Abstract
Phospholipase C-γ2 (PLC-γ2) plays an important role in B-cell signaling. Phosphorylation of various tyrosine residues of PLC-γ2 has been implicated in regulation of its lipase activity. With the use of antibodies specific for each of the putative phosphorylation sites, we have now shown that PLC-γ2 is phosphorylated on Y753, Y759, and Y1217 in response to engagement of the B-cell receptor in Ramos cells, as well as in murine splenic B cells. In cells stimulated maximally via this receptor, the extent of phosphorylation of Y1217 was three times that of Y753 or of Y759. Stimulation of Jurkat T cells or platelets via their immunoreceptors also elicited phosphorylation of Y753 and Y759 but not that of Y1217. A basal level of phosphorylation of Y753 was apparent in unstimulated lymphocytes. The extent of phosphorylation of Y753 and Y759, but not that of Y1217, correlated with the lipase activity of PLC-γ2. Examination of the effects of various pharmacological inhibitors and of RNA interference in Ramos cells suggested that Btk is largely, but not completely, responsible for phosphorylation of Y753 and Y759, whereas phosphorylation of Y1217 is independent of Btk. Finally, phosphorylation of Y1217 and that of Y753 and Y759 occurred on different PLC-γ2 molecules.
The phospholipase C-γ (PLC-γ) isozymes PLC-γ1 and PLC-γ2 are 50% identical in amino acid sequence and share the same domain organization, including the arrangement of an NH2-terminal pleckstrin homology (PH) domain, catalytic X and Y domains, two Src homology 2 (SH2) domains, and one SH3 domain (Fig. 1A). PLC-γ1 is expressed in all tissues examined, whereas the expression of PLC-γ2 is largely restricted to a subset of cells of the hematopoietic lineage (4, 34, 50). Genetic evidence suggests that the functions of the two isozymes may not overlap. Targeted disruption of the PLC-γ1 gene thus results in embryonic death in mice (22), whereas deficiency of PLC-γ2 in mice is not lethal but manifests developmental abnormalities in B cells with consequent severe immunodeficiency as well as dysfunction of platelets and mast cells (48).
FIG. 1.
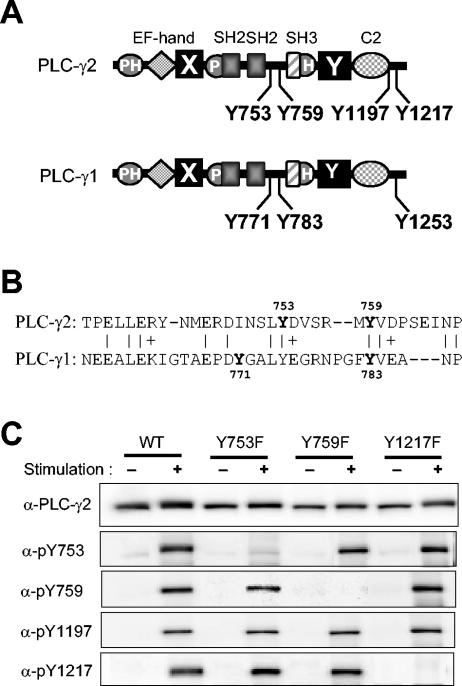
Characterization of antibodies specific for PLC-γ2 phosphorylated at each of four Tyr residues. (A) Domain organization and tyrosine phosphorylation sites of PLC-γ1 and PLC-γ2. The two isozymes share a domain organization that includes an NH2-terminal PH domain, an EF-hand domain, catalytic X and Y domains, a split PH domain (indicated by P and H), two SH2 domains, an SH3 domain, and a C2 domain. (B) Alignment of the amino acid sequences of human PLC-γ1 and PLC-γ2 between the SH2 and SH3 domains. Identical or similar residues are indicated by | and +, respectively. Tyrosine residues in bold are sites of phosphorylation. (C) Specificity of antibodies to phosphorylated forms of PLC-γ2. Null TV-1 cells were infected with recombinant vaccinia viruses encoding either wild-type (WT) PLC-γ2 or Tyr→Phe mutants (Y753F, Y759F, and Y1217F) thereof. They were then deprived of serum overnight and either left unstimulated or stimulated for 10 min with PDGF (0.1 μg/ml) in the presence of 2 mM H2O2 and 1 mM sodium vanadate. Cell lysates were subjected to immunoblot analysis with antibodies to PLC-γ2 or with antibodies specific for PLC-γ2 phosphorylated on Y753, Y759, Y1197, or Y1217, as indicated (α).
Both PLC-γ1 and PLC-γ2 are activated by tyrosine phosphorylation. An essential step in the activation of PLC-γ1 is phosphorylation of tyrosine 783 (Y783), which is induced by stimulation of receptors (such as those for platelet-derived growth factor [PDGF] or epidermal growth factor [EGF]) with intrinsic protein tyrosine kinase (PTK) activity or of receptors (such as B- or T-cell antigen receptors) that are linked to the activation of nonreceptor PTKs (4, 34, 50). PLC-γ1 may be additionally phosphorylated on Y771 and Y1253. The function of phosphorylation on Y771 or on Y1253, which is not required for induction of the lipase activity of PLC-γ1, is thus far unknown. PLC-γ1 phosphorylation on Y1253 occurs substantially in growth factor-stimulated cells but is undetectable in leukocytes stimulated via immunoreceptors. Phosphorylation on Y771 also occurs in growth factor-stimulated cells but to an extent much smaller than that apparent for Y783 and Y1253 phosphorylation (39).
Given that PLC-γ2 is much more abundant than is PLC-γ1 in B cells and that it is an essential component in signaling of the B-cell antigen receptor (BCR), the mechanism of PLC-γ2 activation has been studied most extensively in these cells. The binding of antigen to the BCR induces the activation of Src family PTKs Lyn, Fyn, and Blk (27), which results in phosphorylation of the cytoplasmic tails of the BCR components. These phosphorylated tails provide docking sites for the SH2 domains of the PTK Syk, and BCR-associated Syk then phosphorylates various adapter proteins. The adapter BLNK (B-cell linker protein; also known as SLP-65) appears particularly important for PLC-γ2 activation (23, 28). Phosphorylated BLNK provides a scaffold for the assembly of a macromolecular complex that includes PLC-γ2 and Btk, a member of the Tec family of PTKs. Activated Syk also contributes to the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) by activating phosphatidylinositol (PI) 3-kinase (11, 32, 38). An important function of PIP3 is to bind the PH domain of Btk, thereby facilitating recruitment of the kinase to the cell membrane, probably at lipid rafts. Tyrosine-phosphorylated BLNK and PIP3 thus promote nucleation of a signaling complex known as a signalosome (12); formation of the signalosome concentrates PLC-γ2 in lipid rafts, where it is phosphorylated by colocalized nonreceptor PTKs and gains access to its substrate.
Engagement of the BCR results in the recruitment into the signalosome and consequent activation of members of three distinct families (Src, Syk, and Tec) of nonreceptor PTKs. All three types of PTKs are able to phosphorylate PLC-γ2 in vitro (29, 35, 49). However, it is not clear which kinase (or kinases) is responsible for PLC-γ2 phosphorylation in B cells. Gene disruption of Lyn or Syk resulted in inhibition of BCR-induced phosphorylation of PLC-γ2 (45). However, given that tyrosine phosphorylation of BLNK, production of PIP3, and activation of Btk all occur downstream of Lyn/Syk activation, whether or not PLC-γ2 is a direct substrate of Lyn or Syk in B cells is difficult to prove. PLC-γ2 has been proposed to be a substrate for Btk on the basis of the observations that the extent of BCR-induced tyrosine phosphorylation of PLC-γ2 was increased in a murine B-cell line that overexpresses Btk (10, 37), and was substantially (but not completely) decreased in a chicken B-cell line (DT-40) deficient in Btk (44). BCR stimulation also failed to induce the formation of inositol 1,4,5-trisphosphate (IP3) or an increase in the cytosolic free Ca2+ concentration in the Btk-deficient DT-40 cells. Results obtained with human B cells that express mutant forms of Btk, however, appeared inconsistent with a major role for this kinase in the phosphorylation of PLC-γ2. Mutations in the Btk gene are responsible for X-linked agammaglobulinemia (XLA) in humans, and, as in the Btk-deficient DT-40 cells, the IP3 and Ca2+ responses of B cells derived from individuals with XLA were shown to be markedly attenuated. In contrast to the chicken cells, however, the XLA cells exhibited a near-normal increase in PLC-γ2 tyrosine phosphorylation in response to BCR stimulation (10).
We previously identified Y753 and Y759 as the sites of PLC-γ2 phosphorylation (31). Subsequently, Rodriguez et al. (35) showed that phosphorylation of Y753 and Y759 is essential for the activation of PLC-γ2 by comparing the abilities of wild-type and Tyr→Phe double-mutant (Y753F/Y759F) forms of human PLC-γ2 to restore the Ca2+ response to BCR stimulation in a derivative of DT-40 cells made deficient in PLC-γ2. Immunoblot analysis with antibodies to tyrosine phosphate failed to detect phosphotyrosine residues in the Y753F/Y759F mutant immunoprecipitated from the BCR-stimulated cells, suggesting that Y753 and Y759 are the major sites of phosphorylation in stimulated B cells. In contrast, Watanabe et al. (49) proposed Y1197 and Y1217, in addition to Y753 and Y759, as sites of phosphorylation by Btk on the basis both of in vitro phosphorylation assays and of the observation that mutation of all four Tyr residues was necessary to eliminate completely the BCR-induced phosphorylation of human PLC-γ2 expressed in DT-40 cells.
