Abstract
Amdoxovir [(−)-β-d-2,6-diaminopurine dioxolane (DAPD)] is a nucleoside analogue reverse transcriptase inhibitor of human immunodeficiency virus type 1 (HIV-1) replication. DAPD is deaminated by adenosine deaminase to the guanosine analogue dioxolane guanosine (DXG), which is subsequently phosphorylated to the corresponding 5′ triphosphate (DXG-TP). DXG-TP competes with the natural substrate dGTP for binding to the enzyme-nucleic acid complex. Mycophenolic acid (MPA) and ribavirin (RBV), inhibitors of inosine monophosphate dehydrogenase (IMPDH), inhibit the de novo synthesis of guanine nucleotides, including dGTP. Reducing the intracellular levels of dGTP would be expected to augment the antiviral activity of analogues of deoxyguanosine. In this study we examined the effect of MPA and RBV on the anti-HIV activity of DAPD and DXG. When tested against wild-type virus, both MPA and RBV decreased the 50% effective concentration (EC50) for DXG by at least 10-fold. In contrast, both MPA and RBV increase the EC50 value for zidovudine. MPA and RBV completely reversed the resistance to DXG observed with HIV isolates containing mutations which confer partial resistance to DAPD and DXG. Similarly, when tested against a mutant virus fully resistant to inhibition by DAPD (K65R/Q151M), MPA and RBV reduced the EC50 for DAPD to within twofold of that for the wild type. The combination of MPA or RBV with DAPD or DXG did not result in increased cytotoxicity or reduced levels of mitochondrial DNA when tested at physiologically relevant concentrations. These studies suggest a potential role for the use of IMPDH inhibitors in combination therapy with amdoxovir in the treatment of HIV.
Human immunodeficiency virus (HIV) replication is dependent upon the host cell to provide the necessary substrates for viral replication, including deoxynucleoside triphosphates for the reverse transcription of viral RNA into double-stranded DNA by the HIV-encoded reverse transcriptase (HIV-RT). Nucleoside analogue inhibitors (NRTIs) of the HIV-RT, such as zidovudine (AZT; also called azidothymidine and Retrovir), lamivudine, didanosine (ddI), stavudine, abacavir (ABC; also called Ziagen), and more recently tenofovir and emtricitabine, have formed the cornerstone of anti-HIV therapy. These NRTIs must first be converted to their 5′ triphosphates and must be able to successfully compete with the natural nucleotides of the host cell to effectively inhibit virus replication. Reducing the level of endogenous nucleotides would shift the competition in favor of the nucleotide analogue. Hydroxyurea, and more recently mycophenolic acid (MPA), have been used to target enzymes involved in the de novo synthesis of deoxynucleotides, and both are approved for other pharmaceutical applications. Hydroxyurea has been used to target cellular ribonucleotide reductase that, through a complex pattern of regulation, provides the appropriate supply of the four deoxynucleotides needed for the synthesis of DNA (37). Presumably, hydroxyurea exerts its effect by decreasing the endogenous deoxynucleotide pools, thereby resulting in a relative increase in the intracellular concentration of the 5′ triphosphate of the NRTI (13). In vitro, synergistic anti-HIV activity was observed between ddI and hydroxyurea (19). In addition, HIV variants resistant to ddI are significantly more sensitive to the drug in the presence of hydroxyurea. The combination of hydroxyurea and ddI has been evaluated in a number of clinical studies and the results from these studies confirm the efficacy of the combination (7, 11, 18). While toxicities associated with hydroxyurea have limited its use in treatment-naïve patients, it has been successfully used to augment therapy in treatment-experienced patients failing their current regimens as part of a simplified regimen in patients on fully suppressive highly active antiretroviral therapy (HAART).
In the de novo purine synthesis pathway, inosine monophosphate dehydrogenase (IMPDH) is the first of two enzymes responsible for the conversion of IMP to GMP, which is normally converted to GDP, GTP, and dGTP. Studies with various cell types, including lymphocytes, have shown that inhibiting IMPDH causes a reduction in the intercellular levels of GTP and dGTP (20, 36). Mycophenolate mofetil (MMF) is a prodrug which is rapidly converted to mycophenolic acid, a potent and reversible uncompetitive inhibitor of IMPDH (1). Lymphocytes and monocytes rely primarily on the de novo pathway of guanosine synthesis, and therefore MPA selectively inhibits lymphocyte and monocyte proliferation (1, 20). In addition, MPA inhibits HIV replication in these cells presumably through reduction of dGTP pools (8, 17). MPA has been shown to increase the in vitro anti-HIV activity of ABC when used in combination (21). Several pilot clinical studies have been conducted to evaluate the effect of MPA (given as the prodrug MMF) in combination with HAART (8, 9, 22). Overall, therapy with MMF resulted in a transient decrease in HIV viral load in the majority of patients. In addition, there were no significant changes in the levels of CD4+ cells following 24 weeks of treatment.
Ribavirin (RBV), a purine analogue with a broad spectrum of antiviral activity (32), has been approved for the treatment of respiratory syncytial virus and, in combination with interferon, for the treatment of hepatitis C infection. RBV monophosphate, the active metabolite of the antiviral agent RBV, is a substrate mimic of IMP and as such functions as a competitive inhibitor of IMPDH (33). RBV monophosphate has also been shown to enhance the anti-HIV activity of ddI in vitro (5, 25). As with MPA and hydroxyurea, the synergistic effects of RBV in combination with specific NRTIs is attributed to changes in the intracellular dNTP pools (4). Alternatively, inhibition of IMPDH also results in accumulation of IMP which would in turn facilitate the phosphorylation of (−)-β-d-dioxolane guanosine (DXG) to its 5′ monophosphate form by high-Km 5′ nucleotidase, a proposed key enzyme in the DXG activation pathway (10).
