Abstract
The purpose of this study was to establish a reliable and cost-effective microplate proliferation assay for in vitro antimicrobial testing of bone cement samples. Cement samples devoid of antimicrobial agents, loaded with 2% gentamicin or with different concentrations of high-porosity silver, were incubated in a 96-well microplate with several staphylococcal, Pseudomonas aeruginosa, and Enterococcus faecium isolates exhibiting different susceptibilities to gentamicin. After being rinsed, the samples were brought into a soy medium in which adherent cells on the cement surface either were killed by the antimicrobial surface or started to release clonal counterparts. The medium was monitored in real time by recording a time proliferation curve for each well. Microplate testing revealed no antibacterial effect of plain bone cement. The antibacterial activity of gentamicin-loaded bone cement was shown by the microplate test to depend on the gentamicin susceptibilities of the strains. The effect of high-porosity silver was dose dependent. Bactericidal activity against all tested strains was found for bone cement loaded with 1% high-porosity silver. The accuracy of this new proliferation assay was shown by the high correlation between the types of proliferation curves and antibiotic susceptibility. In contrast to routine agar diffusion testing, it assesses the dynamic response of microorganisms to antimicrobial agents in biomaterials and allows high-throughput screening and detection of antimicrobial properties of poorly water-soluble compounds like silver.
Infections in total joint arthroplasty are devastating situations (19), and many strategies have been undertaken to reduce infection rates, including the use of helmet aspirator suits (22), laminar airflow (5, 10, 18), and prophylactic intravenous antibiotics (9, 13, 15). Loading polymethylmethacrylate (PMMA) bone cement with antibiotics to reduce infection rates has also been postulated in the literature (6, 16, 24, 26).
As in all other fields of medicine, new ideas in orthopedics should be evaluated stepwise, with in vitro and subsequent in vivo investigations of antimicrobial properties occurring before clinical trials. Therefore, testing the antimicrobial activity of bone cement with new antibiotics or other antimicrobial agents should begin with in vitro studies. Agar diffusion testing has been the standard method for many years for in vitro assessment of antibiotic-loaded bone cements (17, 23, 27, 28). However, the assessment by agar diffusion testing of the anti-infective activities of some antimicrobial agents requires accurately defined standard conditions (20). For example, large molecules, such as vancomycin and teicoplanin, harbor a reduced diffusion capacity and their susceptibilities are difficult to determine exactly. Other anti-infective agents, e.g., fosfomycin, are able to interact with components of the culture medium in agar plates, resulting in reduced activity. Therefore, adequate culture media are required (20). New anti-infective agents like microdispersed elementary silver are undetectable in agar diffusion tests (2, 4), which makes new in vitro methods necessary. The purpose of the present study was to introduce a new microplate proliferation assay for in vitro antimicrobial testing of bone cement samples.
MATERIALS AND METHODS
Bone cements.
Plain PMMA bone cement (VersaBond; Smith & Nephew, Memphis, Tenn.), gentamicin-loaded PMMA bone cement (Palacos R with gentamicin [2%]; Schering-Plough, Brussels, Belgium), and PMMA cement (Versa Bond) loaded with 0.1, 0.5, and 1% (wt/wt) high-porosity silver were used for the present study. High-porosity silver comprised of stable agglomerates of 2 to 10 μm was provided by Fraunhofer Institute for Manufacturing and Advanced Materials, Bremen, Germany (IFAM). All cements were prepared in a vacuum-mixing system (Easymix; Coripharm, Dieburg, Germany) in accordance with the manufacturer's instructions. The liquid cement was subsequently brought into molds in order to create bone cement samples with a diameter of 4 mm and a height of 7 mm.
Microorganisms.
Various clinical isolates of several gram-positive and gram-negative bacteria with different susceptibilities to gentamicin were collected at the Institute of Medical Microbiology, Giessen, Germany, to study in vitro antimicrobial properties of the different bone cements (Table 1).
TABLE 1.