We have now generated antibodies specific for PLC-γ2 phosphorylated on either Y753, Y759, Y1197, or Y1217 and have used these antibodies to study the BCR-induced phosphorylation of PLC-γ2.
MATERIALS AND METHODS
Materials and cells.
Convulxin was obtained from Sigma. PDGF-BB (rat, recombinant) was from R&D Systems. LY294002, α-cyano-β-hydroxy-β-methyl-N-(2,5-dibromophenyl)propenamide (LFM-A13), phorbol myristate acetate (PMA), bisindolylmaleimide X (BIM), and PP1 were from Calbiochem.
Ramos, an Epstein-Barr virus-negative Burkitt's lymphoma cell line (26), and J.gamma1, a PLC-γ1-deficient derivative of the Jurkat E6 cell line (19) kindly provided by R. T. Abraham, were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml). Null TV-1 cells, which were derived from PLC-γ1−/− mouse embryonic fibroblasts (22) and were kindly provided by G. Carpenter, were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Platelets were isolated as described previously (9), without aspirin treatment, from the blood of healthy volunteers with acid-citrate-dextrose as an anticoagulant.
Mouse spleens (5 to ∼10 spleens combined for each preparation) were taken from anesthetized animals and dispersed through a nylon mesh to generate a single-cell suspension. After hemolysis by osmotic shock, splenocytes were washed and incubated with anti-CD43 and anti-Mac-1 antibody-conjugated magnetic microbeads (Miltenyi Biotec, Auburn, Calif.). Resting B cells were recovered in the flow-through fraction after separation with an AutoMacs magnetic cell sorter (Miltenyi Biotec) as suggested by the manufacturer.
Antibodies.
Antibodies to PLC-γ2 phosphorylated on Y753, Y759, Y1197, or Y1217 (anti-pY753, anti-pY759, anti-pY1197, and anti-pY1217, respectively) were prepared by injection of rabbits with the phosphorylated peptides INSL(pY)DVSR (corresponding to residues 749 to 757 of human PLC-γ2, with pY representing phosphorylated Tyr), VSRM(pY)VDPS (residues 755 to 763), EEEL(pY)SSSR (residues 1193 to 1201, with Cys1200 replaced with Ser to avoid conjugation with hemocyanin through this residue), and QLFL(pY)DTHQ (residues 1213 to 1221), respectively. All peptides were acetylated at their NH2 termini, had a Gly-Gly-Gly linker sequence followed by a Cys-amide added to their COOH termini, and were conjugated with keyhole limpet hemocyanin via the SH group of the terminal Cys. Peptides were manufactured by BioSynthesis.
Antisera were depleted of cross-reactive antibodies by incubation with the phosphorylated peptides not used as the antigen (for example, pY759, pY1197, and pY1217 peptides for anti-pY753) in order to prevent possible recognition of phosphorylated sites other than the target. Although specific antibodies are usually purified with the use of the immobilized peptide antigen (with the nonphosphorylated peptide for negative selection and then with the phosphorylated peptide for positive selection) (42), we found that this approach resulted in the loss of high-affinity antibodies for the pY753 and pY759 peptides as a result of difficulty in eluting them from the affinity gel.
Rabbit polyclonal anti-PLC-γ2 antibody (Q20) was obtained from Santa Cruz Biotechnology, mouse monoclonal anti-Btk was obtained from BD Transduction Laboratories, mouse monoclonal anti-human CD19 was obtained from BD Biosciences Pharmingen, rabbit polyclonal anti-Syk and mouse monoclonal antiphosphotyrosine (4G10) were obtained from Upstate Biotechnology, rabbit polyclonal anti-phosphoAkt (pThr308) was obtained from Cell Signaling, and F(ab′)2 fragments of goat anti-human immunoglobulin M (IgM) (Fc5μ specific) and F(ab′)2 fragments of goat anti-mouse IgM (μ-chain specific) were obtained from Jackson ImmunoResearch. Mouse monoclonal anti-CD3 (OKT-3) was kindly provided by L. Samelson. A mixture of anti-PLC-γ1 monoclonal antibodies was described previously (41). Anti-pY783 PLC-γ1 antibody was from Biosource/QCB.
Expression of PLC-γ2 mutants.
Point mutations that result in the substitution of phosphorylation site Tyr residues with Phe were introduced into human PLC-γ2 cDNA with the use of the pMT2 vector and a QuikChange site-directed mutagenesis kit (Stratagene). The coding sequences of the mutant and wild-type PLC-γ2 cDNAs were then transferred to the pSC11 vector for the production of recombinant vaccinia viruses (6). Null TV-1 cells were exposed for 3 h to the resulting viruses and then cultured for 24 h in DMEM containing 1% FBS.
Cell stimulation, cell lysis, immunoprecipitation, and immunoblot analysis.
Stimulation of vaccinia virus-infected Null TV-1 cells and preparation of cell lysates were described elsewhere (39). Ramos and Jurkat cells were harvested by centrifugation, washed twice, and resuspended in ice-cold RPMI 1640 supplemented with 25 mM HEPES-NaOH (pH 7.4) at a density of 5 × 107 cells/ml. Platelets were suspended in Tyrode's solution. The cells were equilibrated at 37°C for 10 min and then stimulated, after which 0.25 volume of ice-cold 5× lysis buffer [100 mM HEPES-NaOH (pH 7.4), 5% Triton X-100, 1% sodium dodecyl sulate (SDS), 5 mM EGTA, 5 mM EDTA, 100 mM NaF, 5 mM sodium vanadate, 25-μg/ml leupeptin, 25-μg/ml aprotinin, 0.5 mM 4-(2-aminoethyl)benzenesulfonylfluoride] was added and the resulting mixture was incubated for 10 min on ice before centrifugation at 40,000 × g for 10 min at 4°C. The resulting supernatants (cell lysates) were subjected to SDS-polyacrylamide gel electrophoresis. The separated proteins were transferred electrophoretically to a piece of nitrocellulose membrane, which was then probed with appropriate antibodies. Bound antibodies were detected with alkaline phosphatase-conjugated secondary antibodies (KPL) and the chemiluminescent reagent CDP-Star (Tropix). Images were captured and analyzed with a Kodak Image Station 440 equipped with a cooled charge-coupled device camera.
For immunoprecipitation, cell lysates were incubated with rocking for 1 h at 4°C with an excess amount of antibodies and then for an additional 2 h with protein G-Sepharose (Amersham Pharmacia Biotech). The beads were recovered by centrifugation, washed twice with lysis buffer and once with water, suspended to the original lysate volume in SDS sample buffer, and boiled. The eluted proteins were subjected to immunoblot analysis as described above.
RNAi.
For RNA interference (RNAi), a small interfering RNA (siRNA) that targets human Btk mRNA was based on nucleotides 895 to 913 relative to the translation start site (16). Custom SMARTpool Plus siRNAs that target human Syk as well as a control RNA (Scramble) were supplied by Dharmacon. Cells were washed once with ice-cold phosphate-buffered saline, suspended in electroporation buffer (Amaxa) at a density of 108 cells/ml, and subjected to electroporation with siRNA (1 μM) with an Amaxa Nucleofector apparatus and a 2.0-mm electroporation cuvette.
Measurement of inositol phosphates.
Ramos cells were labeled for 36 h with myo-[2-3H]inositol (2.5 μCi/ml) (Amersham) in inositol-free DMEM containing 2% FBS. After washing, the cells were suspended in DMEM containing 25 mM HEPES-NaOH (pH 7.4) at a density of 1 × 106 to 2 × 106 cells/ml and LiCl (20 mM) and test agents were added. The cells (100 μl) were equilibrated at 37°C for 10 min, stimulated, and then mixed with 9 volumes of 5% perchloric acid. The perchloric acid extracts of cells were centrifuged to remove debris, and the resulting supernatants were neutralized with 2 M KOH before analysis of [3H]inositol phosphates by high-performance liquid chromatography (HPLC) on a Partisil SAX-10 ion-exchange column and with a Flo-One on-line radiometric detector (Packard), as described previously (39).
HPLC analysis of PLC-γ2.
Ramos cell lysates (prepared from 4 × 108 cells) were mixed with 3 ml of packed heparin-Sepharose CL-6B (Amersham Pharmacia Biotech) that had been equilibrated with a solution containing 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 1 mM EGTA. After incubation for 1 h at 4°C with rocking, the mixture was poured into a column and washed with equilibration buffer supplemented with 20 mM NaCl. The bound proteins were eluted with 5 ml of equilibration buffer supplemented with 0.5 M NaCl. The eluted proteins were diluted 10-fold with equilibration buffer and then subjected to fractionation by HPLC on a heparin-5PW column (7.5-mm inside diameter by 7.5 cm; TosoHaas) that had been equilibrated with the equilibration buffer. Bound proteins were eluted with a linear gradient of NaCl (0 to 1 M) in the equilibration buffer over 50 min at a flow rate of 1 ml/min. Fractions were collected at 1-min intervals, and those containing PLC-γ2 were identified by immunoblot analysis.
