Abstract
Agar dilution MIC was used to compare activities of OPT-80, linezolid, vancomycin, teicoplanin, quinupristin/dalfopristin, amoxicillin/clavulanate, imipenem, clindamycin, and metronidazole against 350 gram-positive and -negative anaerobes. OPT-80 was active against gram-positive strains only, especially Clostridium spp. (85 strains tested, including 21 strains of C. difficile), with MICs ranging between ≤0.016 and 0.25 μg/ml.
Clostridium difficile is a leading cause of antibiotic-associated diarrhea, especially in hospitals and long-term facilities (8, 11, 12). The organism accounts for about 20% of hospitalized patients who develop diarrhea after treatment with anti-infectives (and occasionally cytotoxic chemotherapeutic agents) and the majority of cases of antibiotic-associated colitis (3, 7, 10, 17). The rising incidence of C. difficile-associated diarrhea has been attributed to the increasingly common prescription of broad-spectrum antibiotics (16). It is also important to note that the etiology of pseudomembranous colitis appears to be multifactorial and not dependent only on the in vitro activity of an agent against C. difficile (17).
Initial treatment involves discontinuation of the offending medication, as well as supportive therapy, but antimicrobial therapy is necessary if these measures fail to alleviate the symptoms (7, 10). The two most commonly used antimicrobial therapies are vancomycin and metronidazole, and while both are effective in treating the infection, both have shortcomings, including high (approximately 20%) relapse rates (6). In addition, metronidazole may have significant side effects, including nausea, neuropathy, leukopenia, and seizures (6, 18), while widespread use of vancomycin may lead to increased vancomycin resistance in enterococci and staphylococci (2, 4). The above-described shortcomings have necessitated a search for new therapeutic options for this disease.
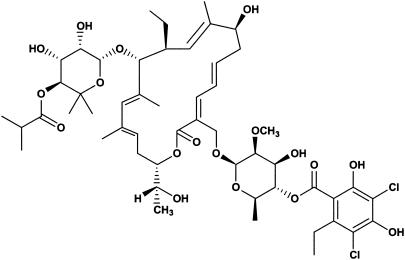
OPT-80 (Fig. 1) is a novel macrocycle which is inactive against gram-negative organisms with moderate activity against gram-positive organisms, such as staphylococci and enterococci, but excellent activity against clostridia. This study compares the in vitro activity of OPT-80 to those of linezolid, vancomycin, teicoplanin, quinupristin/dalfopristin, amoxicillin/clavulanate, imipenem, clindamycin, and metronidazole against 350 anaerobes.
FIG. 1.
Structure of OPT-80.
All anaerobes were clinical strains identified by standard procedures (9) and kept frozen in double-strength skim milk (dehydrated skim milk: Difco Laboratories, Detroit, Mich.) at −70°C until use. All organisms, including the 21 C. difficile strains, were separate isolates and not clonally related. Prior to testing, strains were subcultured twice onto enriched Brucella agar plates (13). OPT-80 was obtained from Optimer Pharmaceuticals, Inc., San Diego, Calif., and other drugs were obtained from respective manufacturers. Agar dilution susceptibility testing was carried out according to the latest method recommended by the NCCLS (13), using Brucella agar with 5% sterile defibrinated laked sheep blood. Clavulanate was combined with amoxicillin in a 1:2 ratio. All quality-control gram-negative and -positive strains recommended by NCCLS (13) were included with each run: in every case, results (where available) were in range.