Amdoxovir [(−)-β-d-2,6-diaminopurine dioxolane (DAPD)] is an aqueous, soluble, and bioavailable prodrug that is rapidly absorbed and converted in vivo to DXG (12, 15). In vitro, the anti-HIV activity observed with DAPD is almost entirely due to the generation of DXG following deamination of DAPD by the action of adenosine deaminase. DXG is subsequently phosphorylated to the 5′ triphosphate, DXG-TP, a strong alternative competitive inhibitor of HIV-RT (Ki = 0.019 μM). In this study, we investigated the effects of MPA and RBV on the in vitro antiviral activity of DAPD and DXG. We demonstrated that when DAPD or DXG is combined with MPA or RBV, a synergistic anti-HIV response occurred. Moreover, when cells infected with mutant HIV-1 resistant to DAPD or DXG were exposed to these combinations, the 50% effective concentration (EC50) for DAPD and DXG reverted to near wild-type values. These results suggest that the combination of MMF and DAPD could prove to be an effective approach to treating experienced patients who are not responding to current NRTI-containing regimens.
MATERIALS AND METHODS
Reagents.
DAPD and DXG were synthesized at Gilead Sciences Inc. AZT and ABC were generously provided by Glaxo SmithKline. Test compounds were prepared in dimethyl sulfoxide at a final concentration of 10 mM. MPA and RBV were purchased from Sigma-Aldrich.
Cells.
Cytotoxicity and activity assays were performed in the T-cell line MT2 and in peripheral blood mononuclear cells (PBMC). Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum and 20 μg of gentamicin (Life Technologies)/ml. PBMC were obtained from HIV-seronegative donors by banding on Ficoll (Amersham Pharmacia Biotech) and were activated by phytohemagglutinin (PHAP; Sigma-Aldrich) for 2 days prior to infection. Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 20 μg of gentamicin/ml, and 10% interleukin-2.
Viruses.
Recombinant viruses were created by subcloning the HIV protease and RT coding sequences obtained from patient plasma HIV RNA into a modified version of the plasmid xxLAI (31). Amplification and cloning strategies have been previously described (23). Recombinant viruses contained mutations in the HIV-RT at positions F116Y, Q151M, and T215Y. Viruses containing mutations at positions K65R, L74V, Q151M, and K65R/Q151M of the HIV-RT were generated by site-directed mutagenesis of the xxLAI plasmid using a QuickChange site-directed mutagenesis kit from Stratagene. Genotypic analysis of the recombinant viruses and of viruses obtained by site-directed mutagenesis was performed by dideoxy-sequencing using ABI Prism 377 technology.
Antiviral assays.
Anti-HIV assays were performed using two different assay methodologies. A cytotoxicity-based (XTT) assay was performed to evaluate activity in MT2 cells (26) and an HIV-1 p24-based enzyme-linked immunosorbent assay (Organon Teknika Corporation) was performed to evaluate activity in PBMC. For the XTT assay, MT2 cells were infected with either the mutant virus or wild-type LAI at a multiplicity of infection of 0.03 in RPMI 1640 medium containing 10% fetal bovine serum, 20 μg of gentamicin/ml, and 2 μg of Polybrene (Sigma)/ml for 2 h at 37°C. Following infection, cells were seeded into 96-well plates containing test compounds at 3 × 104 cells/well. Within each 96-well plate, test compounds were tested in triplicate at fivefold serial dilutions. The infected cells were cultured for 5 days in the presence of test compounds. On day 5, XTT {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5[(phenylammino)carbonyl]-2H-tetrazolium hydroxide} was added, and the plates were incubated for 3 h at 37°C and then analyzed for absorption (A450). A dose-response curve for each test compound was generated using the absorption values of the uninfected cell controls as 100% protection and no drug, virus-infected cells as 0% protection. From the dose-response curve, an EC50 was calculated and defined as the concentration of drug that inhibited viral induced cytopathic effect by 50%.
Anti-HIV activity was also assessed in human PBMC by using an enzyme-linked immunosorbent assay for the detection of HIV-1 p24 core antigen. PBMC were infected with HIV-1 virus at a multiplicity of infection of 0.001 for 4 h at 37°C and plated in the presence of drugs as described above. Infected cells were cultured for 4 days. On day 4, the amount of HIV-1 p24 was determined in each well. Uninfected cells were used as background and readings from the virus control were considered 100% infection. Dose-response curves and EC50s were determined as described above.
The effect of MPA and RBV on the in vitro anti-HIV activity of DAPD, DXG, ABC, and AZT was evaluated using the MT2/XTT and PBMC/p24 assays described above. Combination assays were performed in triplicate with various concentrations of the test compounds, alone or with a fixed concentration of MPA or RBV. Results from the antiviral assays in PBMC were analyzed using the MacSynergy II program to determine potential synergistic and/or antagonistic activities (27, 28). This program calculates a theoretical additive value for each drug combination based on the values generated by the drugs alone using an independent effects model (Bliss independence). The theoretical additive values are subtracted from the experimental values generated by each drug combination to give a value of synergy (positive value) or antagonism (negative value). A synergy volume is calculated by adding all of the synergy values (positive values) for each drug combination. Likewise, all of the antagonistic values (negative values) are added to give an antagonistic volume. These synergy and antagonism volumes are then statistically evaluated using confidence levels of 95, 99, and 99.9% (expressed as μM2%).