RESULTS
Characterization of antibodies specific for phosphorylated PLC-γ2.
The four residues Y753, Y759, Y1197, and Y1217 of PLC-γ2 have been implicated as the sites of phosphorylation on the basis of evidence from various indirect experimental approaches (31, 35, 49). Like Y771 and Y783 of PLC-γ1, Y753 and Y759 of PLC-γ2 are located in the linker region between the catalytic X and Y domains (Fig. 1A). Alignment of the sequences of PLC-γ1 and PLC-γ2 reveals that Y759 of PLC-γ2 corresponds to Y783 of PLC-γ1 (Fig. 1B). However, Y753 of PLC-γ2 aligns poorly with Y771 of PLC-γ1; it corresponds instead to Y775 of PLC-γ1, but there is no evidence that this site is phosphorylated. PLC-γ2 does not contain a Tyr residue corresponding to Y771 of PLC-γ1. The COOH-terminal regions of PLC-γ1 and PLC-γ2 share little sequence similarity; PLC-γ2 does not contain a Tyr residue corresponding to Y1253 of PLC-γ1, and, conversely, PLC-γ1 does not possess Tyr residues corresponding to Y1197 and Y1217 of PLC-γ2. Attempts to map the phosphorylation sites of PLC-γ2 molecules isolated from stimulated B cells have not been successful (35), probably because the extent of phosphorylation is low in BCR-stimulated cells (see below).
To confirm the identity of the tyrosine phosphorylation sites of PLC-γ2 and to provide a means with which to monitor phosphorylation at each site quantitatively, we prepared rabbit polyclonal antibodies specific for PLC-γ2 phosphorylated on either Y753, Y759, Y1197, or Y1217. The specificity of these various antibodies was tested with PLC-γ1-deficient fibroblasts (Null TV-1 cells) that had been infected with recombinant vaccinia viruses encoding either wild-type PLC-γ2 or Tyr→Phe phosphorylation site mutants (Y753F, Y759F, and Y1217F). The cells were stimulated with a combination of PDGF and pervanadate (an irreversible inhibitor of protein tyrosine phosphatases [PTPs] that induces the activation of a variety of receptor and nonreceptor PTKs), and cell lysates were then subjected to immunoblot analysis (Fig. 1C). None of the four antibody preparations recognized wild-type PLC-γ2 before cell stimulation, but they all yielded a single immunoreactive band at a position corresponding to the molecular size of PLC-γ2 after cell stimulation, indicating that they are specific for phosphorylated forms of the enzyme. The sequence specificity of anti-pY753, anti-pY759, and anti-pY1217 was demonstrated by the observation that mutation of the target Tyr residue prevented the recognition of PLC-γ2 by the corresponding antibodies but not that by the other three antibody preparations. The corresponding specificity test could not be performed with anti-pY1197 because, for an unknown reason, we failed to establish the viral vector for the Y1197F mutant.
Phosphorylation of PLC-γ2 in various cell types.
We next compared the patterns of PLC-γ2 phosphorylation among Ramos (human B lymphoma) cells stimulated via the BCR, human platelets stimulated via the collagen receptor, a PLC-γ1-deficient derivative of Jurkat (19) (human T lymphoma) cells stimulated via the T-cell antigen receptor (TCR), and PDGF-stimulated Null TV-1 fibroblasts expressing human PLC-γ2. (Given that PLC-γ1 is much more abundant than is PLC-γ2 in parental Jurkat cells, we used a PLC-γ1-deficient version of this cell line to avoid possible interference.) Each cell type was stimulated with a saturating concentration of ligand, and the duration of stimulation was chosen to maximize the extent of PLC-γ2 phosphorylation. Substantial phosphorylation of Y759 was apparent in each cell type in response to receptor stimulation (Fig. 2A).
FIG. 2.
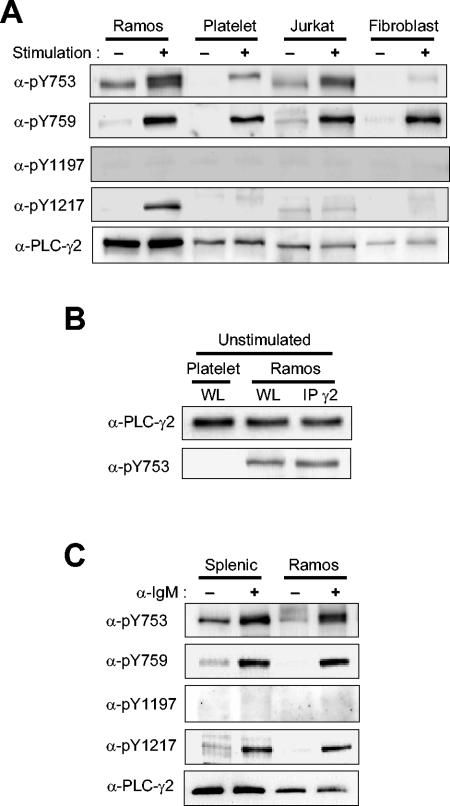
Phosphorylation of PLC-γ2 in various cell types. (A) Ramos cells, platelets, Jurkat cells, and human PLC-γ2-expressing Null TV-1 cells were stimulated (or not) with anti-IgM (25 μg/ml) for 1 min, with convulxin (100 μg/ml) for 2 min, with OKT-3 (0.3 μg/ml) for 1 min, or with PDGF (0.1 μg/ml) for 10 min, respectively. Cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-). The amounts of protein applied to the gel were adjusted so that the lysates from the different cells yielded similar intensities on the blot with anti-pY759. (B) Lysates of unstimulated platelets and Ramos cells (WL) and proteins immunoprecipitated (IP) with anti-PLC-γ2 from the lysate of Ramos cells (IP γ2) were subjected to immunoblot analysis with anti-PLC-γ2 and anti-pY753. The amounts of protein loaded onto the gel were adjusted to give similar blot intensities with anti-PLC-γ2. (C) Ramos cells and mouse splenic B cells were stimulated with anti-IgM (25 μg/ml) for 1 min. Cell lysates were then subjected to immunoblot analysis with indicated antibodies. The amounts of protein applied to the gel were adjusted as in panel A.
In Fig. 2A, the amounts of lysate protein applied to the gel were adjusted to yield similar immunoblot intensities with anti-pY759 for the different cell types. Taking this fact into account, the extent of receptor-induced Y759 phosphorylation decreased in the rank order of Null TV-1 cells > Jurkat cells = platelets > Ramos cells. Phosphorylation of Y1197 was not detected in any of the four cell types. Given that marked phosphorylation of Y1197 was detected in Null TV-1 cells stimulated with PDGF plus pervanadate (Fig. 1C) as well as in B cells stimulated with pervanadate (Fig. 3A), the lack of phosphorylation of this residue in cells stimulated via receptors was not likely due to the unavailability of Y1197 for phosphorylation. Ligand-induced phosphorylation of the other COOH-terminal Tyr residue, Y1217, was apparent in Ramos cells but not in platelets, Jurkat cells, or Null TV-1 cells; the faint bands apparent with a slightly lower (platelets and Null TV-1 cells) or higher (Jurkat cells) mobility relative to that of PLC-γ2 were attributable to nonspecific interactions of anti-pY1217 or the secondary antibodies. Ligand-induced phosphorylation of PLC-γ2 on Y753 was observed in Ramos cells, Jurkat cells, and platelets; the band intensity for pY753 was very low compared with that for pY759 in Null TV-1 fibroblasts, however. Anti-pY753 yielded a single band in unstimulated Ramos and Jurkat cells but a doublet in the stimulated cells. The band detected in the unstimulated cells appeared not to be attributable to recognition of nonphosphorylated PLC-γ2, because the antibodies did not yield this band with unstimulated platelets or Null TV-1 cells. Even when the amount of unstimulated platelet lysate loaded onto the gel was increased so that the amount of PLC-γ2 was similar to that for the Ramos cell sample, no positive band was observed (Fig. 2B). Furthermore, PLC-γ2 immunoprecipitated from unstimulated Ramos cells with anti-PLC-γ2 was equally reactive with anti-pY753 as was PLC-γ2 in the lysate, suggesting that the band was not due to an unknown lysate protein (Fig. 2B). Serum deprivation did not reduce the level of Y753 phosphorylation in Ramos cells (data not shown). These results suggest that some PLC-γ2 molecules are constitutively phosphorylated on Y753 in lymphocytes and that receptor stimulation further increases the extent of Y753 phosphorylation as well as induces phosphorylation at other sites, resulting in the production of at least two different populations of pY753-containing PLC-γ2 molecules with different electrophoretic mobilities.
FIG. 3.