Results of MIC testing are presented in Table 1. As can be seen, OPT-80 was active only against gram-positive anaerobes, especially against Clostridium species (including C. difficile), with MICs ranging between ≤0.016 and 0.25 μg/ml. For the 21 C. difficile strains, 12 required MICs of ≤0.016 μg/ml, 1 required an MIC of 0.03 μg/ml, 4 required MICs of 0.06 μg/ml, 3 required MICs of 0.125 μg/ml, and 1 required an MIC of 0.25 μg/ml. Against gram-positive non-spore-forming rods and peptostreptococci, OPT-80 MICs were higher, ranging between ≤0.016 and 16.0 μg/ml. Gram-positive anaerobes for which OPT-80 MICs were ≥4.0 μg/ml comprised the Propionibacterium acnes species as well as lactobacilli; lactobacilli also required higher linezolid, vancomycin, teicoplanin, and quinupristin/dalfopristin MICs. Linezolid, vancomycin, teicoplanin, and quinupristin/dalfopristin were (with the exception of the latter) active mainly against gram-positive anaerobes, while amoxicillin/clavulanate and imipenem were active against both groups. Some Bacteroides fragilis group strains and clostridia (notably C. difficile) were clindamycin resistant, while all strains except gram-positive non-spore-forming rods were susceptible to metronidazole. Results with other agents reflect previously published findings (5).
TABLE 1.
MICs (μg/ml) of agents
| Organism (na) | Drug | MIC range | MIC50g | MIC90h |
|---|---|---|---|---|
| Bacteroides fragilis (19) | OPT-80 | 64.0->128.0 | >128.0 | >128.0 |
| Linezolid | 4.0-8.0 | 4.0 | 4.0 | |
| Vancomycin | >16.0 | >16.0 | >16.0 | |
| Teicoplanin | >16.0 | >16.0 | >16.0 | |
| Quinupristin/dalfopristin | 8.0->8.0 | 8.0 | >8.0 | |
| Amoxicillin/clavulanate | 0.25-0.5 | 0.5 | 0.5 | |
| Imipenem | 0.06-0.5 | 0.125 | 0.25 | |
| Clindamycin | 0.25->32.0 | 1.0 | 4.0 | |
| Metronidazole | 0.25-1.0 | 1.0 | 1.0 | |
| Non-fragilis B. fragilis group species (38)b | OPT-80 | 64.0->128.0 | >128.0 | >128.0 |
| Linezolid | 2.0-8.0 | 4.0 | 4.0 | |
| Vancomycin | >16.0 | >16.0 | >16.0 | |
| Teicoplanin | 8.0->16.0 | >16.0 | >16.0 | |
| Quinupristin/dalfopristin | 2.0->8.0 | 8.0 | >8.0 | |
| Amoxicillin/clavulanate | 0.5-16.0 | 1.0 | 2.0 | |
| Imipenem | 0.25-2.0 | 0.5 | 1.0 | |
| Clindamycin | 0.03->32.0 | 2.0 | >32.0 | |
| Metronidazole | 0.25-2.0 | 1.0 | 1.0 | |
| Prevotella/Porphyromonas species (42)c | OPT-80 | 16.0->128.0 | >128.0 | >128.0 |
| Linezolid | 0.25-4.0 | 1.0 | 2.0 | |
| Vancomycin | 2.0->16.0 | >16.0 | >16.0 | |
| Teicoplanin | 0.125-8.0 | 2.0 | 4.0 | |
