Abstract
The first-dose pharmacokinetic properties of intramuscular (i.m.) artesunate (ARTS; 2.4 mg/kg immediately [stat], followed by 1.2 mg/kg i.m. daily) and artemether (ARM; 3.2 mg/kg i.m. stat, followed by 1.6 mg/kg i.m. daily) were compared in Vietnamese adults with severe falciparum malaria. A total of 19 patients were studied; 9 received ARTS, and 10 received ARM. ARTS was absorbed very rapidly; concentrations in plasma peaked between 1,362 and 8,388 nmol/liter (median, 5,710 nmol/liter) within 20 min of injection and then declined with a median (range) half-life (t1/2) of 30 (3 to 67) min. ARTS was hydrolyzed rapidly and completely to the biologically active metabolite dihydroartemisinin (DHA). Peak DHA concentrations in plasma ranged between 1,718 and 7,080 nmol/liter (median, 3,060 nmol/liter) and declined with a t1/2 of 52 (26 to 69) min. In contrast, ARM was slowly and erratically absorbed. The absorption profile appeared biphasic. Maximum ARM concentrations in plasma ranged between 67 nmol/liter (a value close to the 50% inhibitory concentration for some Plasmodium falciparum isolates) and 1,631 nmol/liter (median, 574 nmol/liter) and occurred at a median (range) of 10 (1.5 to 24) h. There was relatively little conversion to DHA. After i.m. injection in cases of severe malaria, absorption of the water-soluble ARTS is rapid and extensive, whereas the oil-based ARM is slowly and erratically absorbed, with relatively little conversion to the more active DHA. On the basis of this pharmacological study, parenteral ARTS is preferable to ARM as an initial antimalarial therapy, particularly in the most seriously ill patients. These findings should be formally assessed by a randomized clinical trial.
Severe malaria kills between one and two million people each year. The qinghaosu derivatives artesunate (ARTS) and artemether (ARM) are being used increasingly for the parenteral treatment of severe falciparum malaria (23, 24). They are intrinsically more potent as antimalarials, have a broader stage specificity of action against the malaria parasite (20), and are simpler to administer and safer than parenteral quinine (1, 6, 25). ARM has been more intensively evaluated, although ARTS is more widely used. ARM is formulated in an oil base because of its poor water solubility and can only be injected by the intramuscular (i.m.) route. There is evidence that i.m. ARM may not be absorbed adequately in severely ill children with falciparum malaria who are hypotensive or acidotic (13). Nevertheless, i.m. ARM has proved as effective as parenteral quinine in the largest randomized trials ever conducted in severe malaria (1, 6, 21) and in adult patients with multiorgan dysfunction ARM was therapeutically superior (1). ARTS is a hemisuccinate artemisinin derivative formed by the addition of sodium bicarbonate to lyophilized artesunic acid. ARTS is water soluble and can be given by either intravenous (i.v.) or i.m. injection. Both of these routes of administration give rapid therapeutic responses in severe malaria (7). The data from patients with uncomplicated malaria suggest that i.m. ARTS is well absorbed (8), but there have been no detailed pharmacokinetic studies in severely ill patients and, despite extensive use, there have been no large clinical trials comparing the efficacy of ARTS with other antimalarial drugs in severely ill patients.
In areas of the rural tropics where health care facilities are basic, i.m. injection of an artemisinin derivative may represent the best therapeutic option for severe malaria. We have, therefore, investigated the pharmacokinetic properties of ARM and ARTS given by i.m. injection in severely ill Vietnamese adults with falciparum malaria.
MATERIALS AND METHODS
Patients.
We studied 19 adult patients between June 1998 and September 1999. All were admitted to the Malaria Ward at the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam, with microscopically confirmed severe falciparum malaria. The diagnosis of severe malaria was based on modifications of World Health Organization criteria reported previously (6, 22). Briefly, severe malaria was defined as one or more of the following: (i) Glasgow Coma Score (GCS) of <11, (ii) jaundice (serum bilirubin of >50 μmol/liter; a level of aspartate aminotransferase in serum more than twice the upper limit of the reference range), (iii) acute renal failure (level of creatinine in serum of >250 μmol/liter), (iv) anemia (venous hematocrit of <15%), (v) hyperparasitemia (>250,000 asexual forms/μl of whole blood), and (vi) a lactate level in plasma of >4.0 mmol/liter. Subjects who had been treated with an artemisinin derivative within 24 h were excluded, as were pregnant women and children under the age of 14 years. All patients or attendant relatives gave informed consent prior to participation in the study, which was approved by the Ethical and Scientific Committee of the Hospital for Tropical Diseases and the Health Services of Ho Chi Minh City, Viet Nam.
