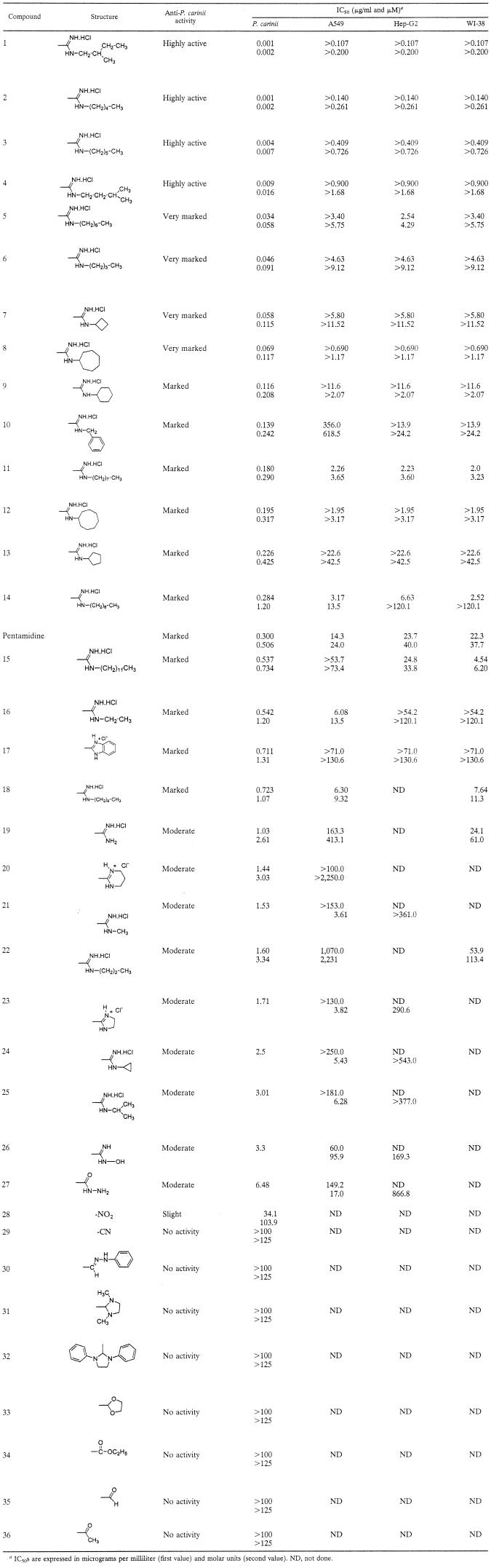
TABLE 1.
Structures and biological activities of pentamidine, piperazine-linked bisbenzamidines, and structurally related 1,4-diarylpiperazines
IC50s are expressed in micrograms per milliliter (first value) and molar units (second value). ND, not done.
The anti-P. carinii activities of these compounds were evaluated in a cell-free ex vivo maintenance system that relies on the assessment of viability by measurement of P. carinii ATP with a luciferase-luciferin bioluminescence assay (8). Several of the compounds exhibited very high anti-P. carinii activity without toxicity to three mammalian cell lines. These compounds hold strong promise for new therapeutic modalities for Pneumocystis pneumonia and are currently being tested in rodent models for evaluation of in vivo efficacy.
MATERIALS AND METHODS
Organism sources.
P. carinii were obtained from chronically immunosuppressed Long Evans and Brown Norway rats housed under conventional conditions at the Cincinnati VA Medical Center or from CD rats (Charles River Laboratories, Hollister, Calif.) inoculated intratracheally with P. carinii and maintained under barrier conditions at the University of Cincinnati Laboratory Animal Medicine Unit (Cincinnati, Ohio) (10). P. carinii were extracted and purified from the lungs of rats after 8 to 12 weeks of immunosuppression, enumerated, cryopreserved, and stored in liquid nitrogen as previously described (7, 9). Typically, infected rat lungs yield up to 2 × 1010 organism nuclei, with the vast majority (ca. 95%) of the life cycle forms present as trophic forms and the remainder (ca. 5%) being composed of cysts. P. carinii preparations were evaluated for microbial contamination, ATP content, karyotype, and host cell content prior to use in the ATP assay (7).
ATP assay.
Cryopreserved organisms were thawed rapidly at 37°C, centrifuged to remove the cryoprotectants, and resuspended in RPMI medium with 20% calf serum and other additives (e.g., nonessential amino acids) (pH 7.5 to 8.0), 380 mOsm (7, 20). Drugs to be tested were prepared in culture medium or in dimethyl sulfoxide (DMSO; final concentration, <0.2% [vol/vol]), and 108 nuclei/ml were added in 0.5 ml of culture medium to each well of a 48-well plate. Each drug concentration was studied in triplicate in at least two different assays with two different batches of P. carinii. Media without drug, with P. carinii, and with 10 μg of ampicillin/ml served as negative controls. For each assay, a set of triplicate wells received pentamidine isethionate at 1 μg/ml as the positive drug activity control. The plates were incubated at 35°C in 5% CO2. At 24, 48, and 72 h, the wells were agitated, and 10-μl samples from each well removed and placed directly into individual wells of a 96-well white plate (Greiner Scientific, Ocala, Fla.) containing 30 μl of 3.5% trichloroacetic acid in 2 mM EDTA (pH 1.5) to release intracellular ATP. The samples were automatically mixed with the luciferin/luciferase reagent via an injector and immediately measured for light emission at 562 nm with a FluoSTAR Optima plate reader (BMG Labtechnologies, Inc.). Each well was read in triplicate,and values are expressed as the average relative light units (RLU).