Type, source, and susceptibilities to gentamicin of microorganisms used in this study
| Microorganism | Strain | Source | MIC of gentamicin (μg/ml) |
|---|---|---|---|
| S. epidermidis | EDCC 5245 | Blood culture from patient with sepsis | <0.064 |
| MRSA | EDCC 5244 | Bronchial lavage fluid from patient with respiratory tract infection | 16 |
| MRSA | EDCC 5246 | Foot swab from patient with wound infection | 64 |
| MRSA | EDCC 5248 | Oral cavity swab from colonized patient | 64 |
| E. faecium | EDCC 5271 | Catheter from patient with urinary tract infection | >1,024 |
| P. aeruginosa | EDCC 5272 | Rump swab from patient with decubitus ulcer | 128 |
Staphylococcus epidermidis Eugen Domann culture collection strain EDCC 5245 was susceptible to gentamicin (MIC < 0.064 μg/ml), methicillin-resistant Staphylococcus aureus (MRSA) EDCC 5244 exhibited intermediate gentamicin susceptibility (MIC = 16 μg/ml), and MRSA EDCC 5246 and MRSA EDCC 5248 showed low-level gentamicin susceptibility (MIC = 64 μg/ml). All staphylococci were identified by API biochemical characteristic testing (bioMerieux, Marcy L'Etoile, France), by sequencing the 16S rRNA, and by specific PCRs to detect the femB, coa, and mecA genes (8; data not shown).
Pseudomonas aeruginosa and Enterococcus faecium were identified by API biochemical characteristic testing. The gentamicin MIC for P. aeruginosa EDCC 5272 was 128 μg/ml, and that for E. faecium EDCC 5271 was >1,024 μg/ml.
The MICs of gentamicin for the different strains were determined under standard conditions by the epsilometer test (E-test; AB BIODISK, Solna, Sweden) as recommended by the vendor.
Biofilm formation.
A biofilm assay based on the ability of bacteria to form biofilms on polystyrene plastic, i.e., on their ability to develop microcolonies that can be detected macroscopically, was used to determine the ability of the tested bacteria to produce biofilms on the surfaces of biomaterials (25). Overnight cultures of the microorganisms were diluted 1:100 into fresh medium (Trypticase soy broth [TSB] plus 0.25% glucose), and 200 μl of the freshly inoculated medium was subsequently dispensed into the wells of a microtiter dish. The inoculated microtiter dish was incubated at 37°C for 48 h without agitation and then removed, and the wells were washed thoroughly and vigorously with biofilm buffer (2 mM CaCl2/MgCl2) to remove unattached cells. The addition of 200 μl of biofilm buffer and 20 μl of crystal violet staining solution (0.1% [wt/vol] crystal violet in water) was used to detect biofilm formation. The plates were incubated for 15 min at room temperature and then rinsed with biofilm buffer to remove residual staining. Bacteria with biofilm activity coated the inner surfaces of the wells, and the microcolonies were visible in purple color.
Microplate proliferation assay.
The microplate proliferation testing used in the present study for bone cement samples was based on an assay introduced for screening of antimicrobial biomaterials (2). Bone cement samples (with a length of 7 mm and a diameter of 4 mm) were incubated with 106 bacterial CFU in 200 μl of cell suspension in each well of a 96-well microplate at 37°C for 1 h to allow adherence of the microorganisms to the cement surface. Every cement-bacterium combination was tested in six different wells of each lane per microplate. After incubation, rinsing of the cement samples with phosphate-buffered saline removed loosely attached cells from the cement's surface. The remaining adherent cells were incubated in phosphate-buffered saline with 0.25% glucose, 0.2% (NH4)2SO4, and 1% sterile TSB for 18 h at 37°C in another 96-well microplate. During this second incubation step, the attached cells on the surface of the bone cement either started to multiply and to release clonal counterparts into the well or were killed by the antimicrobial surface. After removal of the samples, the released bacteria were amplified by adding 50 μl of TSB medium to each well for 36 h. Proliferation of the released daughter cells was monitored at a wavelength of 578 nm online by a microplate reader (VersaMax; Molecular Devices, Sunnyvale, Calif.) for the next 36 h and provided a time-proliferation curve as a test result for each well of the microplate. Whether bacteria were partially or completely inactivated by bone cement samples supplemented with anti-infectiva, they were able to release only a few or even no clonal counterparts, which resulted in the diminishment or the absence of bacterial growth, respectively. Bacteria that remained viable on the supplemented bone cement surface released clonal counterparts whose proliferation resulted in characteristic proliferation curves (Fig. 1).
FIG. 1.