Cytotoxicity assays.
MT2 cells and PHAP-stimulated human PBMC were seeded at densities of 3 × 104 and 1 × 105 cells/well, respectively, in 96-well plates containing twofold serial dilutions of DAPD or DXG. For combination cytotoxicity assays, a fixed concentration of RBV (1, 5, 10, 20, 40, and 60 μM) or MPA (0.01, 0.1 0.25, 0.5, and 1 μM) was added to the DAPD or DXG dilutions. The cultures were incubated for 5 days at 37°C in a humidified 5% CO2 atmosphere and were then incubated with XTT for 3 h. Cytotoxicity was determined by comparing absorption values (A450) obtained from the treated cultures with that of the untreated control.
Mitochondria DNA assay.
The effect of DAPD and DXG alone or in combination with MPA or RBV on mitochondrial DNA (mtDNA) synthesis was assessed using HepG2 cells. Cells were seeded (5 × 104 cells/well) into 12-well tissue culture plates and incubated for 4 days at 37°C in a humidified 5% CO2 atmosphere with RPMI 1640 supplemented with 10% fetal calf serum and 2 mM glutamine. On day 4, the medium was replaced with fresh medium containing various concentrations of test compound. For combination experiments, the concentrations of MPA and RBV were held constant (0.25 μM MPA and 20 μM RBV) and the concentrations of DAPD and DXG were varied (0.01, 0.1, 1, 10, 25, and 50 μM DAPD or DXG). The cells were incubated for 2 weeks and during the incubation period medium was changed every other day. At the end of the 2 weeks, cells were collected and total cellular DNA was extracted using a DNeasy kit (QIAGEN). DNA from each sample was denatured by adding an equal volume of denaturing buffer (0.8 M NaOH, 20 mM EDTA) and heating to 100°C for 10 min. The DNA samples were blotted onto positive-charged nylon membranes washed once with 0.4 M NaOH followed by a single wash with 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]). The DNA was cross-linked to the membrane by exposing it to UV light. A specific 32P probe encompassing nucleotide positions 4212 to 4242 of human mtDNA was used to quantitate the level of mtDNA in each sample. Cellular DNA was quantitated using a 32P probe specific for human glyceraldehyde-3-phosphate dehydrogenase. The quantity of mtDNA was normalized to the amount of cellular DNA resulting in relative mtDNA levels. The levels of mtDNA from the treated cells were compared to those of the untreated cell controls. The data from three experiments were combined, and the data were reported as the means ± the standard deviations.
RESULTS
Anti-HIV activity and cytotoxicity of mycophenolic acid and RBV.
Mycophenolic acid and RBV were tested for activity against the LAI strain of HIV-1 using the MT2/XTT and PBMC/p24 assays described above. Neither MPA nor RBV demonstrated anti-HIV activity when tested in the MT2/XTT assays. The highest concentration of MPA and RBV tested was 1 and 100 μM, respectively. However, both MPA and RBV demonstrated activity against HIV replication when tested in the PBMC/p24 assay. Median EC50s of 0.95 ± 0.29 μM and 20.5 ± 11.8 μM were determined for MPA and RBV, respectively. Inhibition of HIV replication is presumed to occur due to inhibition of cell proliferation caused by decreased levels of dGTP (17) and not by a direct effect of MPA on HIV. Using steady-state kinetic analysis with wild-type HIV-RT, we have shown that MPA alone does not directly inhibit HIV-RT (data not shown).
Mycophenolic acid and RBV were also tested for cytotoxicity in the MT2 cell line and PBMC as described above. MPA was found to be toxic to both cell lines with concentrations required for 50% cell death of 5.7 μM (MT2) and 4.5 μM (PBMC). RBV was found to not be toxic to either cell line at concentrations up to 1 mM.
Activity of DAPD and DXG in combination with MPA and RBV.
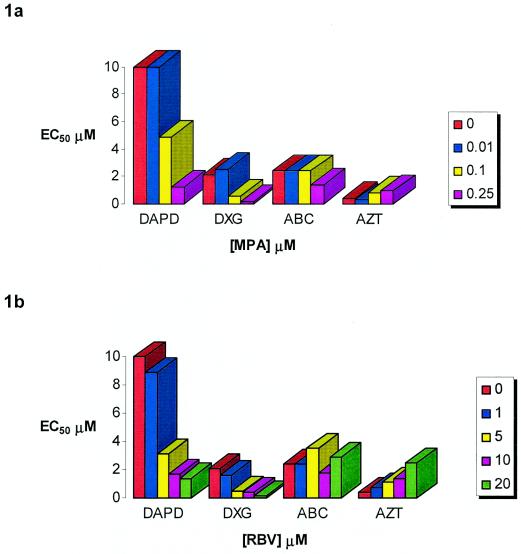
We evaluated the effect of MPA and RBV on the activity of DAPD and DXG in the MT2/XTT and PBMC/p24 assays as described above. The combination of MPA and RBV with abacavir or AZT was also evaluated. Abacavir was used as a positive control as its active form, carbovir triphosphate, is also a guanosine analogue and has been shown to have synergistic anti-HIV activity when combined with MPA (21). AZT was used as a control to demonstrate antagonism. Previous studies have demonstrated that the combination of RBV and AZT was antagonistic with regards to HIV replication, presumably as a consequence of increased levels of dTTP which acts as a feedback inhibitor of thymidine kinase activity (3, 34). In the MT2 cell line, addition of low concentrations of MPA (0.01 μM) had no effect on the anti-HIV activity of any of the compounds tested. We observed that there was a direct relationship between the concentration of MPA or RBV and the activity observed for both DAPD and DXG. The combination of 0.25 μM MPA with DAPD or DXG produced the greatest results (16.7- and 10.5-fold increases in the antiviral activity of DAPD and DXG, respectively) without any cytotoxicity. By contrast, addition of 0.25 μM MPA resulted in a 2.3-fold decrease in the activity of AZT. Little effect was noted on the antiviral activity of abacavir (less than twofold) at any of the MPA concentrations tested. Results are shown in Fig. 1a. No cytotoxicity, as determined by >90% cell viability, was observed at any of the drug combinations tested (data not shown).