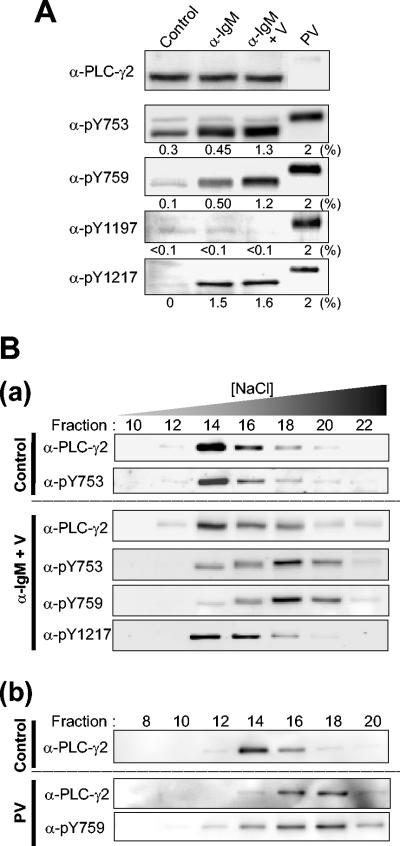
PLC-γ2 phosphorylation in Ramos cells stimulated by BCR engagement. (A) Ramos cells were left unstimulated (control) or were stimulated either for 1 min with anti-IgM (25 μg/ml) in the absence (α-IgM) or presence (α-IgM + V) of 5 mM sodium vanadate or for 10 min with pervanadate (PV; derived from 5 mM sodium vanadate and 2 mM H2O2). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-). The amount of lysate protein applied to the gel for the pervanadate-treated cells was 2% of that applied in the other lanes. Numbers under each panel represent the percentage of PLC-γ2 molecules phosphorylated on the corresponding residue calculated on the basis of the assumption that PLC-γ2 molecules were fully phosphorylated in the pervanadate-treated cells; they are means of values from three independent experiments. (B) Ramos cells were either left unstimulated (Control) or stimulated for 1 min with anti-IgM (25 μg/ml) in the presence of 5 mM sodium vanadate (α-IgM + V) (a). In a separate experiment, they were either left unstimulated (Control) or stimulated with pervanadate (PV; 5 mM sodium vanadate and 2 mM H2O2) for 10 min (b). Lysate proteins were subjected to fractionation by HPLC on a heparin-5PW column with a linear NaCl gradient. The resulting fractions were subjected to immunoblot analysis with the indicated antibodies.
BCR-induced PLC-γ2 phosphorylation was also examined in mouse splenic B cells. Although the extents of phosphorylation were lower than those in Ramos cells, PLC-γ2 was phosphorylated on Y753, Y759, and Y1217 but not on Y1197, as in Ramos cells (Fig. 2C). Constitutive phosphorylation of Y753 was also apparent in unstimulated splenic B cells. Faint bands seen in the blots with anti-pY759 and anti-pY1217 are likely due to a small fraction of cells activated during isolation from spleen (Fig. 2C).
BCR-induced PLC-γ2 phosphorylation.
The stoichiometry of phosphorylation at each site of PLC-γ2 was studied in BCR-stimulated Ramos cells. To obtain fully phosphorylated PLC-γ2, we stimulated the cells with pervanadate. PLC-γ2 appeared maximally phosphorylated after this treatment at all sites; no further increase in the reactivity of each phospho-specific antibody was observed with higher concentrations of pervanadate or with longer incubation periods. In all five immunoblots with anti-PLC-γ2, anti-pY753, anti-pY759, anti-pY1197, or anti-pY1217, the PLC-γ2 bands obtained with the pervanadate-treated cells were sharper and migrated much more slowly than those obtained with untreated cells or those stimulated via the BCR (Fig. 3A). These results strongly suggested that most PLC-γ2 molecules in the pervanadate-treated cells were phosphorylated at all possible sites, probably including serine phosphorylation sites: PLC-γ2 can be heavily phosphorylated on unidentified serine residue(s) in B cells upon stimulation (H. W. Rho, C.-W. Lee, D. J. Park, P.-G. Sue, and S. G. Rhee, unpublished observation). We therefore defined 100% phosphorylation at a given position as the corresponding immunoblot intensity obtained with PLC-γ2 from cells stimulated with pervanadate. The amount of lysate of pervanadate-treated cells applied to the gel shown in Fig. 3A was only 2% of the amounts of the lysates applied to the other lanes.
Stimulation with a saturating concentration of anti-IgM for 1 min (time needed for maximal phosphorylation) resulted in the phosphorylation of 0.45, 0.50, and 1.5% of PLC-γ2 molecules on Y753, Y759, and Y1217, respectively. Incubation of cells with vanadate, a reversible inhibitor of PTPs, before stimulation with anti-IgM increased the level of phosphorylation on Y753 and Y759 by a factor of 2 to 3, whereas that of Y1217 phosphorylation was little affected. Consistent with the low level of PLC-γ2 phosphorylation in BCR-stimulated cells even in the presence of vanadate, no noticeable broadening or mobility shift of the PLC-γ2 band was apparent in the blot with anti-PLC-γ2. However, the PLC-γ2 band detected by anti-pY753 in stimulated cells appeared broad (Fig. 3A) or as a doublet (Fig. 2A), suggesting that pY753 is distributed among PLC-γ2 molecules with different states of overall phosphorylation. The PLC-γ2 band detected by anti-pY759 in stimulated cells was also broad, albeit less so than that detected by anti-pY753. Phosphorylation on Y1197 was not detected in BCR-stimulated cells even in the presence of vanadate; this failure to detect phosphorylation on Y1197 was not due to insensitivity of anti-pY1197, given that the intensity of the PLC-γ2 band detected with anti-pY1197 was similar to those of the bands detected by the other phospho-specific antibodies when cells were treated with pervanadate.
In contrast to those detected by anti-pY753 or anti-pY759, the PLC-γ2 band detected by anti-pY1217 in stimulated cells was sharp. In response to BCR stimulation in the absence of vanadate, 1.5% of PLC-2 molecules were phosphorylated on Y1217 and 0.45 to 0.5% were probably phosphorylated on both Y753 and Y759 (phosphorylation of Y753 and Y759 appears to occur on the same molecules, as shown below). If molecules phosphorylated on Y753 and Y759 were also phosphorylated on Y1217, the immunoreactivity detected with anti-pY1217 would be expected to be resolved into two bands (one for the ∼1% of PLC-γ2 monophosphorylated on Y1217 and the other for the 0.45 to 0.5% of the enzyme that is trisphosphorylated) or at least to appear as a broad band. The sharpness of this band thus argues against this possibility.
We attempted to investigate whether phosphorylation on Y753, Y759, and Y1217 is present in the same molecules by precipitating PLC-γ2 with one phospho-specific antibody and probing the precipitates with the other antibody. However, none of the three antibody preparations proved suitable for immunoprecipitation. We therefore took another approach. Ramos cells were left unstimulated or stimulated either with anti-IgM or with pervanadate, and the resultant cell lysates were then fractionated by HPLC on a heparin column with an NaCl gradient (Fig. 3B). PLC-γ2 molecules derived from the resting cells peaked in fraction 14. This preparation consisted of mostly unphosphorylated protein but contained small amounts (∼0.3%) of PLC-γ2 phosphorylated on Y753 (Fig. 3A). Distribution of this Y753 monophosphorylated protein was indistinguishable from the bulk of unphosphorylated PLC-γ2 detected by anti-PLC-γ2 (Fig. 3Ba, control), indicating that phosphorylation of Y753 alone had no effect on the behavior on the heparin column. When stimulated with anti-IgM in the presence of vanadate, 1 to ∼1.5% of PLC-γ2 molecules were phosphorylated on each of three sites (Fig. 3A). The HPLC analysis of the anti-IgM-stimulated sample revealed that phosphorylated PLC-γ2 molecules detected by anti-pY753 or by anti-pY759 peaked in fraction 18, whereas those detected by anti-pY1217 overlapped with PLC-γ2 molecules detected with anti-PLC-γ2 (Fig. 3Ba, α-IgM + V). This result indicates that the pool of PLC-γ2 molecules phosphorylated on Y1217 is different from that phosphorylated on Y753 and Y759. Phosphorylation on Y753 alone was insufficient to cause the shift from fraction 14 to fraction 18 (as seen with the resting cell samples), but the elution profiles of pY753- and pY759-containing molecules were similar, with both peaking in fraction 18. This finding, together with the finding that activation of PLC-γ2 requires phosphorylation on both Y753 and Y759, suggests that Y753 and Y759 are phosphorylated within the same molecules.
While the stimulation through the BCR could elicit tyrosine phosphorylation of only small fractions of PLC-γ2 molecules, the treatment with a high dose of pervanadate was able to induce phosphorylation of most, if not all, of them (Fig. 3A). When the lysate of pervanadate-treated cells was subjected to the HPLC analysis, PLC-γ2 protein was indeed recovered in the fraction that peaked at 18, and almost no PLC-γ2 was detected in fraction 14 (Fig. 3Bb). This peak of protein detected by anti-PLC-γ2 overlapped with the profile detected with anti-pY759 (Fig. 3Bb) and with anti-pY753 or anti-pY1217 (not shown).