In addition to the negative and positive controls for drug activity mentioned above, a quench control was run for every drug tested. This control evaluated whether the drug had an inhibitory effect on the enzyme-substrate reaction. The highest concentration of a drug used in an assay was placed in triplicate wells containing 10−7 M ATP, luciferin, and luciferase and compared to wells without the drug but containing the ATP and reagents. Any reduction in RLU from wells with the drug versus wells without the drug would indicate inhibition of the reaction. No such inhibition was observed with the compounds in the present study.
Each compound was initially evaluated at 100 μg/ml to screen out inactive compounds. If a 50% or greater decrease in ATP compared to untreated organisms was observed for the compound at 100 μg/ml, a three-concentration series was then run to determine the 50% inhibitory concentration (IC50; 10, 1, and 0.1 μg/ml). Compounds with a high degree of activity (reduction to <50% of control levels at all concentrations) were tested again at further reduced concentrations (nanogram levels).
The effects of drugs on the ATP of Pneumocystis pools were calculated by using the following formula: {[(A − B) − (C − B)]/(A − B)} × 100 = percent decrease in ATP, where A is the average RLU of medium/ampicillin control, B is the background RLU, and C is the average RLU of drug treatment.
Data analysis.
The percent decrease in ATP content of a compound was used in a linear regression formula with the log drug concentrations to determine the IC50 (GraphPad Software v2 for Science; GraphPad, San Diego, Calif.). Based on the IC50 values, each agent was classified by using an activity scale. The original scale had four categories: very marked, marked, moderate, and none (8). A fifth category of “slight” activity was added to expand the scale to accommodate drugs that are able to attain levels higher than 10 μg/ml in serum and were effective in vitro at between 10 and 50 μg/ml in our ATP assay (39). In the present study, we further extended the scale to accommodate compounds with the highest in vitro activity observed to date, i.e., highly active. The categories in the current drug activity scale are thus as follows: highly active (compounds with an IC50 of <0.010 μg/ml), very marked (IC50s of 0.011 to 0.099 μg/ml), marked (IC50s from 0.10 to 0.99 μg/ml), moderate (IC50s from 1.0 to 9.99 μg/ml), slight (IC50s from 10.0 to 49.9 μg/ml), and none (i.e., inactive; IC50s of ≥50 μg/ml).
Toxicity to mammalian cells.
Compounds demonstrating high, very marked, and marked activities on the rating scale were subsequently tested for toxicity to mammalian cells before consideration for in vivo testing in the mouse (Pneumocystis murina) pneumonia model. The ATP assay was used to evaluate viability of mammalian cell monolayers in a manner similar to that established for assessment of anti-P. carinii activity. Confluent monolayers of the lung cell carcinoma cell line A549 (ATCC CCL-185), the liver cell line Hep-G2 (ATCC HB-8065), and the primary diploid lung fibroblast line WI-38 (ATCC CL-75) were established in 48-well plates containing 1 ml of the appropriate American Type Culture Collection-recommended growth medium (www.atcc.org). Media containing 1, 10, and 100 times the concentration of the IC50 calculated for anti-P. carinii activity were added to the plate wells in triplicate. Medium alone served as a negative control, and antimycin A (75 μg/ml) was used as a positive control. The plates were incubated at 35°C, 5% CO2. At 24, 48, and 72 h, media in each well were aspirated, and 1 ml of 3.5% trichloroacetic acid in 2 mM EDTA (pH 1.5) was added to each well. After incubation at room temperature for 10 min, the plates were stored at −20°C until completion of the experiment. The plates were processed by thawing them to room temperature with agitation. Then, 5-μl samples from each well were removed and placed directly into individual wells of a 96-well opaque white plate containing 100 μl of buffer (200 mM Tris, 2.5 mM EDTA [pH 7.75]). The RLU value corresponding to the ATP content was determined as described above.
Chemical syntheses.
The key step for the preparation of 1,4-diarylpiperazines was a double nucleophilic displacement of fluorine in 4-fluoro derivatives by the nitrogen atoms of piperazine. That reaction, performed in boiling dimethyl formamide, produces the expected tricyclic molecules in good yields (e.g., compound 29 was obtained in 70% yield), provided the aromatic precursor bears a strong electron-withdrawing group in the para position. Conversion of the bisbenzonitrile derivative into the targeted bisbenzamidines was effected by the Pinner reaction (29). 1H nuclear magnetic resonance (NMR) spectra were obtained by using a Varian Inova instrument (500 MHz), chemical shifts (δ) are given in parts per million (ppm) with tetramethylsilane (TMS) as an internal reference. Infrared (IR) spectra were recorded on a Perkin-Elmer Spectrum One instrument operating in the diffuse reflectance mode. Solvents and reagents are commercially available (Aldrich Co., Acros Organics, Fisher Scientific, and Sigma Chemical Co.) and were used without further purification.
Compounds 6 (25%), 10 (50%), 13 (50%), and 20 (30%) (24), compounds 23 (40%) and 19 (45%) (31), compounds 9 (35%), 22 (40%), 24 (35%), 25 (55%), 26 (20%), 33 (45%), and 36 (80%) (34), compounds 17 (65%), 21 (40%), 27 (10%), 31 (60%), 32 (70%), and 34 (10%) (25), and compounds 28 (85%) (40), 29 (70%) (4), 30 (65%) (30), and 35 (80%) (32) were prepared according to published procedures, and the overall percent yields obtained in the present study are indicated in parentheses. Elemental analyses were performed by M-H-W Laboratories, Phoenix, Ariz. There is a patent pending for compounds 1 through 36 (M. T. Cushion, P. D. Walzer, T. L. Huang, J. J. Vanden Eynde, and A. Mayence, 25 November 2003, U.S. patent application 60/525-089, patent pending).