Typical time-proliferation curves of microorganisms for the microplate proliferation assay. Curve 1 shows uninhibited growth, curve 2 shows delayed proliferation, and curve 3 shows completely inhibited proliferation. The time needed for a population to proliferate to an OD of 0.2 is defined as the onset OD time.
For internal controls of antimicrobial activity, nylon-knitted fabric plated with 99.9% pure silver was used as a positive control (Shieldex Trading GmbH, Bremen, Germany), and the same nylon material devoid of silver was used as a negative control.
Statistical evaluation.
All measurements were performed six times. The time needed for a population to proliferate to an optical density (OD) of 0.2 was defined as the onset OD time, which was used for quantitative assessment of bacterial proliferation. This time was recorded for each well of all tested bone cement-microorganism combinations, and the mean value and the coefficient of variation (from standard deviations and averages) were determined from these six measurements.
RESULTS
Biofilm activities of the tested strains.
With the biofilm formation assay, MRSA EDCC 5246, MRSA EDCC 5248, E. faecium EDCC 5271, and P. aeruginosa EDCC 5272 showed biofilm formation activity, whereas S. epidermidis EDCC 5245 and MRSA EDCC 5244 did not.
Microplate testing revealed no antibacterial effect of plain bone cement.
Plain bone cement devoid of any anti-infectiva did not inhibit the proliferation of any tested strains. The proliferation curves commonly showed a short lag phase of 1 to 2 h followed by a log phase with a steep slope. Onset OD time was reached between 5 and 10 h (Table 2). The stationary phases were reached in all assays after 14 to 21 h.
TABLE 2.
Average onset OD times for every tested bone cement-microorganism combination
| Strain | Avg onset OD time (h) (% CV)a
|
||
|---|---|---|---|
| Plain bone cement | Gentamicin-loaded bone cement | High-porosity silver bone cement | |
| S. epidermidis EDCC 5245 | 5.8 (11) | >36 (0) | >36 (0) |
| MRSA EDCC 5244 | 6.7 (14) | 22 (28) | >36 (0) |
| MRSA EDCC 5246 | 8.2 (6.9) | 8.7 (11) | >36 (0) |
| MRSA EDCC 5248 | 7.7 (5.1) | 9.6 (13) | >36 (0) |
| P. aeuroginosa EDCC 5272 | 5.0 (1.5) | 5.1 (2.1) | >36 (0) |
| E. faecium EDCC 5271 | 10 (13) | 9.5 (13) | >36 (0) |
CV, coefficient of variation.
Microplate testing revealed that the antibacterial activity of gentamicin-loaded bone cement depended on the gentamicin susceptibilities of the tested strains.
Bacterial proliferation in gentamicin-loaded bone cement depended on the susceptibilities of the strains to gentamicin. No inhibition of bacterial proliferation was found in the MRSA strains that had low levels of susceptibility to gentamicin, EDCC 5246 (Fig. 2) and MRSA EDCC 5248 (MIC = 64 μg/ml). Also, the proliferation of P. aeruginosa EDCC 5272 (MIC = 128 μg/ml) and E. faecium EDCC 5271 (MIC > 1,024 μg/ml) could not be inhibited by gentamicin-loaded bone cement. All proliferation curves were similar to those found for plain cement, with a short lag phase and subsequent log and stationary phases. Onset OD time was reached between 5.1 and 9.6 h (Table 2). The stationary phase was reached after about 13 to 20 h.
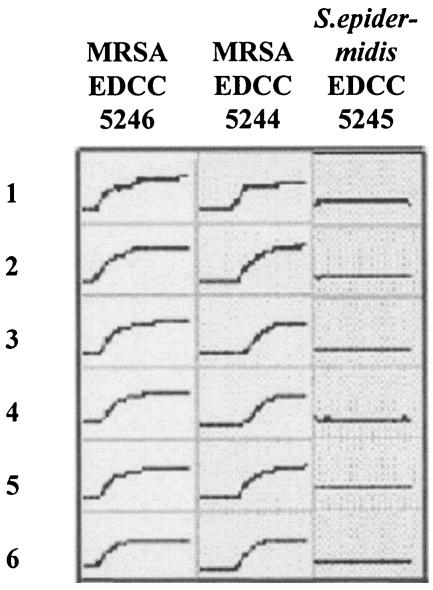
FIG. 2.