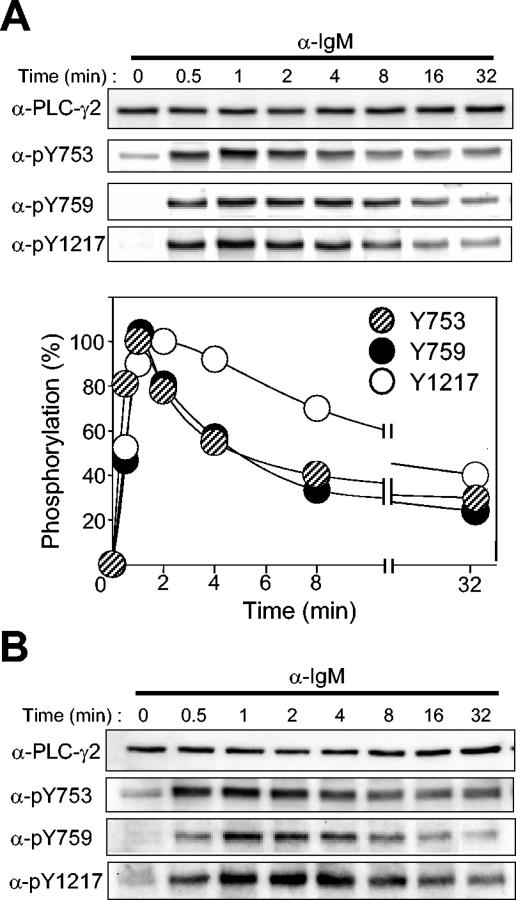
The time courses of phosphorylation of PLC-γ2 at each site were examined in Ramos cells stimulated with a saturating concentration of anti-IgM. The extent of phosphorylation was maximal within 1 min for Y753, Y759, and Y1217 (Fig. 4A). The time courses for Y753 phosphorylation and Y759 phosphorylation were similar, whereas the extent of phosphorylation of Y1217 decreased at a slower rate. This result, together with the observation that vanadate enhanced BCR-induced phosphorylation at Y753 and Y759 but not that at Y1217, suggests that the linker region residues might be dephosphorylated by a PTP different from that responsible for dephosphorylation of the COOH-terminal residue. Stimulation of murine splenic B cells with anti-IgM yielded a pattern of PLC-γ2 phosphorylation similar to that obtained with Ramos cells (Fig. 4B).
FIG. 4.
Time courses of BCR-induced PLC-γ2 phosphorylation. (A) Ramos cells were stimulated with anti-IgM (25 μg/ml) for the indicated times, after which cell lysates were subjected to immunoblot analysis with the indicated antibodies (α-) (upper panel). The intensities of the PLC-γ2 bands were quantified and plotted against time (lower panel); data are means of four independent determinations and are expressed as a percentage of the maximal value for each site after subtraction of the corresponding basal level of phosphorylation. (B) Mouse splenic B cells were stimulated with anti-IgM (25 μg/ml) for the indicated times, after which cell lysates were subjected to immunoblot analysis with the indicated antibodies.
Identification of PTKs that phosphorylate PLC-γ2 in response to BCR stimulation.
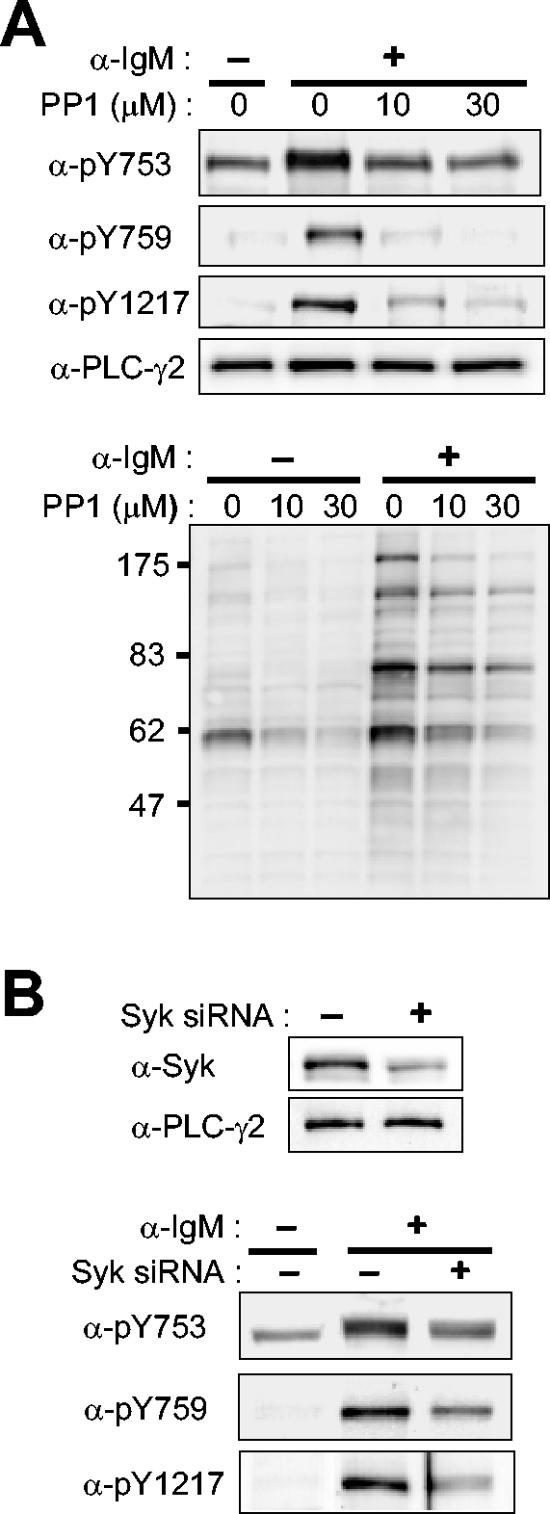
PTKs that belong to the Src family, most notably Lyn, initiate BCR signaling by phosphorylating several components in the BCR. Syk is then recruited by the phosphorylated receptor chains and activated to phosphorylate many cellular substrates such as BLNK (28). We tested the effect of PP1, an inhibitor of Src family kinases, on the phosphorylation of PLC-γ2 in Ramos cells. Phosphorylation of Y753, Y759, and Y1217 induced by anti-IgM was markedly inhibited by PP1 (Fig. 5A). The higher-mobility band observed with anti-pY753, which corresponds to constitutively phosphorylated PLC-γ2 molecules, was resistant to PP1 under the experimental condition, however. As expected, BCR-induced tyrosine phosphorylation of cellular proteins in general was inhibited by PP1, as revealed by immunoblot analysis with antiphosphotyrosine (Fig. 5A).
FIG. 5.
Effects of inhibition of Src family kinases or of Syk depletion on BCR-induced PLC-γ2 phosphorylation in Ramos cells. (A) Effects of Src family inhibition. Ramos cells were treated for 10 min with the indicated concentrations of PP1 or vehicle before stimulation for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-) to PLC-γ2 (upper 4 panels) or with antiphosphotyrosine (lower panel). The positions of molecular size standards are shown in kilodaltons in the bottom panel. (B) Effects of Syk depletion. Ramos cells were transfected (or not) twice with an interval of 2 days with Syk siRNA. Four days after the initial transfection, they were subjected to immunoblot analysis with anti-Syk or anti-PLC-γ2 (upper panel); alternatively, they were stimulated (or not) for 1 min with anti-IgM (25 μg/ml) before immunoblot analysis with the indicated antibodies (lower panel).
Given the lack of specific pharmacological inhibitors of Syk family kinases, we used RNAi to deplete Ramos cells of Syk. It was necessary to transfect the cells with the Syk siRNA twice with an interval of 2 days to achieve a substantial reduction (∼70%) in the extent of Syk expression 4 days after the initial transfection (Fig. 5B). Depletion of Syk resulted in marked inhibition of BCR-induced phosphorylation of PLC-γ2 at all three sites (Fig. 5B). Again, the lower-mobility (more highly phosphorylated) band detected with anti-pY753 was more responsive to Syk depletion than was the higher-mobility band.
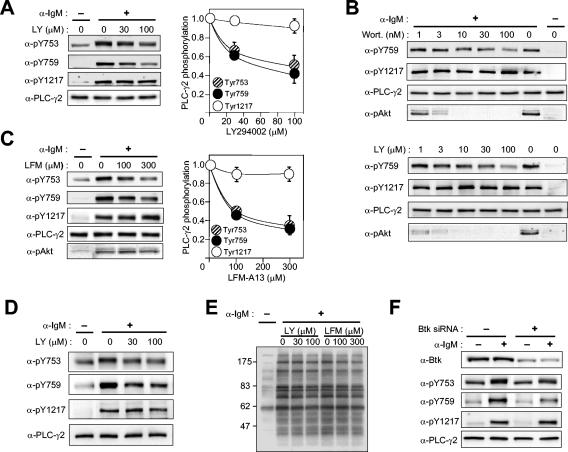
The Tec family kinase Btk has been implicated in the phosphorylation and activation of PLC-γ2 in chicken DT-40 cells (44). Experiments with XLA cells also revealed that activation of PLC-γ2 required functional Btk, although the overall level of phosphorylation of PLC-γ2 measured with antiphosphotyrosine did not appear to be affected in these cells (10). We evaluated the role of Btk in the phosphorylation of PLC-γ2 at each site by using PI 3-kinase inhibitors. Given that PIP3 binding to Btk's PH domain is required to recruit Btk to plasma membranes where Btk phosphorylates its substrates, inhibition of PIP3 synthesis is expected to block Btk function. At saturating concentrations of LY294002, an inhibitor of PI 3-kinase, BCR-induced phosphorylation of PLC-γ2 on Y753 and Y759 in Ramos cells was inhibited by ∼60 to 70%, whereas that of Y1217 was minimally affected (Fig. 6A). We also employed wortmannin, another potent PI 3-kinase inhibitor, and observed a similar result (Fig. 6B).
FIG. 6.