General procedures for preparation: 4,4′-(1,4-piperazinediyl)bisbenzenecarboximidamides.
A mixture of 4,4′-(1,4-piperazinediyl)bisbenzonitrile (2 mmol; 0.6 g) in dichloromethane (250 ml) and methanol (10 ml) was saturated with HCl gas, and the reaction medium was left at room temperature for 4 days. The precipitate (crude imidate) was filtered, washed with acetone, and treated with the appropriate amine (20 mmol) in refluxing ethanol (50 ml) for 1 h. After it cooled, the precipitate was filtered and thoroughly washed. When no precipitation occurred, the solution was concentrated under reduced pressure, and the residue was triturated with ether; the solid was filtered and thoroughly washed. Pure analytical samples were obtained without further purification.
4,4′-(1,4-Piperazinediyl)bis[N-(2-methylbut-1-yl) benzenecarboximidamide], dihydrochloride salt 1.
The overall yield (based on compound 29) was as follows: 55%; melting point (mp) > 300°C; 1H NMR (DMSO-d6) δ 9.4 (br s, 2 H), 9.2 (br s, 2 H), 8.8 (br s, 2 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (m, 4 H), 1.8 (m, 2 H, J = 7 Hz),1.5 (m, 2 H), 1.2 (m, 4 H), and 0.8 (m, 12 H, J = 7 Hz) ppm; IR = 3,062, 1,667, 1,606, 1,515, 1,450, and 1,235 cm−1. Anal. Calc. for C28H42N60.2 HCl (535.59) C, 62.79; H, 8.28; and N, 15.69. Found: C, 62.52; H,7.94; and N, 15.49.
4,4′-(1,4-Piperazinediyl)bis(N-pentyl benzenecarboximidamide), dihydrochloride salt 2.
The overall yield (based on compound 29) was as follows: 45%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.5 (br s, 2 H), 9.2 (br s, 2 H), 8.7 (br s, 2 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.3 (t, 4 H, J = 7 Hz), 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 8 H, J = 7 Hz), and 0.9 (t, 6 H, J = 7 Hz) ppm; IR = 3,063, 1,672, 1,606, 1,515, 1,396, and 1,235 cm−1. Anal. Calc. for C28H42N60.2 HCl (535.59) C, 62.79; H, 8.28; and N, 15.69. Found: C, 62.59; H,8.44; and N, 15.48.
4,4′-(1,4-Piperazinediyl)bis(N-hexyl benzenecarboximidamide), dihydrochloride salt 3.
The overall yield (based on compound 29) was as follows: 75%; mp 295 to 297°C; 1H NMR (DMSO-d6) δ 9.0 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H, J = 7 Hz), 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 8 H), and 0.9 (t, 6 H, J = 8 Hz) ppm; IR = 3,171, 1,674, 1,620, 1,520, 1,394, and 1,166 cm−1. Anal. Calc. for C30H46N60.2 HCl.1H2O (581.66) C, 61.95; H, 8.66; and N, 14.45. Found: C, 61.69; H, 8.29; and N, 14.28.
4,4′-(1,4-Piperazinediyl)bis[N-(4-methylbut-1-yl) benzenecarboximidamide], dihydrochloride salt 4.
The overall yield (based on compound 29) was follows: 40%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.4 (br s, 2 H), 9.2 (br s, 2 H), 8.7 (br s, 2 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H), 1.7 (m, 2 H), 1.5 (m, 4 H, J = 7 Hz), and 0.9 (m, 12 H, J = 7 Hz) ppm; IR = 3,093, 1,668, 1,605, 1,515, 1,393, and 1,235 cm−1. Anal. Calc. for C28H42N60.2 HCl (535.59) C, 62.79; H, 8.28; and N, 15.69. Found: C, 62.52; H, 8.37; and N, 15.83.
4,4′-(1,4-Piperazinediyl)bis(N-heptyl benzenecarboximidamide), dihydrochloride salt 5.
The overall yield (based on compound 29) was follows: 40%; mp 286°C (decomp); 1H NMR (DMSO-d6) δ 9.6 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H), 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 16 H), and 0.8 (t, 6 H, J = 7 Hz) ppm; IR = 3,099, 1,678, 1,608, 1,518, 1,392, and 1,240 cm−1. Anal. Calc. for C32H50N60.2 HCl.1 H2O (609.72) C, 63.03; H, 8.93; N, 13.78. Found: C, 62.59; H,8.73; and N, 13.66.
4,4′-(1,4-Piperazinediyl)bis(N-cyclobutyl benzenecarboximidamide), dihydrochloride salt 6.
The overall yield (based on compound 29) was follows: 25%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.6 (br s, 2 H), 9.1 (br s, 2 H), 8.6 (br s, 2 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 4.2 (m, 2 H, J = 8 Hz), 3.5 (s, 8 H), 2.4 and 2.2 (2 m, 8 H, J = 8 Hz), and 1.8 (2 m, 4 H, J = 8 Hz) ppm; IR = 3,076, 1,667, 1,601, 1,519, and 1,236 cm−1. Anal. Calc. for C26H34N60.2 HCl (503.51) C, 62.02; H, 7.21; and N, 16.69. Found: C, 62.24; H,7.27; and N, 16.85.