Methods. (i) Clinical procedures.
On admission, a full clinical examination was performed, and blood samples were taken for full blood count, biochemistry, blood culture, parasite count, and baseline antimalarial drug assay. An indwelling intravenous catheter was inserted into the antecubital vein of the arm. Patency was maintained by flushing with small volumes of heparinized saline. Patients were randomized to receive either (i) ARTS (Guilin No. 2 Factory, Guangxi, Peoples Republic of China) at 2.4 mg/kg i.m. immediately (stat), followed by 1.2 mg/kg i.m. daily (corresponding to 6.25 and 3.13 μmol/kg, respectively; injection volumes were all 5 ml) or (ii) ARM (Kunming Pharmaceutical Factory, Kunming, Peoples Republic of China) at 3.2 mg/kg i.m. stat (10.7 μmol/kg; injection volume of 0.04 ml/kg), followed by 1.6 mg/kg i.m. daily (5.4 μmol/kg, respectively; injection volume of 0.02 ml/kg).
All injections were given to the anterior thigh. After treatment was initiated, vital signs and parasitemia were monitored every 4 h (or more frequently, if indicated clinically) for 24 h and then every 6 h. Complications were managed as described previously (6)
Blood samples were taken at 0, 5, 10, 15, 20, 30, 45, 60, 75, 90, and 120 min and then at 2.5, 3, 3.5, 4, 5, 6, 8, 10, and 12 h after drug administration. In patients randomized to ARM, initial plasma concentration profiles from the first five patients (last sample at 10 h in two patients and at 12 h in three patients) showed that further samples were needed to characterize the terminal elimination phase, and hence sampling in this group was extended midway through the study to include 12, 18, 20, and 24 h. At each time point, 4 ml of whole blood was collected in lithium heparin tubes (L.I.P, Northampton, United Kingdom). These samples were centrifuged immediately at 1,500 rpm for 5 min at 4°C, and aliquots of separated plasma were frozen at −80°C in Corning 1.8-ml cryotubes (Corning BV Life Sciences, Schiphol-Rijk, The Netherlands) until analyzed.
Thick blood films were stained with Giemsa. The number of asexual parasites per microliter of blood was determined by counting the white blood cells (WBC) in a high-powered field containing 500 parasites in which the ratio of parasites/WBC was <1. The parasitemia was calculated as the product of the parasite/WBC ratio and the WBC count. The time to a 50% reduction in the original parasite count was determined by linear interpolation of the parasite count-time data. Fever clearance time was taken to be the time of the first two axillary temperature readings of <37.5°C.
(ii) Analysis of ARTS, DHA, and ARM.
Fully validated high-performance liquid chromatographic (HPLC) or gas chromatographic-mass spectrometric (GC-MS) methods were used to analyze ARTS, dihydroartemisinin (DHA), and ARM.
ARTS and DHA in plasma from patients who received i.m. ARTS were assayed by the HPLC method of Batty et al. (2). The between-run coefficients of variation (relative standard deviations) were 5 and 6% at 900 and 4,970 nmol/liter, respectively, for ARTS and 11 and 9% at 1,070 and 4,730 nmol/liter, respectively, for DHA. Quality control samples were included with each assay batch, with a requirement that the run was acceptable only if the QC was within ±15% of the nominal value.