FIG. 1.
Effect of increasing MPA and RBV concentrations on the antiviral activity of DAPD, DXG, ABC, and AZT in MT2 cells. Results represent the means of two experiments.
Similar experiments were performed with RBV in combination with DAPD, DXG, abacavir, and AZT. In these assays, the addition of RBV (up to 20 μM) had no effect on anti-HIV activity of abacavir. By contrast, the addition of 20 μM RBV increased the apparent activity of DAPD and DXG by 14.3- and 11.7-fold, respectively. Combining 20 μM RBV with AZT resulted in a >6-fold decrease in activity. These results are consistent with previous reports that have demonstrated the antagonistic activity of RBV and AZT (3, 34). Results are shown in Fig. 1b. No cytotoxicity, as determined by >90% cell viability, was observed at any of the drug combinations tested (data not shown).
The effects of MPA and RBV on the anti-HIV activity of DAPD, DXG, and abacavir were also evaluated in PBMC. At the lowest concentration of MPA tested, 0.01 μM, there was no change in the EC50 observed for abacavir. In addition, increasing the concentration of MPA to 0.1 μM had a minimal effect on abacavir activity (threefold decrease in EC50). By contrast, addition of 0.01 μM MPA resulted in a 4.6- and 9.3-fold increase in the antiviral activity of DAPD and DXG, respectively, while 0.1 μM MPA resulted in a 34- and 23-fold increase in activity for these compounds. In all cases, the addition of 0.25 μM MPA resulted in complete suppression of viral replication at all concentrations of DAPD, DXG, or abacavir tested. The combination of 1 μM RBV had only a minimal effect on the anti-HIV activity of DXG, DAPD, and abacavir. Increasing the concentration of RBV to 10 μM resulted in a significant increase in the activity of DAPD, DXG, and abacavir. These results are summarized in Table 1. The results obtained from the combination of MPA or RBV with DAPD and DXG in PBMC were also evaluated using the MacSynergy II software program. The MacSynergy II program uses an independent effects model (Bliss independence) to generate theoretical volumes of synergy or antagonism for each drug combination. Synergy volumes greater than 25 μM2% are considered synergistic, while volumes between −25 and 25 μM2% are indicative of an additive interaction. Likewise, volumes less than −25 μM2% are considered indicative of antagonism. In this analysis, all of the combinations tested demonstrated synergistic interactions as demonstrated by synergy volumes >25 μM2% (Table 2). No cytotoxicity, as determined by >90% cell viability, was observed at any of the drug combinations tested (data not shown).
TABLE 1.
Effect of MPA and RBV on the activity of DAPD, DXG, and ABC against wild-type HIV-1 in PBMC
| Compound | EC50a (μM) | Fold Δb | Compound | EC50 (μM) | Fold Δ |
|---|---|---|---|---|---|
| DAPD | 4.1 ± 2 | 1 | DAPD | 4.1 ± 2 | 1 |
| DAPD+0.01 μM MPA | 0.9 ± 0.6 | 4.6 | DAPD+1 μM RBV | 2.05 ± 1.5 | 1.5 |
| DAPD+0.10 μM MPA | 0.12 ± 0.08 | 34 | DAPD+10 μM RBV | 0.15 ± 0.05 | 27 |
| DAPD+0.25 μM MPA | <0.02 | >200 | DAPD+20 μM RBV | <0.02 | >200 |
| DXG | 0.14 ± 0.09 | 1 | DXG | 0.14 ± 0.09 | 1 |
| DXG+0.01 μM MPA | 0.015 ± 0.006 | 9.3 | DXG+1 μM RBV | 0.07 ± 0.005 | 2 |
| DXG+0.10 μM MPA | 0.006 ± 0.006 | 23 | DXG+10 μM RBV | <0.001 | >140 |
| DXG+0.25 μM MPA | <0.001 | >140 | DXG+20 μM RBV | <0.001 | >140 |
| ABC | 1.2 ± 0.8 | 1 | ABC | 1.2 ± 0.8 | 1 |
| ABC+0.01 μM MPA | 1.1 ± 0.3 | 1 | ABC+1 μM RBV | 0.39 ± 0.1 | 3 |
| ABC+0.10 μM MPA | 0.4 ± 0.2 | 3 | ABC+10 μM RBV | 0.028 ± 0.02 | 43 |
| ABC+0.25 μM MPA | <0.01 | >120 | ABC+ 20 μM RBV | <0.01 | >120 |
Results represent the means of three experiments ± standard deviations.
n fold change, EC50 DAPD/EC50 DAPD + MPA or RBV.