Time-proliferation curves for gentamicin-loaded bone cement. The proliferation of MRSA EDCC 5246 with low-level susceptibility to gentamicin (MIC = 64 μg/ml) could not be inhibited by gentamicin bone cement, whereas for MRSA EDCC 5244 (MIC = 16 μg/ml) and S. epidermidis EDCC 5245 (MIC < 0.064 μg/ml), delayed proliferation and the absence of growth were detected, respectively. Tests were carried out six times for each strain (lanes 1 to 6). In conclusion, the proliferation curves showed good correlation with the gentamicin susceptibility of the respective strain. y axis, OD; x axis, 0 to 36 h.
The intermediate-level susceptibility to gentamicin (MIC = 16 μg/ml) of MRSA EDCC 5244 (Fig. 2) resulted in a delayed onset of proliferation with an average onset OD time of 22 h. The stationary phase was reached after 26 to 35 h.
For the gentamicin-susceptible strain S. epidermidis EDCC 5245 (MIC < 0.064 μg/ml), a complete inhibition of bacterial proliferation resulted in “flat-line” proliferation curves in all assays (Fig. 2) with an average onset OD time of >36 h (Table 2).
Microplate testing revealed the activity of high-porosity silver bone cement against all tested strains.
The effects of bone cement loaded with high-porosity silver depended on the concentration of silver with all tested strains. The addition of 0.1% high-porosity silver did not lead to a considerable delay in the onset of proliferation, whereas 0.5% showed clear signs of a prolonged lag phase (Fig. 3). Loading of bone cement with 1% high-porosity silver completely inhibited bacterial proliferation of all strains tested. Only flat-line curves were found for this kind of bone cement, and no onset of bacterial growth was observed. None of the tested strains started to proliferate within the first 36 h (Table 2).
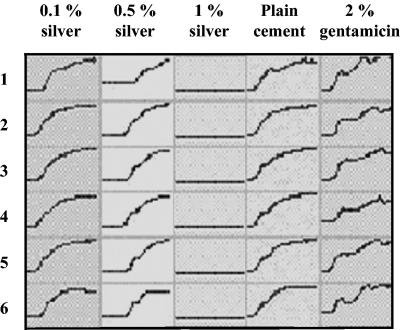
FIG. 3.
Time-proliferation curves for MRSA EDCC 5248. The proliferation of MRSA EDCC 5248 on high-porosity silver-loaded bone cement samples was clearly dose dependent. The addition of 0.1% silver did not lead to a considerable delay in the onset of proliferation compared to the onset for plain bone cement samples in all six lanes. Loading the cement with 0.5% silver resulted in a prolonged lag phase, and the addition of 1% silver led to complete inhibition of bacterial proliferation in all six lanes. y axis, OD; x axis, 0 to 36 h.
Coefficient of variation.
The coefficient of variation, as a statistical instrument for reliability of the test method, was below 15% for 17 of 18 cement-bacterium combinations (Table 2).
DISCUSSION
The hypothesis for the resulting time-proliferation curves for gentamicin-loaded bone cement samples was that bacterial proliferation depended on the respective susceptibilities to gentamicin of the different strains. Low-level susceptibility to gentamicin was expected to result in uninhibited bacterial growth in the microplate proliferation assay for gentamicin-loaded bone cement. Lagging or complete inhibition of bacterial growth was expected for strains with intermediate or high levels of susceptibility to antibiotic. Indeed, MRSA EDCC 5246, MRSA EDCC 5248 (MIC = 64 μg/ml), P. aeruginosa EDCC 5272 (MIC = 128 μg/ml), and E. faecium EDCC 5271 (MIC > 1,024 μg/ml) showed uninhibited bacterial growth with a short lag phase followed by a steep-slope log phase for gentamicin-loaded bone cement. Also, no plain bone cement samples could inhibit or delay the growth of any tested strains. There were always comparable proliferation onset times for these strains on the plain bone cement and the gentamicin bone cement samples.
The proliferation onset OD time for MRSA EDCC 5244 was 22 h for gentamicin-loaded bone cement compared to 6.7 h for plain bone cement. The most likely reason for this difference is the moderate gentamicin susceptibility (MIC = 16 μg/ml) of MRSA EDCC 5244 delaying the onset of growth with a longer lag phase than that of MRSA EDCC 5246 in gentamicin-loaded bone cement samples.