Effects of inhibition or depletion of Btk on BCR-induced PLC-γ2 phosphorylation in Ramos cells. (A) Effect of LY294002 (LY) on PLC-γ2 phosphorylation. Ramos cells were treated for 10 min with vehicle or the indicated concentrations of LY294002 before stimulation (or not) for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-) (left panels). The immunoblot intensities were corrected for the basal level of phosphorylation, normalized by the corrected value for stimulated cells pretreated with vehicle, and plotted against inhibitor concentration (right panels); data are means ± standard error of values from four independent experiments. (B) Effects of wortmannin (Wort.) and LY294002 on PLC-γ2 phosphorylation and Akt phosphorylation on Thr308. Ramos cells were treated for 10 min with the indicated concentrations of inhibitors before stimulation (or not) for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies. (C) Effect of LFM-A13 on PLC-γ2 and Akt phosphorylation. Ramos cells were treated for 10 min with vehicle or the indicated concentrations of LFM-A13 before stimulation (or not) for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies. (D) Effect of LY 294002 on PLC-γ2 phosphorylation in splenic B cells. Murine B cells were treated with indicated concentrations of the inhibitor for 10 min before stimulation for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies. (E) Effects of LY294002 and LFM-A13 on overall tyrosine phosphorylation of cellular proteins. Lysates of Ramos cells similar to those in panels A and C were subjected to immunoblot analysis with antiphosphotyrosine. The positions of molecular size standards are shown in kilodaltons. (F) Effects of Btk depletion on PLC-γ2 phosphorylation. Ramos cells were transfected with a control RNA or Btk siRNA. Three days after transfection, the cells were left unstimulated or stimulated for 1 min with anti-IgM (25 μg/ml). Cell lysates were subjected to immunoblot analysis with the indicated antibodies.
As a measure of PI 3-kinase inhibition, phosphorylation of Akt on Thr308 was monitored in parallel. Phosphorylation of Akt appeared much more sensitive to the PI 3-kinase inhibitors than was PLC-γ2 phosphorylation (Fig. 6B). The serine/threonine kinase Akt possesses a PH domain, which recognizes PIP3 as well as PI(3,4)P2, and translocates to membranes upon cellular synthesis of these phosphoinositides. Akt is then phosphorylated on Thr308 by PDK1 (3-phosphoinositide-dependent protein kinase 1) and on Ser473 by another kinase, and both reactions also depend on PI(3,4)P2 and/or PIP3 (1). Thus, a relatively small change in PI 3-kinase activity can have a more profound effect on Akt activation than on Btk activation. Alternatively, because intracellular concentration of PIP3 is the net result of phosphorylation by PI 3-kinase and dephosphorylation by PTEN and because PIP3 is not evenly distributed throughout the plasma membranes (14, 15, 18, 51), Akt and Btk might be activated by different pools of PIP3, and the PIP3 pool that promoted nucleation of the BCR signalsome of Btk, BLNK, PLC-γ2, etc., might be less sensitive to PI 3-kinase inhibition than the pool responsible for Akt activation. We also tested the effect of PI 3-kinase inhibitors in mouse splenic B cells. As observed in Ramos cells, LY 294002 (Fig. 6D) or wortmannin (not shown) caused a large, but incomplete, reduction in BCR-induced phosphorylation on Y753 and Y759 but not on Y1217.
Involvement of Btk was studied by treating Ramos cells with LFM-A13, a Btk inhibitor rationally designed on the basis of the structure of the kinase (30). Similar to the PI 3-kinase inhibitors, LFM-A13 caused ∼70% inhibition of PLC-γ2 phosphorylation on Y753 and Y759 without affecting Y1217 phosphorylation (Fig. 6C). LFM-A13 did not alter Akt phosphorylation on Thr308, however. The actions of LY 294002 and LFM-A13 appeared to be selective even at the high concentrations used, given that the overall pattern of BCR-induced tyrosine phosphorylation was not affected (Fig. 6E). These results suggest that phosphorylation of Y753 and Y759 residues largely depends on Btk, whereas phosphorylation of Y1217 is independent of Btk. Given that the phosphorylation of the linker region residues was not completely blocked by saturating doses of LY294002, wortmannin, or LFM-A13, however, PTKs other than Tec family members likely participate directly in the phosphorylation of these two sites.
The role of Btk was also studied by RNAi. Three days after transfection of Ramos cells with a Btk-specific siRNA, the expression of Btk was reduced to 31% ± 4% (mean ± standard error; n = 4) of that apparent in control cells (Fig. 6F). The BCR-induced phosphorylation of PLC-γ2 on Y753 or Y759 was reduced by 55% ± 9% and 63% ± 2%, respectively, in these Btk-depleted cells, whereas the level of Y1217 phosphorylation was 99% ± 5% of that in control cells (Fig. 6F). These data thus provide further support for the notion that BCR-induced phosphorylation of PLC-γ2 is achieved by both Btk-dependent and Btk-independent mechanisms.
Effects of changes in PKC activity on the phosphorylation and activation of PLC-γ2.
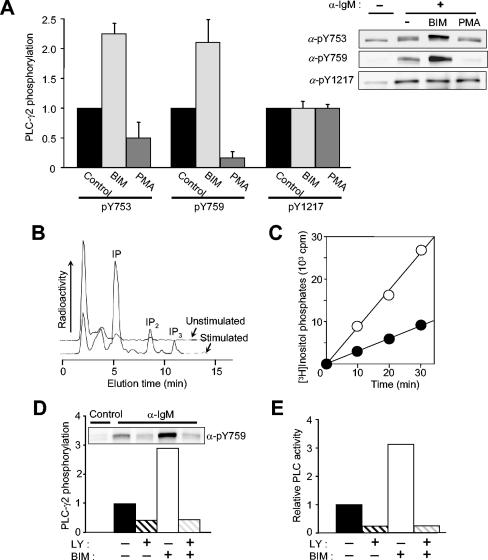
Protein kinase C (PKC) down-regulates Btk activity by directly phosphorylating Btk on Ser180 (25). We tested the effects of the PKC inhibitor BIM and the PKC activator phorbol myristate acetate (PMA) on PLC-γ2 phosphorylation in Ramos cells (Fig. 7A). BCR-induced phosphorylation of PLC-γ2 on Y753 and Y759 was markedly potentiated by BIM and inhibited by PMA. In contrast, phosphorylation of Y1217 was not affected by either reagent, consistent with the operation of Btk-dependent and Btk-independent mechanisms of PLC-γ2 phosphorylation. We also investigated the consequences of the BIM effect of PLC-γ2 phosphorylation. Exposure of Ramos cells that had been labeled with myo-[3H]inositol to anti-IgM in the presence of LiCl resulted in the accumulation of [3H]inositol phosphates (Fig. 7B). The sum of the amounts of inositol monophosphate (IP), inositol bisphosphate (IP2), and inositol trisphosphate (IP3) increased linearly with time for up to 30 min (Fig. 7C). BIM promoted the BCR-induced production of inositol phosphates (Fig. 7C), indicating that the activation of PLC-γ2 is dependent on the phosphorylation of Y753 and Y759 but is not affected by Y1217 phosphorylation. Neither PMA nor BIM, by themselves, had any effect on basal PLC-γ2 phosphorylation or [3H]inositol phosphate generation.
FIG. 7.
Effects of changes in PKC activity on the phosphorylation and activation of PLC-γ2 in Ramos cells. (A) Effects of BIM and PMA on PLC-γ2 phosphorylation. Ramos cells were incubated for 10 min in the absence or presence of 2.5 μM BIM or 1 μM PMA before stimulation (or not) for 1 min with anti-IgM (25 μg/ml). Cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-) (right panel). The relative blot intensities for stimulated cells (after correction for basal values) were determined as means ± standard error from three independent experiments (left panel). (B) Detection of [3H]inositol phosphates produced in unstimulated and BCR-stimulated cells. Ramos cells that had been metabolically labeled with myo-[3H]inositol were left unstimulated or were stimulated for 30 min with anti-IgM (25 μg/ml) in the presence of 20 mM LiCl. [3H]inositol phosphates in cell extracts were analyzed with an HPLC system equipped with an on-line radioactivity detector. (C) Time course of phosphoinositide hydrolysis. Cells labeled with myo-[3H]inositol were treated for 10 min with LiCl in the absence (closed circles) or presence (open circles) of 2.5 μM BIM and then stimulated for the indicated times with anti-IgM (25 μ/ml). [3H]inositol phosphates (IP + IP2 + IP3) in cell extracts were then measured as in panel B. (D and E) LY294002 sensitivity of the effect of BIM on BCR-induced PLC-γ2 phosphorylation (D) and on PLC activity (E). Ramos cells were treated for 10 min in the absence or presence of 2.5 μM BIM, 100 μM LY294002, or 2.5 μM BIM plus 100 μM LY294002 before stimulation for 1 min with anti-IgM (25 μ/ml). Cell lysates were subjected to immunoblot analysis with anti-pY759 (inset). The relative blot intensities were determined as means from three independent experiments. In panel E, cells labeled with myo-[3H]inositol were treated with BIM or LY294002 and then stimulated with anti-IgM as in panel D in the presence of 20 mM LiCl for 30 min. The amounts of [3H]inositol phosphates in cell extracts were then measured. Data are expressed as relative PLC activity after subtraction of basal values.