| Quinupristin/dalfopristin | 0.125-4.0 | 1.0 | 4.0 | |
| Amoxicillin/clavulanate | ≤0.125-2.0 | 0.25 | 1.0 | |
| Imipenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Clindamycin | ≤0.016->32.0 | ≤0.016 | 8.0 | |
| Metronidazole | ≤0.125-2.0 | 0.5 | 2.0 | |
| Fusobacterium nucleatum (14) | OPT-80 | 64.0->128.0 | >128.0 | >128.0 |
| Linezolid | 0.125-1.0 | 0.5 | 0.5 | |
| Vancomycin | >16.0 | >16.0 | >16.0 | |
| Teicoplanin | 16.0->16.0 | >16.0 | >16.0 | |
| Quinupristin/dalfopristin | 0.5-8.0 | 2.0 | 8.0 | |
| Amoxicillin/clavulanate | ≤0.125-0.5 | ≤0.125 | 0.5 | |
| Imipenem | ≤0.016-0.06 | 0.03 | 0.06 | |
| Clindamycin | 0.03-0.125 | 0.06 | 0.125 | |
| Metronidazole | ≤0.125-0.25 | ≤0.125 | 0.25 | |
| Fusobacterium mortiferum (10) | OPT-80 | 64.0->128.0 | >128.0 | >128.0 |
| Linezolid | 0.25-0.5 | 0.25 | 0.25 | |
| Vancomycin | >16.0 | >16.0 | >16.0 | |
| Teicoplanin | >16.0 | >16.0 | >16.0 | |
| Quinupristin/dalfopristin | 4.0->8.0 | 8.0 | 8.0 | |
| Amoxicillin/clavulanate | 1.0-4.0 | 1.0 | 2.0 | |
| Imipenem | 0.5-1.0 | 0.5 | 1.0 | |
| Clindamycin | 0.06-0.125 | 0.125 | 0.125 | |
| Metronidazole | <0.125-0.5 | 0.25 | 0.5 | |
| Fusobacterium species, miscellaneous (14)d | OPT-80 | 16.0->128.0 | >128.0 | >128.0 |
| Linezolid | 0.25-2.0 | 1.0 | 1.0 | |
| Vancomycin | 16.0->16.0 | >16.0 | >16.0 | |
| Teicoplanin | >16.0 | >16.0 | >16.0 | |
| Quinupristin/dalfopristin | 0.5->8.0 | >8.0 | >8.0 | |
| Amoxicillin/clavulanate | ≤0.125-2.0 | 1.0 | 2.0 | |
| Imipenem | ≤0.016-1.0 | 0.5 | 1.0 | |
| Clindamycin | ≤0.016-32.0 | 2.0 | 16.0 | |
| Metronidazole | ≤0.125-0.5 | ≤0.125 | 0.25 | |
| Peptostreptococcus tetradius (16) | OPT-80 | 0.25-2.0 | 1.0 | 1.0 |
| Linezolid | 0.5-1.0 | 0.5 | 1.0 | |
| Vancomycin | 0.5-2.0 | 1.0 | 1.0 | |
| Teicoplanin | ≤0.03-1.0 | ≤0.03 | ≤0.03 | |
| Quinupristin/dalfopristin | 0.5-2.0 | 1.0 | 2.0 | |
| Amoxicillin/clavulanate | ≤0.125-2.0 | ≤0.125 | 1.0 | |
| Imipenem | ≤0.016-0.5 | 0.06 | 0.5 | |
| Clindamycin | 0.5-1.0 | 1.0 | 1.0 | |
| Metronidazole | 0.5-4.0 | 1.0 | 1.0 | |
| Peptostreptococcus asaccharolyticus (15) | OPT-80 | 0.25-1.0 | 0.5 | 1.0 |
| Linezolid | 0.5-2.0 | 1.0 | 1.0 | |
| Vancomycin | 0.125-0.5 | 0.125 | 0.5 | |
| Teicoplanin | 0.06-0.25 | 0.25 | 0.25 | |
| Quinupristin/dalfopristin | 0.25-1.0 | 0.25 | 1.0 | |
| Amoxicillin/clavulanate | ≤0.125-0.5 | ≤0.125 | 0.5 | |
| Imipenem | ≤0.016-0.125 | 0.03 | 0.125 | |
| Clindamycin | 0.06->32.0 | 0.125 | >32.0 | |
| Metronidazole | ≤0.125-1.0 | 1.0 | 1.0 | |