The proliferation of S. epidermidis EDCC 5245 with its high-level susceptibility to gentamicin (MIC < 0.064 μg/ml) was completely inhibited by gentamicin bone cement. The proliferation onset OD time was >36 h, compared to only 5.8 h for the plain cement samples.
All these findings confirmed the hypothesis that bacterial growth in the microplate proliferation assay depended on the levels of susceptibility of the test bacteria.
Agar diffusion is the most common in vitro testing method for studying the antimicrobial activities of loaded bone cement samples (23, 27). The correlation of the MICs of gentamicin determined by agar gel tests and the proliferation behavior of bacteria in the proliferation assay of the present study showed the accuracy of this new testing method. A coefficient of variation below 15% for 17 of 18 cement-bacterium combinations showed the reliability of this proliferation assay.
Compared to the agar diffusion method, microplate proliferation has three distinctive advantages. First, microplate proliferation testing allows assessment of the dynamic response of microorganisms to antimicrobial agents in biomaterials. Not only the adherence but also the proliferation of bacteria is a crucial step in the development of infection (7). The proliferation potential and dynamic behavior of adherent bacteria on the cement's surface are illustrated by resulting time-proliferation curves in this assay. This real-time monitoring of proliferation assesses the dynamic responses of microorganisms to antimicrobial agents in the biomaterial, which cannot be done by standard agar diffusion methods. Furthermore, the potential biofilm activity of the tested strains is considered to be assessed in a better manner than that of routine agar diffusion testing. The production of a biofilm is an important factor in virulence and a crucial step in the pathophysiology of biomaterial-associated infections (12). Formation of a biofilm may alter the activities of antimicrobial agents added to biomaterials (1, 11). Therefore, the impact of biofilm production should be considered by antimicrobial testing methods. Adequate consideration of biofilm activity cannot be done by routine agar diffusion testing, due to the fact that biofilm formation after preincubation of the cement samples with bacteria would be destroyed in the absence of a culture medium on the drying bone cement samples in the agar plates. This new microplate proliferation assay allows colonialization of the cement surface in the first incubation step and subsequent proliferation of the microorganisms in the second. If the cement has no antimicrobial activity, the proliferation of the microorganisms is not inhibited and strains with biofilm activity are enabled to start biofilm formation on the cement surface. Thus, initial steps of biofilm-forming activities (e.g., colonization, proliferation of daughter cells) are specially considered by this method. Ramage et al. investigated the effect of biofilm formation by Propionibacterium acnes on antibiotic susceptibility (21). A first incubation for 18 h at 37°C was done to allow the formation of biofilms on the different bone cement samples. Antibiotic susceptibility was determined by a second incubation procedure in which different antibiotics were added to cation-supplemented Mueller-Hinton broth. The number of bacteria was determined after ultrasonication of the samples by the Miles and Misra drop plate count method as discussed in reference 14. The major drawback of this testing method is that the antibacterial effect of an externally added antibiotic which was not added to the biomaterial itself was tested. Therefore, this assay does not reflect a practical clinical situation of antibiotic-loaded implants, although it reflects biofilm formation activities.
The microplate proliferation test also enables the detection of antimicrobial agents, which cannot be done on agar plates. Bechert et al. (2) showed that the antimicrobial activity of central venous catheters with microdispersed elementary silver could not be determined by agar diffusion testing due to the absence of typical inhibition zones on the agar plate. But these silver catheters proved to offer a remarkable reduction in infection rates in clinical trials (4), and a microplate proliferation assay was able to prove its antibacterial properties in vitro (3). Also, the present study showed complete inhibition of bacterial growth in the proliferation assay for 1% high-porosity silver bone cement. A microplate proliferation assay revealed a clear dose-dependent effect of this antimicrobial agent.
Silverberg et al. (23) demurred from the agar diffusion method for in vitro antimicrobial testing of PMMA bone cement. Bone cement samples loaded with 5-flucytosine did not show any inhibition zone in agar diffusion assays for in vitro antifungal analysis of PMMA bone cement in their study. Those authors concluded that 5-flucytosine could have failed to diffuse through the agar. It is also assumed that elementary silver might be unable to diffuse through the agar, possibly due to its poor water solubility (2). Both examples emphasize the importance of alternative in vitro testing methods to replace agar diffusion tests.