We next examined the role of Btk in the effect of BIM on PLC-γ2 phosphorylation and activation. Pretreatment with LY294002 completely inhibited the potentiating effects of BIM both on the phosphorylation of Y759 (Fig. 7D) and Y753 (data not shown) as well as on in vivo PLC activity (Fig. 7E) in BCR-stimulated cells. Similar effects were observed with LFM-A13 as with LY294002 (data not shown). These results provided further support for a mediator role of Btk in BCR-induced PLC-γ2 phosphorylation and activation.
Role of PI 3-kinase in BCR-induced PLC-γ2 activation.
Activation of PI 3-kinase has been considered a requirement for BCR-induced PLC-γ2 activation, and this requirement has been attributed to the role of PIP3 in the membrane recruitment of Btk (12). Our results now indicate that the recruited Btk activates PLC-γ2 by phosphorylating it on Y753/Y759 (Fig. 6 and 7). We have shown that PIP3 does not affect growth factor-induced PLC-γ1 phosphorylation on the critical residue Y783 but serves as an activating factor for the Y783-phosphorylated enzyme (39). We therefore investigated whether tyrosine-phosphorylated PLC-γ2 is also activated by PIP3.
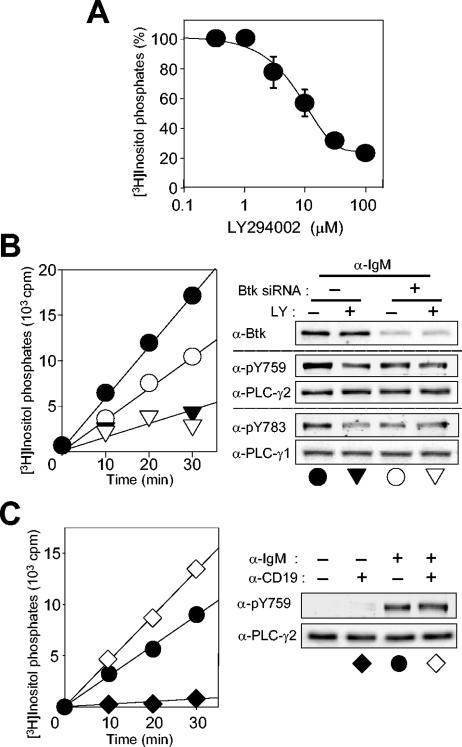
LY294002 inhibited the BCR-induced PLC activation in Ramos cells in a dose-dependent manner (Fig. 8A). At 30 or 100 μM, LY294002 inhibited PLC activity by 70 and 80%, respectively, while the same concentrations of this drug reduced the extent of BCR-induced PLC-γ2 phosphorylation on Y753/Y759 by 40 and 60%, respectively (Fig. 5A). PIP3 thus appears to act both at the level of Btk to up-regulate PLC-γ2 phosphorylation and as an activator of the lipase activity of the phosphorylated PLC-γ2.
FIG. 8.
Role of PI 3-kinase in BCR-induced PLC-γ2 activation in Ramos cells. (A) Concentration dependence of PLC inhibition by LY294002. Cells labeled with myo-[3H]inositol were treated for 10 min with the indicated concentrations of LY294002 in the presence of 20 mM LiCl and then stimulated for 30 min with anti-IgM (25 μg/ml). [3H]inositol phosphates in cell extracts were then measured. Data are means ± standard error of three independent determinations. (B) Effects of Btk RNAi and PI 3-kinase inhibition on BCR-induced PLC activity and phosphorylation of PLC-γ2 and PLC-γ1. Ramos cells were transfected with a control RNA (closed symbols) or Btk siRNA (open symbols). After 3 days, the cells were labeled with myo-[3H]inositol, treated for 10 min with 100 μM LY294002 (triangles) or vehicle (circles) in the presence of LiCl, and then stimulated for the indicated times with anti-IgM (25 μg/ml). [3H]inositol phosphates in cell extracts were then measured (left panel); data are representative of one of three independent determinations. Alternatively, the siRNA-transfected cells were treated with 100 μM LY294002 for 10 min before stimulation with anti-IgM (25 μg/ml) for 1 min; cell lysates were then subjected to immunoblot analysis with the indicated antibodies (α-) (right panel). (C) Effect of CD19 costimulation on BCR-induced PLC activity and PLC-γ2 phosphorylation. Cells labeled with myo-[3H]inositol were stimulated in the presence of LiCl for the indicated times with anti-IgM (25 μ/ml) (circles), anti-CD19 (10 μg/ml) (closed diamonds), or anti-IgM (25 μg/ml) plus anti-CD19 (10 μg/ml) (open diamonds), after which [3H]inositol phosphates in cell extracts were measured (left panel); data are from a representative experiment out of three independent determinations. Alternatively, cells stimulated for 1 min were subjected to immunoblot analysis with the indicated antibodies.
To provide further support for this conclusion, we examined the effects of Btk RNAi followed by treatment with LY294002 (Fig. 8B). Transfection of Ramos cells with Btk siRNA alone inhibited the BCR-induced activation of PLC by 40% and the BCR-induced phosphorylation of PLC-γ2 on Y759 by 45%. Exposure of the Btk-depleted cells to a saturating concentration (100 μM) of LY294002 resulted in only a slight further reduction in the extent of BCR-induced Y759 phosphorylation, whereas the extent of PLC activation was reduced by half compared with that apparent in the cells subjected to RNAi alone. These results are thus consistent with a dual role for PIP3 in BCR-induced PLC-γ2 activation.
Given that Ramos cells express PLC-γ1 and that BCR engagement induces phosphorylation of this isozyme on the critical residue Y783 (39), it was important to assess the possible contribution of PLC-γ1 to BCR-induced PLC activity. The amount of PLC-γ1 in Ramos cells is only one-fourth of that of PLC-γ2; the BCR-induced phosphorylation of PLC-γ1 is similar to that of PLC-γ2 in terms of kinetics and stoichiometry; and the two isozymes exhibit similar specific activities in vitro (data not shown). Furthermore, LY294002 and Btk RNAi each inhibited PLC-γ1 phosphorylation on Y783 (Fig. 8B). On the basis of these observations, the presence of PLC-γ1 in Ramos cells does not appear to undermine the conclusion that PIP3 plays a dual role in BCR-induced PLC-γ2 activation.
CD19 is a BCR-associated coreceptor that functions as an enhancer of BCR signaling. This transmembrane protein contains two Tyr residues that are phosphorylated in response to cross-linking of CD19 molecules and then serve as binding sites for the SH2 domains of the p85 regulatory subunit of PI 3-kinase (3, 47). This interaction with CD19 results in the activation of PI 3-kinase. We tested whether additional stimulation of PI 3-kinase via CD19 would enhance BCR-induced PLC activation. Costimulation of Ramos cells with anti-IgM and anti-CD19 resulted in a greater accumulation of inositol phosphates than that induced by anti-IgM alone (Fig. 8C). Although CD19 stimulation alone has previously been shown to induce Btk activation and subsequent PLC-γ2 phosphorylation in human and mouse B cells (5, 13), it induced neither measurable PLC-γ2 phosphorylation on Y759 nor inositol phosphate production under our experimental conditions (Fig. 8C). The extent of costimulation-induced phosphorylation of PLC-γ2 on Y759 did not differ from that induced by BCR engagement alone. The enhancement of PIP3 production induced by CD19 stimulation thus appeared to augment the lipase activity of PLC-γ2 (likely that of PLC-γ1 also) without affecting the state of PLC-γ2 phosphorylation in BCR-stimulated cells.
DISCUSSION
Identification of phosphorylation sites of PLC-γ2.
We have prepared and established the specificity of antibodies that recognize individual tyrosine phosphorylation sites of PLC-γ2. Studies with these antibodies revealed that engagement of the BCR induces phosphorylation of endogenous PLC-γ2 on Y753, Y759, and Y1217 in mammalian B-cell preparations. We previously identified Y753 and Y759 as the sites of phosphorylation of PLC-γ2 in BCR-stimulated Ramos cells by two-dimensional mapping of tryptic peptides followed by phosphoamino acid analysis; we failed, however, to detect a pY1217-containing peptide by this approach, possibly because pY1217 was distributed among several tryptic peptides as a result of partial digestion (three consecutive Arg residues form potential trypsin cleavage sites that would generate multiple pY1217-containing peptides). Both Y753 and Y759 were also proposed as sites of phosphorylation of PLC-γ2 on the basis of experiments with chicken DT-40 cells expressing human PLC-γ2 (35, 49). However, phosphorylation of Y1197, which was proposed as a step necessary for PLC-γ2 activation in the BCR-stimulated chicken cells (49), did not occur to a measurable extent in Ramos lymphoma cells as well as in murine splenic B cells.
Alignment of the amino acid sequences of PLC-γ1 and PLC-γ2 reveals that Y753 and Y759 of PLC-γ2 correspond to Y775 and Y783, respectively, of PLC-γ1. Y783 is the only residue whose phosphorylation is critical for growth factor-induced lipase activity of PLC-γ1, and there is no evidence that Y775 of this isozyme is phosphorylated in stimulated cells. Residue Y1217 appears to be unique to PLC-γ2.
Phosphorylation of PLC-γ2 on Y753 and Y1217 depends on cell type.