ARM and its metabolite DHA in plasma from patients who received i.m. ARM were quantified by the GC-MS method of Mohamed et al. (12), with minor modifications in the GC-MS conditions. Briefly, separations and quantitations were achieved on a Hewlett-Packard 5890 gas chromatograph coupled to a Hewlett-Packard 5971A mass selective detector operating in selected ion monitoring mode (SIM). A J&W DB-5 30-m-by-0.25-mm (inner-diameter) GC column coated with a 0.25-mm film thickness of 5% phenyl methyl siloxone (part 122-5032; J&W Scientific Products GmbH, Cologne, Germany) was used. The gas carrier was helium at a flow rate of 1 ml/min, and the injection port temperature was 250°C. The oven temperature was 100°C initially and maintained at this temperature for 2 min and then ramped at 16°C/min to 250°C, maintained at the latter temperature for 1 min, and then ramped to a final temperature of 300°C. Under these conditions, the retention times were 10.5 min (m/z 152; dwell time = 40 ms), 11 min (m/z 138; dwell time = 40 ms), and 12.4 min (m/z 166; dwell time = 40 ms) for DHA, ARM, and artemisinin (internal standard), respectively. The within-run coefficients of variation for the assay were 3.7 and 7.8% at 35 and 352 nmol/liter, respectively, for DHA and 3.8 and 6.1% at 67 and 671 nmol/liter, respectively, for ARM. Similarly, between-run coefficients of variation were 16.0, 13.5, and 8.7% at 8, 39, and 176 nmol/liter, respectively, for DHA and 13.0 and 6.4% at 70 and 185 nmol/liter, respectively, for ARM. The limits of quantitation were 40 nmol/liter for ARM and 3 nmol/liter for DHA. Quality control samples were included with each assay batch, with a requirement that the run was acceptable only if the quality control was within ±15% of the nominal value.
(iii) Pharmacokinetic analysis.
Data were plotted graphically and analyzed by using WinNonlin 3.1 (Pharsight, Mountain View, Calif.). Where possible, one- or two-compartment open models were fitted to the plasma concentration-time profiles. The final choice of model was based on the Akaike Information Criterion and on the distribution of residuals. Conventional pharmacokinetic parameters (i.e., the area under the plasma concentration time curve [AUC] from time to infinity [AUC0-∞I; with extrapolation to ∞Clast/λz, where Clast is the final measured concentration, and λz is the terminal elimination rate constant], the t1/2, the apparent clearance after i.m. administration [CL or CLi.m./f], the volume of distribution at pseudodistribution equilibrium [Vd], the maximum concentration in plasma [Cmax], and the time to Cmax [Tmax]) were determined from the plasma concentration-time data by both compartmental and noncompartmental analysis. The estimates of pharmacokinetic parameters for DHA assumed complete conversion of ARTS to DHA as reported previously (4).
Statistical analysis.
Data were analyzed by using the computer package SPSS for Windows (version 10.0; SPSS, Inc., Chicago, Ill.). The data are presented as means ± the standard deviations (SD) or geometric means (SD range). Two-sample comparisons were made by using the Student t test or chi-squared test as appropriate. A two-tailed level of significance of 0.05 was used throughout.
RESULTS
Of the 19 patients studied, 9 received ARTS, and 10 received ARM. There were no adverse reactions to either drug. One patient received peritoneal dialysis in the ARM group. Two patients died, both from the ARM group. The first, a 34-year-old male was admitted obtunded (GCS 7), jaundiced, acidotic (plasma lactate = 11 mmol/liter), in acute renal failure, and in shock. He died 39 h after admission. The parasitemia (proportion of red cells parasitized) was 46% on admission, rose to a peak of 61% at 4 h, and was still above the admission value at 24 h. ARM levels in plasma remained <100 nmol/liter during the first 10 h after admission. The second, a 42-year-old male, was admitted jaundiced, comatose (GCS 6), and in shock and died 30 h postadmission. The admission parasitemia was 47%, falling to 2% at 24 h. ARM levels in plasma remained <20 nmol/liter for the first 10 h and did not rise above 50 nmol/liter during the first day. The other 17 patients made an uneventful recovery. The median (range) times to fever and parasite clearance time were similar in the two groups: 34 (15 to 60) h and 48 (21 to 60) h, respectively, in the ARTS group and 35 (15 to 60) h and 48 (30 to 60) h, respectively, in the ARM group. The baseline characteristics of the patients in the two groups are summarized in Table 1.
TABLE 1.