TABLE 2.
Experimental synergy volumes for the combinations of DAPD and DXG with MPA or RBV in PBMCa
| Drug 1 | Drug 2 | Synergyb (μM2%) | Antagonismb (μM2%) | Net effectc (μM2%) |
|---|---|---|---|---|
| DAPD | MPA | 37.36 | −5.35 | 32.01 |
| DAPD | RBV | 37.68 | −1.01 | 36.67 |
| DXG | MPA | 58.57 | −9.82 | 48.75 |
| DXG | RBV | 27.69 | 0 | 27.69 |
Results from one experiment with three plates.
95% confidence.
Synergy plus antagonism.
Activity of DAPD and DXG in combination with MPA and RBV against drug resistant variants.
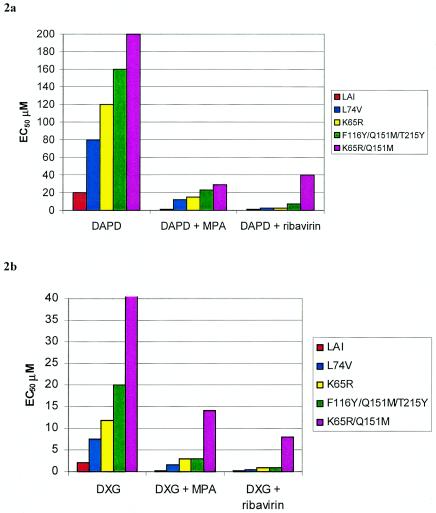
The effect of MPA and RBV on the activity of DAPD and DXG against strains of HIV-1 containing mutations that confer resistance to these analogues was also analyzed. Resistant virus included laboratory isolates containing the K65R and L74V mutations created by site-directed mutagenesis in the LAI backbone. These mutations have been shown to develop in vitro when virus was passed in the presence of increasing concentrations of DXG (6, 16, 23). The Q151M mutation also was shown to cause moderate resistance to DXG (23). A laboratory strain containing the K65R/Q151M double mutation as well as a recombinant virus containing mutations at positions 116Y, 151M, and 215Y were tested. Combination assays were performed with a fixed concentration of MPA (0.25 μM) or RBV (20 μM) in the MT2 assay. The mutant viruses demonstrated increased EC50s for DAPD and DXG, with the K65R/Q151M mutant being the most resistant (>10-fold increase in EC50). However, in the presence of 0.25 μM MPA or 20 μM RBV, the EC50s determined for DAPD were within twofold of the values obtained for wild-type virus. Likewise, the combination of MPA or RBV with DXG resulted in a >10-fold increase in activity against the mutant strains (Fig. 2).
FIG. 2.
Effect of 0.25 μM MPA and 20 μM RBV on the antiviral activity of DAPD and DXG against drug-resistant isolates (MT2). Results represent the means of two experiments.
The effects of MPA and RBV on the activity of DAPD and DXG against the highly resistant K65R/Q151M mutant were also evaluated in PBMC. Addition of increasing concentrations of MPA (up to 0.2 μM) or RBV (up to 10 μM) resulted in increased activity of both DAPD and DXG, with a greater than 50-fold increase in DXG activity and greater than 11-fold increase in DAPD activity observed at the highest concentrations tested (Table 3).
TABLE 3.
Effect of increasing concentrations of MPA and RBV on the antiviral activity of DAPD and DXG against virus containing the K65R/Q151M mutations (PBMC)
| Initial treatment [EC50 (fold Δ)]a | Addition
|
||||||
|---|---|---|---|---|---|---|---|
| Control | 0.05 μM MPA | 0.1 μM MPA | 0.2 μM MPA | 2.5 μM RBV | 5 μM RBV | 10 μM RBV | |
| DAPD | >250 (1) | >250 (1) | 140 ± 30 (>1.8) | 2.9 ± 1.3 (>86) | 225 ± 7 (1) | 120 ± 20 (>2) | 22 ± 2 (>11) |
| DXG | 30 ± 9 (1) | 33 ± 6 (1) | 2 ± 0.7 (15) | <0.2 (>150) | 7.8 ± 0.8 (4) | 3.7 ± 0.4 (8) | 0.22 ± 0.01 (136) |
EC50 values in micromolars data represent the means of two experiments ± standard deviations.
Mitochondrial toxicity of DAPD and DXG in combination with MPA or RBV.
DAPD and DXG were previously reported to have little effect on mitochondrial DNA synthesis (12). The level of mtDNA was evaluated in HepG2 cells incubated with 0.25 μM MPA or 20 μM RBV alone and in combination with DAPD or DXG to determine if the presence of an IMPDH inhibitor would affect mtDNA synthesis. Due to the variability of the assay, a 20 to 30% difference in the level of mtDNA was not uncommon. Therefore, dideoxycytidine, a potent inhibitor of polymerase γ was used as a positive control in each experiment. RBV concentrations up to 80 μM did not significantly affect mtDNA content or cell growth, whereas MPA at a concentration of 0.5 μM and above caused a dose-dependent increase in cell death and decrease in mtDNA content. Consistent with previously reported results, DAPD did not cause a significant reduction in mtDNA content at the clinically relevant concentration of 10 μM (12, 35). However, the combination of 20 μM RBV and DAPD did cause a significant reduction in mtDNA at DAPD concentrations of ≥10 μM (Table 4). DXG did not significantly affect mtDNA levels at the highest concentration used in this study (50 μM). Furthermore, the addition of 0.25 μM MPA did not alter the effect of DXG on mtDNA synthesis. The combination of DXG and RBV did appear to cause some inhibition of mtDNA synthesis at the highest DXG concentration tested (50 μM). Results are shown in Table 4.