| Peptostreptococcus anaerobius (15) | OPT-80 | ≤0.016-0.03 | ≤0.016 | ≤0.016 |
| Linezolid | 0.25-1.0 | 0.5 | 1.0 | |
| Vancomycin | 0.25-0.5 | 0.25 | 0.5 | |
| Teicoplanin | 0.06-0.25 | 0.25 | 0.25 | |
| Quinupristin/dalfopristin | 0.125-0.5 | 0.25 | 0.5 | |
| Amoxicillin/clavulanate | 0.25-64.0 | 1.0 | 32.0 | |
| Imipenem | 0.06-4.0 | 0.125 | 2.0 | |
| Clindamycin | ≤0.016-0.5 | 0.03 | 0.5 | |
| Metronidazole | ≤0.125-1.0 | 0.5 | 1.0 | |
| Finegoldia magna (15) | OPT-80 | 0.25-2.0 | 1.0 | 1.0 |
| Linezolid | 1.0-2.0 | 2.0 | 2.0 | |
| Vancomycin | 0.125-0.5 | 0.25 | 0.25 | |
| Teicoplanin | 0.125-0.25 | 0.25 | 0.25 | |
| Quinupristin/dalfopristin | 0.5-1.0 | 0.5 | 1.0 | |
| Amoxicillin/clavulanate | 0.25-1.0 | 1.0 | 1.0 | |
| Imipenem | 0.06-0.125 | 0.06 | 0.125 | |
| Clindamycin | 0.125->32.0 | 0.25 | >32.0 | |
| Metronidazole | 0.25-1.0 | 0.5 | 1.0 | |
| Micromonas micros (14) | OPT-80 | ≤0.016-0.06 | 0.03 | 0.06 |
| Linezolid | 0.5-2.0 | 1.0 | 2.0 | |
| Vancomycin | 0.5-1.0 | 1.0 | 1.0 | |
| Teicoplanin | 0.125-0.5 | 0.25 | 0.25 | |
| Quinupristin/dalfopristin | 0.5-2.0 | 1.0 | 2.0 | |
| Amoxicillin/clavulanate | 0.25-2.0 | 1.0 | 2.0 | |
| Imipenem | 0.03-0.25 | 0.06 | 0.125 | |
| Clindamycin | 0.125-1.0 | 0.25 | 0.25 | |
| Metronidazole | ≤0.125-1.0 | 0.25 | 0.5 | |
| Peptostreptococcus prevotii (3) | OPT-80 | 0.25-1.0 | ||
| Linezolid | 0.5-2.0 | |||
| Vancomycin | 0.125-0.5 | |||
| Teicoplanin | 0.25 | |||
| Quinupristin/dalfopristin | 0.25-1.0 | |||
| Amoxicillin/clavulanate | 0.25-1.0 | |||
| Imipenem | ≤0.016-0.03 | |||
| Clindamycin | 0.125-0.25 | |||
| Metronidazole | 0.5-1.0 | |||
| Propionibacterium acnes (20) | OPT-80 | 0.5-4.0 | 4.0 | 4.0 |
| Linezolid | 0.5-1.0 | 0.5 | 1.0 | |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |
| Teicoplanin | 0.125-0.5 | 0.5 | 0.5 | |
| Quinupristin/dalfopristin | ≤0.06-0.125 | 0.125 | 0.125 | |
| Amoxicillin/clavulanate | ≤0.125-1.0 | 0.25 | 0.5 | |
| Imipenem | ≤0.016-0.125 | 0.03 | 0.06 | |
| Clindamycin | 0.03-0.125 | 0.06 | 0.06 | |
| Metronidazole | >16.0 | >16.0 | >16.0 | |
| Eggerthella lenta (10) | OPT-80 | ≤0.016-0.06 | ≤0.016 | 0.03 |
| Linezolid | 1.0-2.0 | 1.0 | 2.0 | |
| Vancomycin | 1.0-2.0 | 1.0 | 2.0 | |
| Teicoplanin | 0.06-0.5 | 0.25 | 0.5 | |
| Quinupristin/dalfopristin | 0.25-2.0 | 0.5 | 1.0 | |
| Amoxicillin/clavulanate | 1.0-4.0 | 2.0 | 2.0 | |
| Imipenem | 0.5-1.0 | 0.5 | 1.0 | |
| Clindamycin | 0.125-1.0 | 0.125 | 0.5 | |
| Metronidazole | 0.25-0.5 | 0.5 | 0.5 | |