Patient characteristics
| Parametera | ARM | ARTS | P |
|---|---|---|---|
| No. of patients (no. of males) | 10 (6) | 9 (7) | |
| Age (yr) | |||
| Mean | 36.7 | 31.6 | 0.47 |
| Range | 24-60 | 17-62 | |
| Mean temp on admission (°C) (SD) | 38.9 (1.1) | 38.7 (1.0) | 0.73 |
| Mean hematocrit (%) (SD) | 33.0 (8.9) | 33.8 (9.6) | 0.83 |
| Mean white blood cell count (109/liter) (SD) | 8.4 (3.5) | 7.4 (2.4) | 0.52 |
| No. of patients with: | |||
| GCS of <8 | 1 | 0 | 1.0b |
| GCS of 8-11 | 2 | 0 | 0.4b |
| GCS of 12-!5 | 7 | 9 | 0.2b |
| Serum creatinine (μmol/liter) (SD) | 106.0 (36.3) | 185.6 (238.6) | 0.32 |
| Blood lactate (mmol/liter) (SD) | 5.1 (4.2) | 6.4 (6.7) | 0.67 |
| Total plasma bilirubin (μmol/liter) (SD) | 147.0 (119.7) | 63.2 (76.9) | 0.09 |
| Parasitemia on admission (μl−1)a (range) | 225,944 (30,144-1,775,230) | 57,747 (360-816,750) | 0.32 |
| Fever clearance time (h) (SD) | 56 (34.7) | 65 (67.9) | 0.72 |
| Parasite clearance time (h) (SD) | 48 (11.5) | 38.2 (13.5) | 0.14 |
| Mortality | 2 (20%) | 0 (0%) | 0.4b |
Geometric mean.
Fisher 2-tailed results.
Pharmacokinetics of i.m. ARTS.
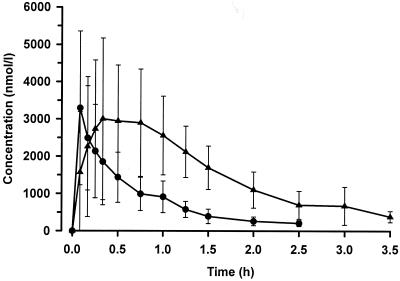
ARTS was absorbed very rapidly with peak concentrations measured in the first sample (5 min) in five of the nine patients. A one-compartment model was fitted satisfactorily to the plasma concentration-time data for both ARTS and DHA (Fig. 1). No significant improvements in fit were obtained by using a two-compartment model, with mean Akaike information criteria for one and two compartmental models of 101 and 95, respectively (95% CI for the difference from −4.3 to 16.8). Derived pharmacokinetic parameters are summarized in Table 2. There was a wide variation between individuals in peak ARTS concentrations in plasma (range, 1,362 to 8,385 nmol/liter). Elimination rates were rapid and varied relatively little between patients (coefficient of variation, 11.6%). ARTS was converted rapidly to DHA. By the first sample (5 min) DHA levels were a median (range) of 21% (5 to 122%) of the parent compound concentration. The molar AUC values for dihydroartemisinin were almost double those for ARTS (mean [95% confidence interval] difference of 727 [321 to 1,132] nmol/liter · h). DHA was eliminated significantly more slowly (P = 0.018). The median (range) estimated elimination half-life for DHA was 52 (26 to 69) min compared to 30 (3 to 67) min for ARTS. These two values were significantly correlated (r = 0.79; P = 0.012)
FIG. 1.
Concentration-time profile for ARTS (•) and its principal biologically active metabolite DHA (▴) after the first i.m. dose of 6.25 μmol (2.4 mg) of ARTS/kg to nine patients. The data are summarized as means ± SD.
TABLE 2.