4,4′-(1,4-Piperazinediyl)bis(N-cycloheptyl benzenecarboximidamide), dihydrochloride salt 8.
The overall yield (based on compound 29) was follows: 40%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.0 (br s, 6 H), 7.6 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.9 (m, 2 H, J = 4 Hz), 3.5 (s, 8 H), 1.9 (m, 4 H), 1.6-1.5 (m, 12 H, J = 8 Hz), and 1.4 (m, 8 H) ppm; IR = 3,062, 1,669, 1,605, 1,516, and 1,230 cm−1. Anal. Calc. for C32H46N60.2 HCl (587.67) C, 65.40; H, 8.23; and N, 14.30. Found: C, 65.27; H, 8.04; and N, 14.32.
4,4′-(1,4-Piperazinediyl)bis(N-octyl benzenecarboximidamide), dihydrochloride salt 11.
The overall yield (based on compound 29) was follows: 60%; mp 235°C (decomp); 1H NMR (DMSO-d6) δ 9.0 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H, J = 7 Hz) 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 20 H), and 0.8 (t, 6 H, J = 7 Hz) ppm; IR 3,107, 1,678, 1,611, 1,518, and 1,394 cm−1. Anal. Calc. for C34H54N60.2 HCl.1.5 H2O (646.78) C, 63.14; H, 9.19; and N, 12.99. Found: C, 62.97; H, 8.81; and N, 13.11.
4,4′-(1,4-Piperazinediyl)bis(N-cyclooctyl benzenecarboximidamide), dihydrochloride salt 12.
The overall yield (based on compound 29) was follows: 45%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.0 (br s, 6 H), 7.6 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.9 (m, 2 H, J = 4 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H, J = 7 Hz) 1.7 (m, 12 H), and 1.5 (m, 16 H) ppm; IR = 3,145, 1,661, 1,601, 1,516, and 1,447 cm−1. Anal. Calc. for C34H50N6. 2 HCl.2H2O (651.73) C, 62.66; H, 8.66; and N, 12.90. Found: C, 62.79; H, 8.44; and N, 13.07.
4,4′-(1,4-Piperazinediyl)bis(N-nonyl benzenecarboximidamide), dihydrochloride salt 14.
The overall yield (based on compound 29) was follows: 70%; mp 256°C (decomp); 1H NMR (DMSO-d6) δ 8.9 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H, J = 7 Hz) 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 24 H), and 0.8 (t, 6 H, J = 7 Hz) ppm; IR = 3,112, 1,682, 1,614, 1,519, and 1,394 cm−1. Anal. Calc. for C36H58N60.2 HCl.1.5 H2O (674.83) C, 64.07; H, 9.41; and N, 12.45. Found: C, 63.93; H, 9.06; and N, 12.33.
4,4′-(1,4-Piperazinediyl)bis(N-dodecyl benzenecarboximidamide), dihydrochloride salt 15.
The overall yield (based on compound 29) was follows: 75%; mp 284°C (decomp); 1H NMR (DMSO-d6) δ 8.6 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H) 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 36 H), and 0.8 (t, 6 H, J = 7 Hz) ppm; IR = 3,110, 1,679, 1,614, 1,519, and 1,395 cm−1. Anal. Calc. for C42H70N60.2 HCl.1 H2O (749.98) C, 67.26; H, 9.94; and N, 11.20. Found: C, 67.33; H, 9.91; and N, 10.85.
4,4′-(1,4-Piperazinediyl)bis(N-ethyl benzenecarboximidamide), dihydrochloride salt 16.
The overall yield (based on compound 29) was follows: 60%; mp > 300°C; 1H NMR (DMSO-d6) δ 9.5 (br s, 2 H), 9.1 (br s, 2 H), 8.7 (br s, 2 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (q, 4 H, J = 7 Hz), and 1.2 (t, 6 H, J = 7 Hz) ppm; IR = 3,135, 1,667, 1,621, 1,519, and 1,397 cm−1. Anal. Calc. for C22H30N60.2 HCl.0.5 H2O (460.44) C, 57.39; H, 7.22; and N, 18.25. Found: C, 57.21; H, 7.31; and N, 18.00.
4,4′-(1,4-Piperazinediyl)bis(N-decyl benzenecarboximidamide), dihydrochloride salt 18.
The overall yield (based on compound 29) was follows: 50%; mp 271°C (decomp); 1H NMR (DMSO-d6) δ 8.9 (br s, 6 H), 7.7 (d, 4 H, J = 9 Hz), 7.1 (d, 4 H, J = 9 Hz), 3.5 (s, 8 H), 3.4 (t, 4 H) 1.6 (m, 4 H, J = 7 Hz), 1.3 (m, 28 H), and 0.8 (t, 6 H, J = 7 Hz) ppm; IR = 3,110, 1,679, 1,613, 1,519, and 1,242 cm−1. Anal. Calc. for C38H62N60.2 HCl. (675.86) C, 67.53; H, 9.54; and N, 12.43. Found: C, 67.73; H, 9.46; and N, 12.18.
RESULTS
Chemical syntheses.