| Miscellaneous gram-positive non-spore-forming rods (20)e | OPT-80 | ≤0.016-16.0 | 0.125 | 16.0 |
| Linezolid | 0.125-8.0 | 1.0 | 4.0 | |
| Vancomycin | 0.25->16.0 | 0.5 | >16.0 | |
| Teicoplanin | 0.06->16.0 | 0.25 | >16.0 | |
| Quinupristin/dalfopristin | 0.125-4.0 | 0.25 | 2.0 | |
| Amoxicillin/clavulanate | ≤0.125-2.0 | 0.5 | 2.;0 | |
| Imipenem | 0.06-4.0 | 0.25 | 2.0 | |
| Clindamycin | ≤0.016->32.0 | 0.125 | 8.0 | |
| Metronidazole | 2.0->16.0 | >16.0 | >16.0 | |
| Clostridium perfringens (35) | OPT-80 | ≤0.016-0.06 | ≤0.016 | 0.03 |
| Linezolid | 1.0-4.0 | 2.0 | 2.0 | |
| Vancomycin | 0.25-2.0 | 0.5 | 1.0 | |
| Teicoplanin | ≤0.03-0.25 | 0.06 | 0.125 | |
| Quinupristin/dalfopristin | 0.5-1.0 | 0.5 | 1.0 | |
| Amoxicillin/clavulanate | ≤0.125-0.5 | ≤0.125 | 0.25 | |
| Imipenem | 0.03-1.0 | 0.125 | 0.25 | |
| Clindamycin | 0.06->32.0 | 0.5 | 2.0 | |
| Metronidazole | ≤0.125-2.0 | 1.0 | 1.0 | |
| Clostridium difficile (21) | OPT-80 | ≤0.016-0.25 | ≤0.016 | 0.125 |
| Linezolid | 2.0-4.0 | 2.0 | 2.0 | |
| Vancomycin | 0.5-2.0 | 1.0 | 2.0 | |
| Teicoplanin | 0.06-0.5 | 0.5 | 0.5 | |
| Quinupristin/dalfopristin | 0.5-2.0 | 1.0 | 1.0 | |
| Amoxicillin/clavulanate | ≤0.125-8.0 | 2.0 | 4.0 | |
| Imipenem | 0.5->8.0 | 8.0 | >8.0 | |
| Clindamycin | 1.0->32.0 | 4.0 | >32.0 | |
| Metronidazole | ≤0.125-0.5 | 0.25 | 0.5 | |
| Clostridium tertium (10) | OPT-80 | ≤0.016-0.06 | ≤0.016 | 0.03 |
| Linezolid | 2.0-4.0 | 4.0 | 4.0 | |
| Vancomycin | 1.0-2.0 | 2.0 | 2.0 | |
| Teicoplanin | 0.06-0.25 | 0.125 | 0.25 | |
| Quinupristin/dalfopristin | 1.0-2.0 | 1.0 | 1.0 | |
| Amoxicillin/clavulanate | 0.25-1.0 | 1.0 | 1.0 | |
| Imipenem | 0.125-1.0 | 0.5 | 0.5 | |
| Clindamycin | 1.0->32.0 | 8.0 | 16.0 | |
| Metronidazole | 0.25-2.0 | 1.0 | 1.0 | |
| Clostridium species (19)f | OPT-80 | ≤0.016-0.06 | ≤0.016 | 0.03 |
| Linezolid | 1.0-4.0 | 1.0 | 4.0 | |
| Vancomycin | 0.5-2.0 | 1.0 | 2.0 | |
| Teicoplanin | ≤0.03-8.0 | 0.06 | 0.125 | |
| Quinupristin/dalfopristin | 0.5-1.0 | 1.0 | 1.0 | |
| Amoxicillin/clavulanate | ≤0.125-1.0 | ≤0.125 | 0.25 | |
| Imipenem | 0.125-4.0 | 0.25 | 0.5 | |
| Clindamycin | 0.06-8.0 | 0.5 | 2.0 | |
| Metronidazole | ≤0.125-1.0 | 0.5 | 0.5 | |
| Clostridium spp. (all) (85) | OPT-80 | ≤0.016-0.25 | ≤0.016 | 0.06 |
| Linezolid | 1.0-4.0 | 2.0 | 4.0 | |
| Vancomycin | 0.25-2.0 | 1.0 | 2.0 | |
| Teicoplanin | ≤0.03-8.0 | 0.125 | 0.5 | |
| Quinupristin/dalfopristin | 0.5-2.0 | 1.0 | 1.0 | |
| Amoxicillin/clavulanate | ≤0.125-8.0 | 0.25 | 2.0 | |
| Imipenem | 0.03->8.0 | 0.25 | 8.0 | |