ARTS and DHA pharmacokinetics after intravenous artesunate injection in severe malariaa
| ARTS
|
DHA
|
||
|---|---|---|---|
| Parameter | Median (range) | Parameter | Median (range) |
| Cmax (nmol/liter) | 5,710 (1,362-8,388) | Cmax (nmol/liter) | 3,060 (1,718-7,080) |
| Vd (liter/kg)* | 1.09 (0.47-4.58) | Vd/f (liter/kg)* | 1.79 (0.63-3.53) |
| k10 (/h)* | 1.39 (0.62-120) | Tmax (min) | 35 (10-86) |
| t1/2 (min)* | 30 (67-3.5) | k01 (/h)* | 2.34 (0.70-17.9) |
| MRT (min) | 43 (5-97) | k10 (h)* | 0.79 (0.60-1.59) |
| AUC (nmol · h/liter)† | 2,228 (533-4672) | Elimination t1/2 (min)* | 52.7 (69.3-26.2) |
| CLi.m. (liter/h/kg) | 2.84 (1.33-11.73) | AUC (nmol · h/liter)† | 5,262 (3,812-7,436) |
| CL/f (liter/h/kg/) | 1.18 (1.64-0.84) | ||
Cmax, peak plasma concentration; Vd, total apparent volume of distribution; k10, first-order elimination rate constant; t1/2; half-life; MRT, mean residence time; AUC, area under the plasma concentration-time curve; CL, clearance; Tmax, time of peak concentration; k01, first-order formation rate constant; f, fraction of parent drug converted to DHA (assumed to be 100%). *, estimated from one-compartment modelling (DHA values assume 100% bioavailability); †, none of the artesunate AUC was extrapolated (0 to 10% of the DHA AUC was extrapolated).
Pharmacokinetics of ARM.
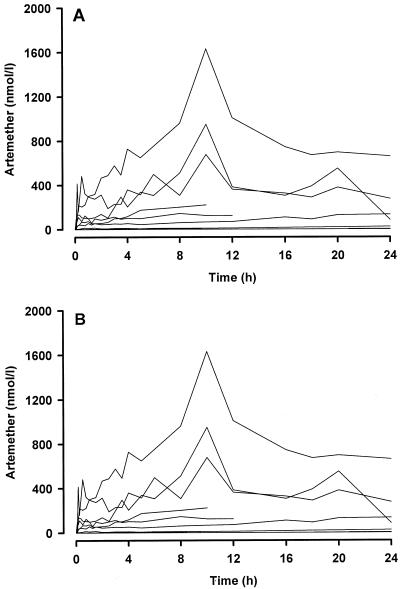
Plasma concentrations of ARM and DHA fluctuated widely after i.m. injection of ARM (Fig. 2). As a result, compartmental modeling was not possible. In general, the absorption profile was biphasic with a relatively rapid initial absorption phase during the first 30 min, followed by a more gradual rise to a peak concentration at a median of 10 h (see upper panel, Fig. 2). Thereafter, mean ARM concentrations tended to decline slowly, but the levels at 24 h were still higher than those during the first 4 h of sampling. In individual patients (lower panel, Fig. 2), peak ARM concentrations in plasma ranged between 67 and 1,631 nmol/liter (median, 574 nmol/liter) and occurred at a median (range) of 10 (1.5 to 24) h. The mean plasma DHA concentration-time profile followed the same pattern as ARM (upper panel, Fig. 3) but at much lower concentrations. The mean ratio of parent drug to metabolite varied between 10 and 30 throughout the 24-h sampling period, a finding consistent with relatively little metabolism of ARM to DHA. In only two patients did peak DHA concentrations exceed 25 nmol/liter and in six patients, the concentration did not rise above 5 nmol/liter.
FIG. 2.
Individual concentration-time profiles for ARM (A) and its metabolite DHA (B) after the first i.m. dose of 10.7 μmol (3.2 mg) of ARM/kg to 10 patients with severe P. falciparum infection. For ARM, two patients had samples collected to 10 h, three patients had samples collected to 12 h, and five patients had samples collected to 24 h. For DHA, two patients had samples collected to 10 h, three patients had samples collected to 12 h, and five patients had samples collected to 24 h. In five of the profiles some of the concentration-time points shown were undetected (zero) or below the assay limit of quantitation.
DISCUSSION
Severe malaria carries a high mortality that can only be reduced by effective antimalarial therapy (22, 24). It is therefore essential that parasiticidal concentrations of antimalarial drugs are achieved as soon as safely possible after admission to hospital or health clinic. ARM is the most widely assessed of the parenteral artemisinin derivatives. Although it is an oil-based formulation that can be given only by the i.m. route, it has proved at least as effective as i.m. quinine in the treatment of severe malaria in large randomized trials that have enrolled collectively nearly 2,000 patients both in Asia and in Africa (1). The dose regimens recommended for both ARM and ARTS and used in the present study were developed empirically before valid analytical techniques were available that allow detailed pharmacokinetic-pharmacodynamic evaluation.