In the syntheses of this series of compounds, the pentamidine structure (Fig. 1A) was modified by inserting a 1,4-piperazinediyl linker between the two benzamidine groups (R = amidine, compound 19). The N-substituted derivatives were obtained by a Pinner reaction performed with 4,4′-(1,4-piperazinediyl)bisbenzonitrile 29 and the appropriate amine. Compounds 20 and 23, in which the amidine moiety was included in a ring, were prepared in a similar way but with 1,3-diaminopropane and 1,3-diaminoethane, respectively. The substance bearing an amidoxime group (compound 26) was also synthesized by a Pinner reaction because the action of hydroxylamine on compound 29 did not produce compound 26. The bisbenzaldehyde 35 (obtained from piperazine and 4-fluorobenzaldehyde) was used as the starting material to afford the bisbenzimidazole derivative 17, the phenylhydrazone-containing substance 30, and products substituted by dioxolanes (compound 33) or imidazolidines (compounds 31, 32) rings. 1,4-Diarylpiperazines 28 (dinitro derivative), 34 (diester), and 36 (diketone) were readily accessible by direct condensation of piperazine with the appropriate 4-fluoroarene. Finally, a reaction between 34 and hydrazine produced the dicarboxhydrazide 27.
Anti-P. carinii activities.
The structure-activity relationships for the piperazine-linked bisbenzamidines and the toxicities of each compound in three different mammalian cell lines are shown in Table 1. Compounds were ranked in order of the lowest concentration necessary to reduce the ATP content of P. carinii populations by 50% (i.e., the IC50) compared to untreated control populations (8). Note that the IC50 values are expressed on a microgram per milliliter and micromolar basis. The activity level was expressed on a scale (from highly active to no activity) modified from previous studies (8, 39). At the time the scale was established, no compounds were effective below an IC50 of 0.012 μg/ml (e.g., potassium cyanide); thus, <0.100 μg/ml was set as the highest level of activity and given a semiqualitative assessment as “very marked.” In the present study, we identified four compounds with IC50 values that were reduced in concentration by a log or more from the very-marked ranking. To differentiate this activity, a rank of highly active was assigned and defined as an IC50 of <0.010 μg/ml.
Three of the four highly active compounds contained branched alkyl groups (compounds 1 and 4) and an n-alkyl group (compound 2) of five carbons, with the other n-alkyl group containing an additional sixth carbon (compound 3). This requirement for a five- to six-carbon chain was quite specific, since the addition of one carbon to the alkyl chain increased the IC50 to 0.034 μg/ml (compound 5) and a reduction to a four-carbon alkyl chain resulted in an IC50 of 0.046 μg/ml (compound 6). However, both of these compounds retained very high inhibitory activities with a ranking of very marked. The other two compounds in the very-marked activity group contained 4 (compound 7) and 7 (compound 8)-carbon cycloalkyl groups. Derivatives bearing a cyclic ring of five (compound 13), six (compound 9), or eight (compound 12) carbon atoms exhibited only a marked activity.
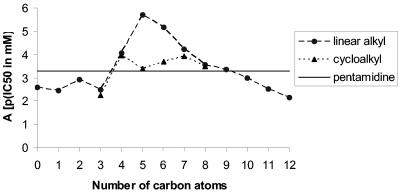
An association with the presence of five to six carbon atoms in the linear alkyl group and the highest inhibitory activity was apparent. A graphic representation of this relationship is shown in Fig. 2. The trend is similar but not as dramatic for the cycloalkyl carbon atom number.
FIG. 2.
Anti-PCP activity of piperazine-linked bisbenzamidines. A graph of the “minus log of the anti-P. carinii activity expressed in mM” versus the number of carbon atoms in the aliphatic moiety of the R substituent, when that moiety is a linear alkyl group or a cycloalkyl group, is shown.
The activity of most of the compounds (12 of 26) fell in the marked category of activity. This level of activity was reported for both of the most clinically efficacious anti-P. carinii treatments, TMP-SMZ and pentamidine isethionate, with IC50 values of 0.104 μg/ml (8) and 0.300 μg/ml (Table 1), respectively. In this series of piperazine-linked bisbenzamidines, the trend of decreased activity associated with alkyl chains shorter than four carbons and longer than seven carbons is readily apparent. A reduction in carbon chain length to two and three carbons resulted in IC50 values of 0.542 μg/ml (compound 16 [marked activity]) and 1.60 μg/ml (compound 22 [moderate activity]), whereas a single carbon chain (compound 21) or lack of an alkyl group (compound 19) resulted in moderate activity at IC50s of 1.53 and 1.03 μg/ml, respectively. Increases of alkyl chain length to 8, 9, 10, and 12 carbons (compounds 11, 14, 18, and 15) all resulted in marked activity. A benzyl ring substituent (compound 10) analog also showed marked activity. When both the amidinium nitrogens are part of a five- or six-membered cyclic system (compounds 17, 20, and 23), the activities were either marked or moderate. Except derivatives 26 (hydroxylamine) and 27 (carboxhydrazide), which were characterized by a moderate activity, the other 1,4-diarylpiperazines that were not substituted by terminal amidines moieties appeared to be devoid of activity against P. carinii.
Toxicity.
Two transformed cell lines derived from two different organ systems and a primary cell line were chosen for evaluation of the relative toxicity of each compound. The A549 cell line is an epithelial lung cell line derived from a human carcinoma and has been used for this purpose in previous studies (39). The Hep-G2 cell line is an epithelial cell line derived from a human hepatocellular carcinoma, and WI-38 is a human diploid cell line derived from normal embryonic (3 months of gestation) lung tissue.