Another advantage of this new testing method is its high-throughput potential of up to 96 different bone cement samples per microplate. The assay is simple, time-saving, and cost-effective.
In summary, this microplate proliferation assay showed reliable and reproducible results in the assessment of antimicrobial activity of bone cement samples. Compared to the agar diffusion test, its three major advantages are its ability to assess the dynamic responses of microorganisms to antimicrobial agents in biomaterials, its ability to test the activities of antimicrobial agents that are difficult or impossible to test by agar diffusion, and its high-throughput capacity.
The necessity for new, innovative antimicrobial agents requires a reliable and fast in vitro screening method to investigate their antimicrobial potential in bone cement. Both preconditions can be fulfilled by this new microplate proliferation assay.
Acknowledgments
We thank T. Konradt and H. Gerauer from Bio-Gate Bioinnovative Materials, Nuremberg, Germany, C. Sattig and E. Wüst from Coripharm, Dieburg, Germany, and K.-S. Bommersheim, Institute of Medical Microbiology, Giessen, Germany, for excellent technical assistance.
REFERENCES
- 1.Arizono, T., M. Oga, and Y. Sugioka. 1992. Increased resistance of bacteria after adherence to polymethyl methacrylate. An in vitro study. Acta Orthop. Scand. 63:661-664. [DOI] [PubMed] [Google Scholar]
- 2.Bechert, T., P. Steinrücke, and J. P. Guggenbichler. 2000. A new method for screening anti-infective biomaterials. Nat. Med. 6:1053-1056. [DOI] [PubMed] [Google Scholar]
- 3.Bechert, T., M. Böswald, S. Lugauer, A. Regenfus, J. Greil, and J. P. Guggenbichler. 1999. The Erlanger silver catheter: in vitro results for antimicrobial activity. Infection 27(Suppl. 1):S24-S29. [DOI] [PubMed] [Google Scholar]
- 4.Behr, A. 1998. Reduktion von katheterassozierten Infektionen durch die Imprägnierung eines zentralvenösen Katheters mit niedrigen Konzentrationen an Silber: Ergebnisse einer europäischen Multizenterstudie. Intensivmedizin 35:475. [Google Scholar]
- 5.Bradley, L. P., W. F. Enneking, and J. A. Franco. 1980. The effect of operating-room environment on the infection rate after Charnley low-friction total hip replacement. J. Bone Jt. Surg. Am. Vol. 57-A:80-83. [PubMed] [Google Scholar]
- 6.Buchholz, H. W., and H. Engelbrecht. 1970. Über die Depotwirkung einiger Antibiotika bei Vermischung mit dem Kunstharz Palacos. Chirurg 40:511-515. [PubMed] [Google Scholar]
- 7.Christensen, G. D., L. Baldassarri, and W. A. Simpson. 1994. Colonisation of medical devices by coagulase-negative staphylococci, p. 45-78. In F. A. Waldvogel and A. L. Bisno (ed.), Infection associated with indwelling medical devices, 2nd ed. ASM Press, Washington, D.C.