Despite the generally good clinical results with ARM, our data suggest that the absorption of i.m. ARM is erratic and unpredictable. Indeed, the plasma concentration profiles were so variable that we were unable to perform pharmacokinetic modeling. In 2 of the 10 ARM-treated patients studied, one of whom died, the concentrations of ARM in plasma did not exceed 50 nmol/liter within 6 h of dosing. Furthermore, conversion to the more active principal metabolite DHA was minimal, and thus the antimalarial efficacy depended almost entirely on the parent ARM.
Although malarial parasites are exquisitely sensitive to artemisinin derivatives, these low levels are very close to the highest reported IC50s for Plasmodium falciparum isolates taken from patients with primary or recrudescent infections on the northwestern border of Thailand (up to 60 nmol/liter for ARTS, 58 nmol/liter for ARM, and 29 nmol/liter for DHA) (3) and therefore below the concentrations required for maximum parasiticidal effect. In general, in vitro MICs are lower than those extrapolated in vivo partly because of differences in protein binding and because drug concentrations are constant in vitro, whereas they fluctuate in vivo. Poor absorption was documented previously in a study in African children with cerebral malaria (4), in which the combined levels of ARM and DHA in plasma peaked at <100 μg/liter (336 nmol/liter). Taken together, these observations give rise to the concern that some severely ill patients may not absorb i.m. ARM adequately for maximum parasite killing in the critical early hours after the start of treatment. The superior intrinsic antimalarial potency of ARM compared to quinine, therefore, may be counterbalanced by its erratic absorption in the most severely ill patients.
There are few other reports of ARM pharmacokinetics after i.m. injection, mostly in healthy subjects. In one, eight volunteers received a dose of 300 mg, with a geometric mean Cmax of 540 μg/liter (1,812 nmol/liter) and mean half-lives of absorption and elimination of 2 and 6.9 h, respectively (9). The corresponding mean peak for DHA was greater than that of ARM (646 μg/liter or 2,275 nmol/liter), but the elimination t1/2 was of similar magnitude (5.1 h). In another study, eight subjects received 5 mg/kg by i.m. injection, and the mean peak concentrations were 588 μg/liter (1,973 nmol/liter) and 142 μg/liter (500 nmol/liter) for ARM and DHA, respectively (18). The erratic absorption of ARM prevented accurate estimation of elimination half-lives (18), as in the present investigation. It is difficult to know why the results of these two published studies differ so markedly, since the same dose of the same formulation of ARM was given and the same assay methodology (HPLC with electrochemical detection) was used.
A third study involving 17 Thai adults with severe malaria, carried out by the same group as the first volunteer study described above (9), reported that the dose-adjusted AUC of ARM was increased in cases of severe malaria, especially in patients with renal impairment (10). All subjects had plasma concentration profiles of both ARM and DHA that exhibited the same pattern as observed in healthy subjects (9), with tmax values all close to 4 h, and concentrations in plasma that fell progressively thereafter to levels that were below, or close to, the limit of detection at 24 h (10). These findings are in marked contrast to those in our patients and the other reported studies with ARM (13, 19) and the closely related compound arteether (11), which suggests that the absorption of the oil based drugs from an i.m. depot is prolonged and erratic, and biotransformation to DHA much reduced compared to that after oral dosing.
Comparison of these results with studies of oral administration (18) suggests that extensive first-pass metabolism of ARM occurs when the drug is given by mouth compared to parenteral routes. After oral administration the AUC of the metabolite considerably exceeds that of the parent compound, whereas the reverse is seen after i.m. administration. The DHA concentrations in plasma measured in our patients with severe malaria were, after we adjusted for the lower dose used (3.2 versus 5 mg/kg), still considerably lower than those measured in healthy volunteers after i.m. administration (18), suggesting that hepatic metabolism of ARM, mainly by CYP3A4, but also by 2B6 and 3A5 (5, 14), is reduced by malaria infection. Acute malaria reduces the hepatic biotransformation of many drugs (16). The metabolism of quinine (also largely via CYP3A4) has been shown to be reduced in proportion to the severity of malaria (19). The effect of severe malaria on ARM pharmacokinetics may be even more pronounced, since both absorption from the i.m. injection site and hepatic conversion to the active metabolite are reduced.