Testing in all three cell lines was conducted with compounds that showed the most promise for in vivo evaluation, i.e., those that had marked or better anti-P. carinii activity. Generally, the toxicity results for a given compound were similar for all three lines. There were only three cases in which a compound was toxic in one or two cell lines and not in the other. These included compound 5, which showed toxicity in the Hep-G2 line at 75 times the IC50; compound 10, which was toxic to the A549 line at 2,560 times the IC50; and compound 15, which was toxic to the Hep-G2 and WI-38 cell lines but not the A549 line at 46 and 8 times the IC50 value. Remarkably, all four of the highly active compounds showed no toxicity in any cell line at 100 times the anti-P. carinii IC50. This lack of toxicity was also apparent in the next level of activity. Only one of the four compounds with very-marked activity (compound 5) showed slight toxicity in the Hep-G2 line at 75 times the IC50 without toxicity in the other two cell lines. As the anti-P. carinii activity decreased, the number of compounds exhibiting toxicity increased. Six of eleven compounds with marked activity, including pentamidine, had toxicity in one or more of the cell lines, and four of the nine compounds with moderate activity showed toxicity. The moderately active compounds were usually screened only in the A549 cell line, since compounds with this level of activity were not selected for further study in animal models. For the same reason, compounds with slight or no activity were not tested in the cell line assays.
DISCUSSION
Pentamidine and related compounds have long been used to treat a variety of infectious diseases, especially trypanosomiasis and leishmaniasis, caused by parasitic protozoans but also infections caused by fungi, bacteria, malarial parasites, some viruses, and Pneumocystis spp. (5, 33). Problems related to toxicity, oral availability, and resistance have hindered the widespread use and development of these compounds, despite promising approaches to synthesis of prodrugs and other substitution strategies (15). However, with increasing evidence for P. jirovecii resistance to TMP-SMZ (1, 27, 42), we considered it worthwhile to revisit the basic pentamidine structure with newly devised substitution strategies. Previously, we showed promising antiprotozoal and anti-P. carinii activities with a 1,4-piperazinediyl skeleton as a rigid linker (24, 25, 31). These results provided the rationale for the synthesis of the series of piperazine-linked bisbenzamidines and related compounds presented here. Several compounds within the library of piperazine-linked bisbenzamidines were found to demonstrate very high anti-P. carinii activity in an in vitro system, with little to no associated toxicity in three mammalian cell lines. Eight compounds (compounds 1 to 8 [Table 1]) exhibited higher activity than TMP-SMX in the same screening system (IC50 = 0.104 μg/ml) (8), and these eight and an additional six compounds demonstrated higher activity than the parent compound, pentamidine (Table 1). A definite structure-activity relationship was observed among the most efficacious compounds, with a five- to six-carbon chain defining the highest activities and a shorter or longer chain resulting in decreased efficacy. Toxicity to the mammalian cell lines began with compounds in the marked category, with one exception: compound 5 in the very-marked category exhibited toxicity in the Hep-G2 cell line.
The mechanism(s) of action of pentamidine appear to be complex and incompletely understood. Because pentamidine and related compounds were shown to bind to the minor groove of the DNA molecule, it was initially thought that the anti-P. carinii properties were reliant upon inhibition of the organism's topoisomerase I (13, 28). This was not found to be the case, and later studies with recombinant P. carinii topoisomerase I as a reagent rather than semipurified extracts provided further evidence that the cytotoxic mechanisms of pentamidine were not targeted to this enzyme (35). Topoisomerase II (3) or inhibition of group I intron splicing (22, 41) were suggested as other potential targets. In some pathogenic protozoa, such as Trypanosoma brucei brucei (5), the efficacy of pentamidine has been linked in part to the function of at least three transporters. Loss of these transporters conferred a resistant phenotype. Collapse of mitochondrial membrane potential leading to loss of mitochondrial activity was associated with pentamidine toxicity in Leishmania species (5, 36).
Data in the present study only permit us to speculate as to the potential mechanisms of action of this series of compounds. The highly active compounds (compounds 1 to 4 in Table 1) are predominantly charged at physiological pH and may be taken up into the target site(s) via transporters (5). DNA-binding affinity measurements to calf thymus DNA and poly(dA-dT) were performed but did not show a direct correlation with the anti-P. carinii activity (data not shown). Several of the more potent compounds, namely, compounds 11, 14, 15, 17, and 18, were poor DNA binders, whereas the less-potent compounds, namely, 19 to 25, were strong DNA binders. This suggests that binding to DNA may not be the major mode of action against P. carinii for this series of compounds.
The nature of the in vitro system, which permits only minimal replication, also does not support a mechanism of action that targets DNA replication. Rather, the rapid and dramatic decreases in ATP levels suggests that the target may be the mitochondria, as we postulated in previous studies (6), or perhaps transporters, as reported in other parasitic pathogens (5). Potential mitochondrial toxicity is supported by our recent observations (23, 24) that bisbenzamidines, including pentamidine, can form complexes with heme (ferriprotoporphyrin IX). Therefore, these compounds could target heme-containing macromolecules such as the cytochrome bc1 complex, an essential respiratory enzyme present in the mitochondrial membrane of eukaryotic organisms. Further experiments are under way to verify the hypothesis that the cytochrome bc1 complex could be a potential target for the bisbenzamidines.
The activities of compounds 1, 2, 3, and 4 were the highest ever observed with the present in vitro assay system. Studies in the mouse model of Pneumocystis pneumonia are ongoing to evaluate in vivo efficacy, and strategies for improving bioavailability are being evaluated.