TABLE 4.
Effects of DAPD, DXG, MPA, and RBV on mitochondrial DNAa
| Compound | mtDNAa at a concentration (μM) of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.25 | 0.5 | 1 | 5 | 10 | 20 | 25 | 40 | 50 | 80 | |
| DAPD | 94 ± 8 | 92 ± 3 | 72 ± 5 | 67 ± 3 | 46 ± 1 | ||||||
| DAPD+MPAb | 74 ± 18 | 96 ± 8 | 97 ± 4 | 78 ± 4 | 53 ± 4 | ||||||
| DAPD+RBVb | 73 ± 1 | 79 ± 6 | 63 ± 13 | 29 ± 11 | 19 ± 6 | ||||||
| DXG | 95 ± 9 | 79 ± 6 | 82 ± 8 | 79 ± 8 | 72 ± 19 | ||||||
| DXG+MPA | 126 ± 16 | 122 ± 8 | 96 ± 9 | 96 ± 11 | 98 ± 3 | ||||||
| DXG+RBV | 83 ± 5 | 69 ± 5 | 69 ± 11 | 61 ± 16 | 55 ± 13 | ||||||
| ddCc | 99 ± 5 | 30 ± 1 | 15 ± 1 | 11 ± 1 | 12 ± 1 | ||||||
| RBV | 93 ± 9 | 102 ± 11 | 88 ± 9 | 84 ± 12 | 80 ± 5 | ||||||
| MPA | 75 ± 15 | 72 ± 12 | 51 ± 16 | 44 ± 23 | 39 ± 20 | ||||||
Data are expressed as percent mtDNA ± standard deviations from the means of three experiments.
MPA, 0.25 μM; RBV, 20 μM.
ddC, dideoxycytidine.
DISCUSSION
DXG is a novel nucleoside analog of deoxyguanosine that has potent activity in vitro and in vivo against wild-type HIV-1 and viruses that carry mutations which confer resistance to AZT, lamivudine, and to non-nucleoside reverse transcriptase inhibitors (23). We examined the ability of two inhibitors of IMPDH, RBV and MPA, to enhance the antiviral activity of DAPD and DXG against both wild-type and resistant virus. Purine nucleoside analogues in combination with inhibitors of IMPDH have previously been shown to give a synergistic antiviral response when tested in vitro against members of the herpesvirus family, human cytomegalovirus, and HIV (21, 24).
Both inhibitors of IMPDH were evaluated in this study in combination with DAPD or DXG in an attempt to determine if either RBV or MPA displayed any advantages over the other. Neither RBV nor MPA displayed any anti-HIV activity in the MT2 cell assay. However, both compounds enhanced the activity of DAPD and DXG against wild-type virus. In these assays, addition of 0.25 μM MPA or 20 μM RBV resulted in a greater than 10-fold increase in the anti-HIV activity of DXG. By contrast, neither MPA nor ribavirin had any effect on the anti-HIV activity of abacavir, another guanosine analogue, in the MT2 cell line.
In contrast to results obtained in MT2 cells, both MPA and RBV demonstrated anti-HIV activity when tested in PBMC. Unlike MT2 cells, PBMC rely primarily on the de novo synthesis of guanosine and are therefore more susceptible to the effects of IMPDH inhibitors (1). In these cells, addition of MPA at a concentration of 0.25 μM completely inhibited HIV replication in PBMC when combined with DXG (data not shown). This concentration of MPA is less than the trough blood concentrations observed for the compound in transplant patients treated with Cellcept and below that required to inhibit T-cell proliferation (1, 30). Lower concentrations of MPA also had synergistic activity with DAPD and DXG. Similar to what was observed in the MT2 assays, the effect of MPA on the activity of abacavir was less pronounced, especially at the lower concentrations. These differences may be attributed to the differences in substrate specificity between the triphosphate forms of the two compounds for HIV-RT. Pre-steady-state kinetic analysis of the rate of incorporation and binding affinity of the compounds for HIV-RT (29; also J. Feng, personal communication) have shown that DXG-TP has a 10-fold selective advantage over carbovir triphosphate. The very low Ki value obtained for DXG-TP against HIV-RT (0.019 μM) suggests that small changes in the intracellular dGTP pools would have a large impact on the activity of the drug observed in cell culture. While RBV was also shown to have similar synergistic anti-HIV activity when combined with DAPD or DXG, the concentrations used in these assays are above the steady-state plasma levels observed in vivo (14).
These studies also demonstrate that the combination of either IMPDH inhibitor restored the ability of DXG to inhibit otherwise resistant HIV. The L74V, K65R, and Q151M mutations have all been shown to confer various levels of resistance to DXG (23). The combination of 0.2 μM MPA with DXG resulted in a decrease in EC50 of DXG for the highly resistant 65R/151M virus to within twofold of the values obtained for wild-type virus when tested in PBMC. This is equivalent to a greater than 100-fold increase in the activity of DXG against this highly resistant virus. Furthermore, these results were obtained with concentrations of DXG and MPA which were clinically relevant. While RBV also increased the activity of DAPD and DXG against the highly resistant virus, the effect was less pronounced at clinically relevant concentrations of RBV.