| Clindamycin | 0.06->32.0 | 1.0 | 16.0 | |
| Metronidazole | ≤0.125-2.0 | 0.5 | 1.0 |
n, no. of strains.
Bacteroides thetaiotaomicron (7), Bacteroides uniformis (6), Bacteroides distasonis (7), Bacteroides ovatus (8), Bacteroides vulgatus (7), and Bacteroides stercoris (3).
Prevotella bivia (7), Prevotella disiens (4), Prevotella intermedia (7), Prevotella melaninogenica (6), Prevotella corporis (4), Prevotella oris (4), Prevotella loeschii(2), Prevotella buccae (5), and Porphyromonas asaccharolytica (3).
Fusobacterium varium (9) and Fusobacterium necrophorum (5).
Lactobacillus species (8), Bifidobacterium species (8), and Actinomyces species (4).
Clostridium bifermentans (4), Clostridium cadaveris (3), Clostridium sordellii (8), Clostridium hastiforme (1), Clostridium innocuum (1), Clostridium paraputrificum (1), and Clostridium histolyticum (1).
MIC at which 50% of isolates tested were inhibited.
MIC at which 90% of isolates tested were inhibited.
OPT-80 (tiacumicin B) is a member of a novel group of 18-membered macrocyclic antibiotics with in vitro activity against pathogenic gram-positive bacteria and selected anaerobes, notably C. difficile (1, 14, 15). Previous studies testing tiacumicin B and C have demonstrated MICs against C. difficile of between 0.125 and 0.25 μg/ml. The resistance frequency was <2.9 × 10−8 at four and eight times the MIC, and both compounds at oral doses of 0.2, 1, or 5 mg per kg of body weight protected 100% of clindamycin-treated hamsters exposed to C. difficile (14). In the present study, MICs of OPT-80 against C. difficile were lower than those reported by Swanson and colleagues (14). In a recent study (1), Ackermann and colleagues reported MICs a few dilutions lower than ours against 207 strains of C. difficile and a more limited number of other gram-positive and gram-negative anaerobic species. The MIC differences in the these three studies may be due to methodology (agar dilution MICs in Wilkins Chalgren agar in the work of Swanson et al. [14], microdilution MICs in Wilkins-Chalgren medium in the work of Ackermann et al. [1], and agar dilution using enriched Brucella blood agar medium in our work). Additionally, Ackermann et al. (1) tested dilutions lower than was the case in our study. Although the MIC distribution for C. difficile differs somewhat in these three studies, all MICs were <0.5 μg/ml. We have no explanation for the species-related MIC differences of OPT-80 among anaerobic gram-positive cocci.