Acknowledgments
This study was supported by Public Health Service grant NO1 AI75319 from the National Institute of Allergy and Infectious Diseases (M.T.C. and P.D.W.), by the Medical Research Service Department of Veterans Affairs (M.T.C. and P.D.W.), and by grants DA13546 from the National Institutes on Drug Abuse and 2S06GM08008 from the National Institutes of General Medical Sciences (T.L.H.).
REFERENCES
- 1.Armstrong, W., S. Meshnick, and P. Kazanjian. 2000. Pneumocystis carinii mutations associated with sulfa and sulfone prophylaxis failures in immunocompromised patients. Microbes Infect. 2:61-67. [DOI] [PubMed] [Google Scholar]
- 2.Assan, R., C. Perronne, D. Assan, L. Chotard, C. Mayaud, S. Matheron, and D. Zucman. 1995. Pentamidine-induced derangements of glucose homeostasis: determinant roles of renal failure and drug accumulation. A study of 128 patients. Diabetes Care 18:47-55. [DOI] [PubMed] [Google Scholar]
- 3.Bell, C. A., C. C. Dykstra, N. A. Naiman, M. Cory, T. A. Fairley, and R. R. Tidwell. 1993. Structure-activity studies of dicationically substituted bis-benzamidazoles against Giardia lamblia: correlation of antigiardial activity with DNA-binding affinity and giardial topoisomerase II inhibition. Antimicrob. Agents Chemother. 37:2668-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, S. S. 1960. Search for chemotherapeutic amidines-(XVII)- alpha, omega-bis (p-amidinoanilino) alkanes. J. Chem. Soc. 5172-5176.
- 5.Bray, P. G., M. P. Barrett, S. A. Ward, and H. P. de Koning. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present, and future. Trends Parasitol. 19:232-239. [DOI] [PubMed] [Google Scholar]
- 6.Chen, F., and M. T. Cushion. 1994. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J. Clin. Microbiol. 32:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, M. S., and M. T. Cushion. 2001. Standardization of an in vitro drug screening assay by use of cryopreserved and characterized Pneumocystis carinii populations. J. Eukaryot. Microbiol. 2001(Suppl.):178S-179S. [DOI] [PubMed] [Google Scholar]
- 8.Cushion, M. T., F. Chen, and N. Kloepfer. 1997. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob. Agents Chemother. 41:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushion, M. T., M. Kaselis, S. L. Stringer, and J. R. Stringer. 1993. Genetic stability and diversity of Pneumocystis carinii infecting rat colonies. Infect. Immun. 61:4801-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cushion, M. T., S. Orr, S. P. Keely, and J. R. Stringer. 2001. Time between inoculations and karyotype forms of Pneumocystis carinii f.sp. carinii influence outcome of experimental coinfections in rats. Infect. Immun. 69:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushion, M. T., and P. D. Walzer. 2004. In vitro and in vivo testing of new compounds. In P. D. Walzer and M. T. Cushion (ed.), Pneumocystis pneumonia, in press. Marcel-Dekker, Inc., New York, N.Y.
- 12.Donkor, I. O., T. L. Huang, B. Tao, D. Rattendi, S. Lane, M. Vargas, B. Goldenberg, and C. J. Bacchi. 2003. Trypanocidal activity of conformationally restricted pentamidine congeners. J. Med. Chem. 46:1041-1048. [DOI] [PubMed] [Google Scholar]
- 13.Dykstra, C. C., and R. R. Tidwell. 1991. Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J. Protozool. 38:78S-81S. [PubMed] [Google Scholar]
- 14.Fishman, J. A. 1998. Treatment of infection due to Pneumocystis carinii. Antimicrob. Agents Chemother. 42:1309-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, J. E., J. E. Kerrigan, K. Ramachandran, B. C. Bender, J. P. Stanko, S. K. Jones, D. A. Patrick, and R. R. Tidwell. 1998. Anti-Pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob. Agents Chemother. 42:666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes, W. T., P. C. McNabb, T. D. Makres, and S. Feldman. 1974. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob. Agents Chemother. 5:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannidis, J. P., D. O. Dixon, M. McIntosh, J. M. Albert, S. A. Bozzette, and S. M. Schnittman. 1999. Relationship between event rates and treatment effects in clinical site differences within multicenter trials: an example from primary Pneumocystis carinii prophylaxis. Control Clin. Trials 20:253-266. [DOI] [PubMed] [Google Scholar]
- 18.Ivady, G., and L. Paldy. 1958. Ein neves Behandlungsverfahren der interstitiellen plasmazelligen Pneumonie Fruhgeborener mit funfwertigen Stibium und aromatischen Diamidinen. Monatsschr. Kinderheilkd. 106:10-15. [PubMed] [Google Scholar]
- 19.Ivady, G., L. Paldy, M. Koltay, G. Toth, and Z. Kovacs. 1967. Pneumocystis carinii pneumonia. Lancet i:616-617. [DOI] [PubMed]