Neither MPA nor RBV was cytotoxic to MT2 or PMBC when combined with DAPD or DXG at any of the concentrations tested. When tested in HepG2 cells, MPA at concentrations of 0.50 μM and above did have an effect on cell viability and mtDNA content while RBV alone had no effect on mtDNA at concentrations up to 80 μM. When tested in combination, addition of 0.25 μM MPA did not decrease mtDNA levels compared to levels seen with either DAPD or DXG alone. By contrast, addition of 20 μM RBV to either DAPD or DXG resulted in decreased mtDNA levels. The initial interpretation of the data may appear in conflict, where MPA in combination did not produce an effect on mtDNA synthesis or cytotoxic effect while ribavirin in combination with DAPD or DXG slightly enhanced the reduction of mtDNA. Firstly, the level of MPA required to achieve either a reduction in mtDNA synthesis or a reduction in cell viability is greater than that used in the combination experiments. Secondly, ribavirin, a nucleoside analog, may act synergistically in combination to inhibit the DNA polymerase γ resulting in reduced levels of mtDNA. Altering intracellular dNTP pools could affect the rates of polymerization for the various cellular polymerases. With the addition of an nucleoside analogue, the polymerase selectivity for either the analogue or the native dNTP would also be affected. Polymerase γ, responsible for replication of mtDNA, has been shown to be more susceptible to inhibition by NRTIs than other cellular polymerases (2). Therefore, in these short term cytotoxicity assays, changes in mtDNA are more readily evident than global cytotoxicity. While DAPD and DXG do not affect the level of mtDNA at relevant serum and plasma concentrations, our results indicate that altering dGTP levels by inhibiting IMPDH could possibly instigate a decrease in mtDNA by DAPD or DXG. However, early studies with MPA and abacavir have not shown evidence of mitochondrial toxicity.
Our findings support the use of MPA in combination therapy with DAPD for the treatment of HIV infection. While RBV was also synergistic with DAPD, the high levels required for this activity combined with the potential for mitochondrial toxicity make it a less likely combination. Due to the favorable resistance profile of DAPD, the combination with MPA may be of use in salvage therapy, particularly as a component of HAART. Clinical studies have been initiated to evaluate the combination of DAPD and MPA against HIV in the salvage setting.
REFERENCES
- 1.Allison, A. C., and E. M. Eugui. 2000. Mycophenolate mofetil and its mechanism of action. Immunopharmacology 47:85-118. [DOI] [PubMed] [Google Scholar]
- 2.Arts, E. J., and M. A. Wainberg. 1996. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob. Agents Chemother. 40:527-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, M., R. Pauwels, J. Balzarini, P. Herdewijn, E. D. Clercq, and J. Desmyter. 1987. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynuclesides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 31:1613-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini, J., C. K. Lee, P. Herdewijn, and E. D. Clercq. 1991. Mechanism of the potentiating effect of ribavirin on the activity of 2′,3′-dideoxyinosine against human immunodeficiency virus. J. Biol. Chem. 266:21509-21514. [PubMed] [Google Scholar]
- 5.Balzarini, J., L. Naesens, M. J. Robins, and E. D. Clercq. 1990. Potentiating effect of ribavirin on the in vitro and in vivo antiretrovirus activities of 2′,3′-dideoxyinosine and 2′,3′-dideoxy-2,6-diaminopurine riboside. J. Acquir. Immune Defic. Syndr. 3:1140-1147. [PubMed] [Google Scholar]
- 6.Bazmi, H. Z., J. L. Hammond, S. C. H. Cavalcanti, C. K. Chu, R. F. Schinazi, and J. W. Mellors. 2000. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (−)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob. Agents Chemother. 44:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron, F., B. Ponceau, D. Bouhour, A. Boibieux, B. Verrier, and D. Peyramond. 2000. Long-term safety and antiretroviral activity of hydroxyurea and didanosine in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 25:329-336. [DOI] [PubMed] [Google Scholar]
- 8.Chapuis, A. G., G. P. Rizzardi, C. D'Agostino, A. Attinger, C. Knabenhans, S. Fleury, H. Acha-Orbea, and G. Pantaleo. 2000. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat. Med. 6:735-736. [DOI] [PubMed] [Google Scholar]
- 9.Coull, J. J., D. Turner, T. Melby, M. R. Betts, R. Lanier, and D. M. Margolis. 2001. A pilot study of the use of mycophenolate mofetil as a component of therapy for multidrug-resistant HIV-1 infection. J. Acquir. Immune Defic. Syndr. 26:423-434. [DOI] [PubMed] [Google Scholar]
- 10.Feng, J., M. L. Krajewski, P. Krishan, Y. Cheng, and K. Borroto-Esoda. Presented at the Gordon Research Conference, Salve Regina University, Newport, R.I., 29 June to 4 July 2003.