- 8.Domann, E., H. Hossain, R. Fussle, and T. Chakraborty. 2000. Rapid and reliable detection of multiresistant Staphylococcus aureus (MRSA) by multiplex PCR. Dtsch. Med. Wochenschr. 125:613-618. [DOI] [PubMed] [Google Scholar]
- 9.Doyon, F., J. Evrard, and F. Mazas. 1989. Evaluation of therapeutic trials published apropos of antibiotic prophylaxis in orthopedic surgery. Rev. Chir. Orthop. Reparatrice Appar. Mot. 75:72-76. [PubMed] [Google Scholar]
- 10.Glynn, M. K., and J. M. Sheehan. 1983. An analysis of the causes of deep infection after total knee and hip arthroplasties. Clin. Orthop. 178:202-206. [PubMed] [Google Scholar]
- 11.Gristina, A. G., R. A. Jennings, P. T. Naylor, L. X. Webb, and Q. N. Myrvik. 1989. Comparative in vitro antibiotic resistance of surface-colonizing coagulase-negative staphylococci. Antimicrob. Agents Chemother. 33:813-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gristina, A. G., C. D. Hobgood, L. X. Webb, and Q. N. Myrnik. 1987. Adhesive colonialization of biomaterials and antibiotic resistance. Biomaterials 6:423-426. [DOI] [PubMed] [Google Scholar]
- 13.Gyssens, I. C., J. T. Knape, G. van Hal, and J. W. ver der Meer. 1997. The anaesthetist as determinant factor of quality of surgical antimicrobial prophylaxis. A survey in a university hospital. Pharm. World Sci. 19:89-92. [DOI] [PubMed] [Google Scholar]
- 14.Hedges, A. J., R. Shannon, and R. P. Hobbs. 1978. Comparison of the precision obtained in counting viable bacteria by the spiral plate maker, the droplette and the Miles & Misra methods. J. Appl. Bacteriol. 45:57-65. [DOI] [PubMed] [Google Scholar]
- 15.Hill, C., R. Flamant, F. Mazas, and J. Evrard. 1981. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet i:795-796. [DOI] [PubMed]
- 16.Josefsson, G., G. Gudmundsson, L. Kolmert, and S. Wijkstrom. 1990. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty. A five-year survey of 1688 hips. Clin. Orthop. 253:173-178. [PubMed] [Google Scholar]
- 17.König, D. P., J. M. Schierholz, R. D. Hilgers, C. Bertram, F. Perdreau-Remington, and J. Rütt. 2001. In vitro adherence and accumulation of Staphylococcus epidermidis RP 62 A and Staphylococcus epidermidis M7 on four different bone cements. Langenbeck's Arch. Surg. 386:328-332. [DOI] [PubMed] [Google Scholar]
- 18.Marotte, J. H., G. A. Lord, J. P. Blanchard, J. L. Guillamon, P. Samuel, J. P. Servant, and P. H. Mercier. 1987. Infection rate in total hip arthroplasty as a function of air cleanliness and antibiotic prophylaxis. 10-year experience with 2,384 cementless Lord madreporic prostheses. J. Arthroplasty 2:77-82. [DOI] [PubMed] [Google Scholar]
- 19.Peersman, G., R. Laskin, J. Davis, and M. Peterson. 2001. Infection in total knee replacement. Clin. Orthop. 392:15-23. [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing, 12th informational supplement. M100-S12. NCCLS, Wayne, Pa.
- 21.Ramage, G., M. M. Tunney, S. Patrick, S. P. Gorman, and J. R. Nixon. 2003. Formation of Propionibacterium acnes on orthopedic biomaterials and their susceptibility to antimicrobials. Biomaterials 24:3221-3227. [DOI] [PubMed] [Google Scholar]
- 22.Ritter, M. A., H. H. Eitzen, J. B. Hart, and M. L. French. 1980. The surgeon's grab. Clin. Orthop. 153:204-209. [PubMed] [Google Scholar]
- 23.Silverberg, D., P. Kodali, J. Dipersio, R. Acus, and M. Askew. 2002. In vitro analysis of antifugal impregnated polymethylmethacrylate bone cement. Clin. Orthop. 403:228-231. [DOI] [PubMed] [Google Scholar]
- 24.Thierse, L. 1978. Erfahrungen mit Refobacin-Palacos im Hinblick auf die tiefen Spätinfektionen nach Hüftoperationen. Z. Orthop. Grenzgeb. 116:847-849. [PubMed] [Google Scholar]
- 25.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 26.Wannske, M., and H. Tscherne. 1979. Ergebnisse prophylaktischer Anwendung von Refobacin-Palacos bei der Implantation von Endoprothesen des Hüftgelenkes in Hannover. Aktuel. Probl. Chir. Orthop. 12:201-208. [PubMed] [Google Scholar]
- 27.Weisman, D. L., M. L. Olmstead, and J. J. Kowalski. 2000. In vitro evaluation of antibiotic elution from polymethylmethacrylate (PMMA) and mechanical assessment of antibiotic-PMMA composites. Vet. Surg. 29:245-251. [DOI] [PubMed] [Google Scholar]
- 28.Welch, A. 1978. Antibiotics in acrylic bone cement. In vitro studies. J. Biomed. Mater. Res. 12:679-700. [DOI] [PubMed] [Google Scholar]