In contrast to ARM, the absorption of ARTS was rapid and reliable. The concentrations of ARTS and DHA in plasma in our patients (median peak values of 5,707 and 3,223 nmol/liter, respectively) were generally similar to those reported previously in uncomplicated malaria (2, 8, 14). The peak concentrations of ARTS and DHA are 50 to 100 times higher than those required for full activity against the parasite. Both are eliminated rapidly, even in cases of severe malaria; yet this brief exposure to the infecting parasite population (several hours) is sufficient for a maximum antiparasitic effect in all cases. There are also important pharmacokinetic differences between the two artemisinin derivatives after oral administration in malaria (15). In a recent crossover study in uncomplicated malaria in which oral ARTS was compared to oral ARM, the relative oral bioavailability of oral ARM was 58% compared to that of ARTS (17).
Unfortunately, there is still only one manufacturer of parenteral ARTS, and this formulation is not yet prepared to an internationally recognized Good Manufacturing Practices standard. Parenteral ARTS has considerable pharmacokinetic advantages over ARM in the treatment of severe falciparum malaria. Since these drugs have a similar cost and are both widely available in the tropics, ARTS is the preferred choice for the treatment of severe falciparum malaria, particularly in patients who are most seriously ill and in whom absorption from an i.m. depot may be compromised. These findings should be formally assessed by a randomized clinical trial
Acknowledgments
We are indebted to Kevin Batty and Shane Powell for expert technical assistance with some of the HPLC assays. We thank Colonel Brian Schuster, Walter Reed Army Institute of Research and Experimental Therapeutics, Washington, D.C., for assay reference standards for ARTS, ARM, artemisinin, and DHA.
This study was supported by a project grant from the National Health and Medical Research Council of Australia (T.M.E.D. and K.FI.) and the Wellcome Trust of Great Britain.
REFERENCES
- 1.Artemether-Quinine Meta-Analysis Study Group. 2001. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:637-650. [DOI] [PubMed] [Google Scholar]
- 2.Batty, K. T., T. M. E. Davis, L. T. Thu, T. Q. Binh, T. K. Anh, and K. F. Ilett. 1996. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J. Chromatogr. B 677:345-350. [DOI] [PubMed] [Google Scholar]
- 3.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive ARTS-mefloquine use. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis, T. M., H. L. Phuong, K. F. Ilett, N. C. Hung, K. T. Batty V. D. Phuong, S. M. Powell, H. V. Thien, and T. Q. Binh. 2001. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob. Agents Chemother. 45:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace, J. M., A. J. Aguilar, K. M. Trotman, J. O. Peggins, and T. G. Brewer. 1998. Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450. Drug Metab. Disp. 26:313-317. [PubMed] [Google Scholar]
- 6.Hien, T. T., N. P. J. Day, N. H. Phu, N. T. H. Mai, T. T. H. Chau, P. P. Loc, D. X. Sinh, L. V. Chuong, H. Vinh, D. Waller, T. E. A. Peto, and N. J. White. 1996. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N. Engl. J. Med. 335:76-83. [DOI] [PubMed] [Google Scholar]
- 7.Hien, T. T., N. H. Phu, N. T. H. Mai, T. T. H. Chau, T. T. M. Trang, P. P. Loc, B. M. Cuong, N. T. Dung, H. Vinh, D. J. Waller, and N. J. White. 1992. An open randomized comparison of intravenous and intramuscular artesunate in severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 86:584-585. [DOI] [PubMed] [Google Scholar]
- 8.Ilett, K. F., K. T. Batty, S. M. Powell, T. Q. Binh, L. Thi, A. Thu, H. L. Phuong, N. C. Hung, and T. M. E. Davis. 2002. The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 53:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karbwang, J., K. Na-Bangchang, K. Congpuong, P. Molunto, and A. Thanavibul. 1997. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur. J. Clin. Pharmacol. 52:307-310. [DOI] [PubMed] [Google Scholar]
- 10.Karbwang, J., K. Na-Bangchang, T. Tin, K. Sukonstason, W. Rimchala, and T. Harinasuta. 1998. Pharmacokinetics of intramuscular ARM in patients with severe falciparum malaria with or without acute renal failure. Br. J. Clin. Pharmacol. 45:597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looareesuwan, S., P. Wilairatana, P. de Boer, A. Pouwels, S. Krudsood, J. J. van Lier, W. Chokejundachai, F. W. M. Smeets, K. Chalermrut, S. Vannaphan, B. Oosterhuis, and C. B. Lugt. 1999. A comparative study of intramuscular artemitol and artemether in the treatment of severe malaria in children, p. 56. Proceedings of the Joint International Tropical Medicine Meeting, Bangkok, Thailand.