Results of this study suggest a potential place for OPT-80 in oral treatment of clostridial infections, especially pseudomembranous colitis caused by C. difficile toxin. Pharmacokinetic-pharmacodynamic and additional experimental animal studies are necessary to further delineate the clinical role of these compounds in treatment of anaerobic infections.
Acknowledgments
This study was supported by a grant from Optimer Pharmaceuticals, Inc., San Diego, Calif.
REFERENCES
- 1.Ackermann, G., B. Löffler, D. Adler, and A. C. Rodloff. 2004. In vitro activity of OPT-80 against Clostridium difficile. Antimicrob. Agents Chemother. 48:2280-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Society of Health-System Pharmacists. 1998. ASHP therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am. J. Health Syst. Pharm. 55:1407-1411. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G. 1992. Antibiotic-associated diarrhea. Clin. Infect. Dis. 15:573-581. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). 1995. Morb. Mortal. Wkly. Rep. Recomm. Rep. 44:1-13. [PubMed] [Google Scholar]
- 5.Ednie, L. M., A. Rattan, M. R. Jacobs, and P. C. Appelbaum. 2003. Antianaerobic activity of RBX 7644 (ranbezolid), a new oxazolidinone, compared with those of eight other agents. Antimicrob. Agents Chemother. 47:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekety, R., et al. 1997. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am. J. Gastroenterol. 92:739-750. [PubMed] [Google Scholar]
- 7.Fekety, R., and A. B. Shah. 1993. Diagnosis and treatment of Clostridium difficile colitis. JAMA 269:71-75. [PubMed] [Google Scholar]
- 8.Guerrant, R. L., J. M. Hughes, N. L. Lima, and J. Crane. 1990. Diarrhea in developed and developing countries: magnitude, special settings and etiologies. Rev. Infect. Dis. 12(Suppl. 1):S41-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jousimies-Somer, H. R., P. Summanen, D. M. Citron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual, 6th ed. Star Publishing Co., Belmont, Calif.
- 10.Kelly, C. P., C. Pothoulakis, and J. T. LaMont. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257-262. [DOI] [PubMed] [Google Scholar]
- 11.McFarland, L. V. 1995. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am. J. Infect. Control 23:295-305. [DOI] [PubMed] [Google Scholar]
- 12.Miller, M. A., M. Hyland, M. Ofner-Agostini, M. Gourdeau, and M. Ishak. 2002. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect. Control Hosp. Epidemiol. 23:137-140. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2001. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 5th ed.; approved standard. NCCLS publication no. M11-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Swanson, R. N., D. J. Hardy, N. L. Shipkowitz, C. W. Hanson, N. C. Ramer, P. B. Fernandes, and J. J. Clement. 1991. In vitro and in vivo evaluation of tiacumicins B and C against Clostridium difficile. Antimicrob. Agents Chemother. 35:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theriault, R. J., J. P. Karwowski, M. Jackson, R. L. Girolami, G. N. Sunga, C. M. Vojtko, and L. J. Coen. 1987. Tiacumicins, a novel complex of 18-membered macrolide antibiotics. J. Antibiot. 40:567-574. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox, M. H. 1996. Cleaning up Clostridium difficile colitis. Lancet 348:767-768. [DOI] [PubMed] [Google Scholar]
- 17.Wistrom, J., S. R. Norrby, E. B. Myhre, S. Eriksson, G. Granstrom, L. Lagergren, G. Englund, C. E. Nord, and B. Svenungsson. 2001. Frequency of antibiotic-associated diarrhea in 2,462 antibiotic-treated hospitalized patients: a prospective study. J. Antimicrob. Chemother. 47:43-50. [DOI] [PubMed] [Google Scholar]
- 18.Yassin, S. F., T. M. Young-Fadok, N. N. Zein, and D. S. Pardi. 2001. Clostridium difficile-associated diarrhea and colitis. Mayo Clin. Proc. 76:725-730. [DOI] [PubMed] [Google Scholar]