- 11.Frank, I. 1999. Clinical use of hydroxyurea in HIV-1 infected patients. J. Biol. Regul. Homeost. Agents 13:186-191. [PubMed] [Google Scholar]
- 12.Furman, P. A., J. Jeffrey, L. L. Kiefer, J. Y. Feng, K. S. Anderson, K. Borroto-Esoda, E. Hill, W. C. Copeland, C. K. Chu, J. P. Sommadossi, I. Liberman, R. F. Schinazi, and G. R. Painter. 2001. Mechanism of action of 1-β-d-2,6-diaminopurine dioxolane, a prodrug of the human immunodeficiency virus type 1 inhibitor 1-β-d-dioxolane guanosine. Antimicrob. Agents Chemother. 45:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, W. Y., A. Cara, R. C. Gallo, and F. Lori. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. USA 90:8925-8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glue, P. 1999. The clinical pharmacology of ribavirin. Semin. Liver Dis. 19:17-24. [PubMed] [Google Scholar]
- 15.Gu, Z., M. A. Wainberg, N. Nguyen-Ba, L. L'Heureux, J. M. de Muys, T. L. Bowlin, and R. F. Rando. 1999. Mechanism of action and in vitro activity of 1′,3′-dioxolanylpurine nucleoside analogues against sensitive and drug-resistant human immunodeficiency virus type 1 variants. Antimicrob. Agents Chemother. 43:2376-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, Z., M. A. Wainberg, P. Nguyen-Ba, L. L'Heureux, J. M. de Muys, and R. F. Rando. 1999. Anti-HIV-1 activities of 1,3-dioxolane guanine and 2,6-diaminopurine dioxolane. Nucleosides Nucleotides 18:891-892. [DOI] [PubMed] [Google Scholar]
- 17.Ichimura, H., and J. A. Levy. 1995. Polymerase substrate depletion: a novel strategy for inhibiting the replication of the human immunodeficiency virus. Virology 211:554-560. [DOI] [PubMed] [Google Scholar]
- 18.Lori, F. 1999. Hydroxyurea and HIV: 5 years later—from antiviral to immune-modulating effects. AIDS 13:1433-1442. [DOI] [PubMed] [Google Scholar]
- 19.Lori, F., A. Malykh, A. Cara, D. Sun, J. N. Weinstein, J. Lisziewicz, and R. C. Gallo. 1994. Hydroxyurea as an inhibitor of human immunodeficiency virus-type 1 replication. Science 266:801-805. [DOI] [PubMed] [Google Scholar]
- 20.Lowe, J. K., L. Brox, and F. F. Henderson. 1977. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 37:736-743. [PubMed] [Google Scholar]
- 21.Margolis, D., A. Heredia, J. Gaywee, D. Oldach, G. Drusano, and R. Redfield. 1999. Abacavir and mycophenolic acid, an inhibitor of inosine monophosphate dehydrogenase, have profound and synergistic anti-HIV activity. J. Acquir. Immune Defic. Syndr. 21:362-370. [PubMed] [Google Scholar]
- 22.Margolis, D. M., S. Kewn, J. J. Coull, L. Ylisastigui, D. Turner, H. Wise, M. M. Hossain, E. R. Lanier, L. M. Shaw, and D. Black. 2002. The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J. Acquir. Immune Defic. Syndr. 31:45-49. [DOI] [PubMed] [Google Scholar]
- 23.Mewshaw, J. P., F. T. Myrick, D. A. C. S. Wakefield, B. J. Hooper, J. L. Harris, B. McCreedy, and K. Borroto-Esoda. 2002. Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails. J. Acquir. Immune Defic. Syndr. 29:11-20. [DOI] [PubMed] [Google Scholar]
- 24.Neyts, J., G. Andrei, and E. De Clercq. 1998. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother. 42:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer, S., and S. Cox. 1994. Intracellular metabolism of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine in combination in the absence and presence of ribavirin. Antivir. Chem. Chemother. 5:403-409. [Google Scholar]
- 26.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. D. Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard, M. N., and C. Shipman, Jr. 1996. Analysis of combinations of antiviral drugs and design of effective multidrug therapies. Antiviral Ther. 1:9-20. [PubMed] [Google Scholar]
- 29.Ray, A. S., A. Basavapathruni, and K. S. Anderson. 2002. Mechanistic studies to understand the progressive development of resistance in human immunodeficiency virus type 1 reverse transcriptase to abacavir. J. Biol. Chem. 277:40479-40490. [DOI] [PubMed] [Google Scholar]
- 30.Sanquer, S., M. Breil, C. Baron, D. Dahmane, A. Astier, and P. Lang. 1998. Trough blood concentrations in long-term treatment with mycophenolate mofetil. Lancet 351:1557. [DOI] [PubMed] [Google Scholar]
- 31.Shi, C., and J. W. Mellors. 1997. A recombinant retroviral system for rapid in vivo analysis of human immunodeficiency virus type 1 susceptibility to reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 41:2781-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole:1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 33.Streeter, D. G., J. T. Witkowski, G. P. Khare, R. W. Sidwell, R. J. Bauer, R. K. Robins, and L. N. Simon. 1973. Mechanism of action of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA 70:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt, M. W., K. L. Hartshorn, P. A. Furman, T. C. Chou, J. A. Fyfe, L. A. Coleman, C. Crumpacker, R. T. Schooley, and M. S. Hirsch. 1987. Ribavirin antagonizes the effect of azidothymidine on HIV replication. Science 235:1376-1379. [DOI] [PubMed] [Google Scholar]
- 35.Wang, L. H., J. W. Bigley, M. Brosnan-Cook, N. D. Sista, and F. Rousseau. Presented at the 8th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 4 to 8 February 2001.
- 36.Yalowitz, J. A., and H. N. Jayaram. 2000. Molecular targets of guanine nucleotides in differentiation, proliferation and apoptosis. Anticancer Res. 20:2329-2338. [PubMed] [Google Scholar]
- 37.Yarbro, J. W. 1992. Mechanism of action of hydroxyurea. Semin. Oncol. 19:1-10. [PubMed] [Google Scholar]