- 12.Mohamed, S. S., S. A. Khalid, S. A. Ward, T. S. Wan, H. P. Tang, M. Zheng, R. K. Haynes, and G. Edwards. 1999. Simultaneous determination of artemether and its major metabolite dihydroartemisinin in plasma by gas chromatography-mass spectrometry-selected ion monitoring. J. Chromatogr. B Biomed. Appl. 731:251-260. [DOI] [PubMed] [Google Scholar]
- 13.Mordi, M. N., S. M. Mansor, V. Navaratnam, and W. H. Wernsdorfer. 1997. Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br. J. Clin. Pharmacol. 43:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy, S., E. Mberu, D. Muhia, M. English, J. Crawley, C. Waruiru, B. Lowe, C. Newton, P. Winstanley, K. Marsh, and W. Watkins. 1997. The disposition of intramuscular artemether in children with cerebral malaria: a preliminary study. Trans. R. Soc. Trop. Med. Hyg. 91:331-334. [DOI] [PubMed] [Google Scholar]
- 15.Navaratnam, V., S. M. Mansor, N. W. Sit, J. Grace, Q. Li, and P. Olliaro. 2000. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 39:255-270. [DOI] [PubMed] [Google Scholar]
- 16.Newton, P., Y. Suputtamongkol., P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. J. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pukrittayakamee, S., S. Looareesuwan, K. Keeratithakul, T. M. E. Davis, P. Teja-Isavadharm, B. Nagachinta, A. Weber, A. L. Smith, D. Kyle, and N. J. White. 1997. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur. J. Clin. Pharmacol. 52:487-493. [DOI] [PubMed] [Google Scholar]
- 18.Suputtamongkol, Y., P. Newton, B. Angus, P. Teja-Isavadharm, D. Keeratithakul, M. Rasameesoraj, S. Pukrittayakamee, and N. J. White. 2001. A comparison of oral artesunate and artemether antimalarial bioactivities in acute falciparum malaria. Br. J. Clin. Pharmacol. 52:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teja-Isavadharm, P., F. Nosten, D. E. Kyle, C. Luxemburger, F. ter Kuile, J. O. Peggins, T. G. Brewer, and N. J. White. 1996. Comparative bio-availability of oral, rectal and intramuscular artemether in healthy subjects-use of simultaneous measurement by high performance liquid chromatography with electro-chemical detection and bioassay. Br. J. Clin. Pharmacol. 42:599-604. [DOI] [PubMed] [Google Scholar]
- 20.ter Kuile, F., N. J. White, P. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in-vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76:86-95. [DOI] [PubMed] [Google Scholar]
- 21.van Hensbroek, M. B., E. Omyiorah, and S. Jaffar et al. 1996. A trial of artemether or quinine in children with cerebral malaria. N. Engl. J. Med. 335:69-75. [DOI] [PubMed] [Google Scholar]
- 22.Warrell, D. A., M. Molyneux, and P. Beales. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1-69. [Google Scholar]
- 23.White, N. J. 1996. The treatment of malaria. N. Engl. J. Med. 335:800-806. [DOI] [PubMed] [Google Scholar]
- 24.White, N. J. 1998. Not much progress in treatment of cerebral malaria. Lancet 352:594-595. [DOI] [PubMed] [Google Scholar]
- 25.White, N. J., S. Looareesuwan, D. A. Warrell, M. J. Warrell, D. Bunnag, and T. Harinasuta. 1982. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am. J. Med. 73:564-572. [DOI] [PubMed] [Google Scholar]