- 20.Kaneshiro, E. S., M. S. Collins, and M. T. Cushion. 2000. Inhibitors of sterol biosynthesis and amphotericin B reduce the viability of Pneumocystis carinii f.sp. carinii. Antimicrob. Agents Chemother. 44:1630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazanjian, P., W. Armstrong, P. A. Hossler, W. Burman, J. Richardson, C. H. Lee, L. Crane, J. Katz, and S. R. Meshnick. 2000. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J. Infect. Dis. 182:551-557. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., R. R. Tidwell, and M. J. Leibowitz. 1994. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J. Eukaryot. Microbiol. 41:31-38. [DOI] [PubMed] [Google Scholar]
- 23.Mayence, A., J. J. Vanden Eynde, and T. L. Huang. 2004. Evidences for the formation of bisbenzamidine-heme complexes in cell-free systems. Bioorg. Med. Chem. Lett. 14:1625-1628. [DOI] [PubMed] [Google Scholar]
- 24.Mayence, A., J. J. Vanden Eynde, F. M. Krogstad, D. J. Krogstad, M. T. Cushion, and T. L. Huang. 2004. Parallel solution-phase synthesis of conformationally restricted congeners of pentamidine and evaluation of their antiplasmodial activities. J. Med. Chem. 47:2700-2705. [DOI] [PubMed] [Google Scholar]
- 25.Mayence, A., J. J. Vanden Eynde, L. LeCour, Jr., L. A. Walker, B. L. Tekwani, and T. L. Huang. 2004. Piperazine-linked bisbenzamidines: a novel class of antileishmanial agents. Eur. J. Med. Chem. 39:547-553. [DOI] [PubMed] [Google Scholar]
- 26.Mei, Q., S. Gurunathan, H. Masur, and J. A. Kovacs. 1998. Failure of co-trimoxazole in Pneumocystis carinii infection and mutations in dihydropteroate synthase gene. Lancet 351:1631-1632. [DOI] [PubMed] [Google Scholar]
- 27.Meshnick, S. R. 1999. Drug-resistant Pneumocystis carinii. Lancet 354:1318-1319. [DOI] [PubMed] [Google Scholar]
- 28.Queener, S. F. 1995. New drug developments for opportunistic infections in immunosuppressed patients: Pneumocystis carinii. J. Med. Chem. 38:4739-4759. [DOI] [PubMed] [Google Scholar]
- 29.Roger, R., and D. G. Neilson. 1961. The chemistry of imidates. Chem. Rev. 61:179-211. [Google Scholar]
- 30.Suzuki, T., H. Ono, and M. Yokoyama. 1986. Bishydrazone compounds. Chem. Abstr. 105:15213g. [Google Scholar]
- 31.Tao, B., T. L. Huang, Q. Zhang, L. Jackson, S. F. Queener, and I. O. Donkor. 1999. Synthesis and anti-Pneumocystis carinii activity of conformationally restricted analogues of pentamidine. Eur. J. Med. Chem. 34:531-538. [Google Scholar]
- 32.Thampi, N. S., F. W. Schueler, and V. B. Vernon. 1967. Photosensitizing activity of N,N′-bis (p-formylphenyl) piperazine. J. Med. Chem. 10:111-112. [DOI] [PubMed] [Google Scholar]
- 33.Tidwell, R. R., J. D. Geratz, W. A. Clyde, Jr., K. U. Rosenthal, and E. J. Dubovi. 1984. Suppression of respiratory syncytial virus infection in cotton rats by bis(5-amido-2-benzimidiazolyl)methane. Antimicrob. Agents Chemother. 26:591-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanden Eynde, J. J., A. Mayence, L. LeCour, Jr., and T. L. Huang. 2004. Synthesis, antituberculosis activity, and DNA binding affinity of a highly diverse library of 1,4-diarylpiperazines. Med. Chem. Res. 12:401-414. [Google Scholar]
- 35.van Dross, R. T., and M. M. Sanders. 2002. Molecular characterization of recombinant Pneumocystis carinii topoisomerase I: differential interactions with human topoisomerase I poisons and pentamidine. Antimicrob. Agents Chemother. 46:2145-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verseci, A. E., and R. Docampo. 1992. Calcium transport by digitonin-permeabilized Leishmania donovani: effects of Ca2+, pentamidine, and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284:463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker, D. J., A. E. Wakefield, M. N. Dohn, R. F. Miller, R. P. Baughman, P. A. Hossler, M. S. Bartlett, J. W. Smith, P. Kazanjian, and S. R. Meshnick. 1998. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J. Infect. Dis. 178:1767-1775. [DOI] [PubMed] [Google Scholar]
- 38.Walzer, P. D. 2000. Pneumocystis carinii, p. 2781-2795. In G. L. Mandell, J. E. Bennet, and P. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Inc., New York, N.Y.
- 39.Walzer, P. D., A. Ashbaugh, M. Collins, and M. T. Cushion. 2001. In vitro and in vivo effects of quinupristin-dalfopristin against Pneumocystis carinii. Antimicrob. Agents Chemother. 45:3234-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkelman, E., W. Raether, W. Dittmar, D. Duewel, D. Gericke, W. Hohorst, H. Rolly, and E. Schrinner. 1975. Chemotherapeutically active nitro compounds. 1. Nitroanilines. Arzneim-Forsch. 25:681-708. [PubMed] [Google Scholar]
- 41.Zhang, Y., Z. Li, D. S. Pilch, and M. J. Leibowitz. 2002. Pentamidine inhibits catalytic activity group I intron Ca.LSU by altering RNA folding. Nucleic Acids Res. 30:2961-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingale, A., P. Carrera, A. Lazzarin, and P. Scarpellini. 2003. Detection of Pneumocystis carinii and characterization of mutations associated with sulfa resistance in bronchoalveolar lavage samples from human immunodeficiency virus-infected subjects. J. Clin. Microbiol. 41:2709-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]