We compared the patterns of PLC-γ2 phosphorylation on Y753, Y759, Y1197, and Y1217 in BCR-stimulated Ramos cells with those apparent in human platelets stimulated via the collagen receptor, Jurkat human T-lymphoma cells stimulated via the TCR, and PDGF-stimulated mouse Null TV-1 fibroblasts expressing human PLC-γ2. The results obtained for Y759 were similar for all four cell types in that phosphorylation of Y759 was induced by cell stimulation. Phosphorylation of Y753 and Y1217, however, differed among the cell types. Residue Y753 was constitutively phosphorylated in both B and T cells and underwent further phosphorylation in response to cell stimulation; pY753-containing molecules were not detected in unstimulated platelets but were apparent after cell stimulation, whereas Y753 phosphorylation was only weakly induced compared to that of Y759 in fibroblasts. Phosphorylation of Y1217 was not detected in platelets, Jurkat cells, or fibroblasts, and it did not appear to be an artifact of transformation in Ramos cells because it was also apparent in freshly isolated mouse splenic B cells. Phosphorylation on Y1197 was undetectable in the four different cell types tested after stimulation with their respective physiological agonists. Although phosphorylation on Y1197 by Btk was observed in an in vitro reaction using PLC-γ2 fragments, it had not been confirmed in the context of full-length protein either in vivo or in vitro (49).
These cell type-dependent differences in PLC-γ2 phosphorylation may not be due to the specificities of kinases expressed in the different cells. PLC-γ1 is phosphorylated both at Y783 and Y1253 by various PTKs, including Src, ZAP-70, and the EGF receptor in vitro. Y1253 phosphorylation is apparent in fibroblasts stimulated with PDGF or EGF but undetectable in lymphoma cells stimulated via immunoreceptors (39). The four PLC-γ2 Tyr residues (Y753, Y759, Y1197, and Y1217) could be phosphorylated in vitro by Btk when peptides containing these residues were used as substrates (49). It thus appears that an aspect of the cellular environment other than the resident kinases plays an important role in determining the pattern of PLC-γ isozyme phosphorylation. Activated receptor PTKs initiate phosphorylation of PLC-γ isozymes through direct association with the latter's SH2 domains. In contrast, in immunoreceptor signaling, PLC-γ isozymes do not associate directly with the kinases responsible for their phosphorylation. Rather, various adapter proteins provide a platform for such an interaction (23, 28, 36). Membrane-anchored proteins such as LAT (linker for activation of T cells) constitute one class of such adapters. LAT becomes phosphorylated on several Tyr residues in activated T cells and mediates the assembly of various SH2 domain-containing proteins, including PLC-γ and Itk, a Tec family PTK (36). Mature B cells do not express LAT but do express NTAL (non-T-cell activation linker), also known as LAB (linker for activation of B cells), which possesses a basic structural organization similar to that of LAT (2, 21). It remains to be determined, however, whether NTAL is the functional counterpart of LAT and links the BCR to PLC-γ in B cells. Another class of adapters implicated in PLC-γ regulation includes cytosolic proteins such as BLNK and SLP-76 (SH2 domain-containing leukocyte phosphoprotein of 76 kDa). BLNK is expressed in B cells, whereas SLP-76 is found in T cells and platelets (23, 28). Although both of these proteins contain multiple Tyr residues to be phosphorylated, SLP-76 associates with PLC-γ through interaction of its proline-rich region with the SH3 domain of PLC-γ (52, 53); BLNK binds PLC-γ as a result of interaction between its phosphotyrosine residues and the SH2 domains of PLC-γ (7, 20), however. A possible simple explanation for the cell-type-specific pattern of PLC-γ2 phosphorylation therefore is that different adapters present the lipase molecules to PTKs in different configurations, with the result that the kinases may or may not have access to Y753 or Y1217.
Distinct features of PLC-γ2 phosphorylation at each site in B cells.
The BCR induces phosphorylation of only a small fraction of PLC-γ2 molecules: ∼0.5% of molecules contained pY753 and pY759 and ∼1.5% of molecules contained pY1217 in Ramos cells stimulated in the absence of vanadate. Given that the pY753-containing enzyme was also present in resting cells and that PLC activity was virtually undetectable in such cells, phosphorylation of PLC-γ2 on Y753 alone does not appear to be sufficient for lipase activity. The phosphorylation of Y753 and that of Y759 occurred to similar extent, exhibited similar time courses, and were enhanced by vanadate inhibition of PTPs in BCR-stimulated cells. In contrast, the characteristics of Y1217 phosphorylation differed from those of phosphorylation of the linker region residues: The maximal level of Y1217 phosphorylation attained in the absence of vanadate was about three times that of Y753 or Y759 phosphorylation, and Y1217 phosphorylation decayed more slowly and was not enhanced by vanadate. In addition, phosphorylation of Y753 and Y759 seems likely to occur on the same PLC-γ2 molecules, which appeared to be different from those phosphorylated on Y1217.
One study with chicken DT-40 cells expressing human PLC-γ2 suggested that phosphorylation of Y753 and Y759 is sufficient for lipase activation (35), whereas another study with the same cell line indicated that phosphorylation of not only Y1217 but also Y1197 is as important as that of the linker region residues (49). PLC-γ2 null mammalian B cells have not been established. Nevertheless, several lines of evidence from the present study exclude a role for the COOH-terminal tyrosine residues in the activation of PLC-γ2. BCR stimulation thus did not elicit Y1197 phosphorylation in Ramos cells as well as in mouse splenic B cells. Phosphorylation of Y1217 was apparent in B cells but was virtually undetectable in other cell types, including platelets, in which PLC-γ2 is much more abundant than is PLC-γ1. Stimulation of the collagen receptor induces PIP2 hydrolysis in platelets through activation of PLC-γ2 (50). If the phosphorylation of Y1217 were required for PLC-γ2 activation, PIP2 hydrolysis would not be expected to occur in stimulated platelets. Moreover, we found that a reduction in the amount of Btk or pharmacological interference of Btk activity resulted in a reduction in the extent of phosphorylation of Y753 and Y759 and in a concomitant reduction in PLC activity, whereas Y1217 phosphorylation was not affected by this treatment. Also, treatment of Ramos cells with the PKC inhibitor BIM resulted in up-regulation of PLC-γ2 phosphorylation on Y753 and Y759 as well as of PLC activity, again without an effect on the level of Y1217 phosphorylation. These results indicate that phosphorylation of Y1217 does not activate the lipase function of PLC-γ2. Phosphorylation of this COOH-terminal residue, which occurs on molecules distinct from those phosphorylated on Y753/Y759, might have a function independent of lipase activity. PLC-γ isozymes exhibit several distinct lipase-independent biological activities (17, 33, 40).
Btk-dependent and Btk-independent phosphorylation of PLC-γ2.
Members of three distinct families (Src, Syk, and Tec) of PTK have been implicated in immunoreceptor-mediated phosphorylation of PLC-γ (28, 46, 50). Lyn, Syk, and Btk have been suggested to be the major PTKs of each family that function in BCR signaling. We have now confirmed that these three PTKs are necessary for normal induction of PLC-γ2 phosphorylation by BCR stimulation. Inhibition of Src family kinases by PP1 resulted in inhibition not only of PLC-γ2 phosphorylation at each site but also of the phosphorylation of many other proteins. Depletion of Syk by RNAi also inhibited PLC-γ2 phosphorylation at all sites, but it did not have a global effect on protein tyrosine phosphorylation (data not shown). However, given that Syk activity is required for the phosphorylation of BLNK and subsequent recruitment of PLC-γ2, whether or not PLC-γ2 is a direct substrate of this PTK remains unclear.
In contrast, Btk appears to phosphorylate certain residues of PLC-γ2 directly. Maximal inhibition of Btk function by LY294002, wortmannin, or LFM-A13 resulted in 60 to 70% inhibition of PLC-γ2 phosphorylation on Y753 and Y759 without an effect on that of Y1217. Moreover, RNAi-mediated depletion of Btk by 70% and inhibition of the remaining enzyme molecules with a saturating concentration of LY294002 did not reduce the extent of phosphorylation of the linker region residues by more than 60 to 70% and did not affect the extent of Y1217 phosphorylation. These results indicate that Btk is the kinase responsible for the phosphorylation of >60% of PLC-γ2 molecules on Y753 and Y759, but that another kinase (or kinases) also phosphorylates these linker region residues. Phosphorylation of Y1217 appeared to be fully independent of Btk, though an in vitro study by Watanabe et al. (49) suggested this residue was a preferred Btk site. Chiu et al. (7) recently showed that Btk and PLC-γ2 must assemble on the same BLNK molecules to achieve functional Ca2+ signaling. It is thus possible that PLC-γ2 molecules either recruited to the signalosome but not bound to Btk-occupied BLNK or recruited by some other means might be phosphorylated by other kinases such as Lyn or Syk.
Although we used PI 3-kinase inhibitors to downregulate Btk function, the mechanism by which this is achieved is not clear. There is a report suggesting that the tyrosine phosphorylation and activation of Btk are not affected by the absence of PI 3-kinase (43). Nevertheless, a large body of evidence including our present study supports the involvement of PI 3-kinase in PLC-γ2 activation (8, 24). Therefore it is a possibility that PIP3 might be required for the proper localization of Btk (likely in the BCR signalsome) necessary for PLC-γ2 phosphorylation but not for the activation of Btk via tyrosine phosphorylation.
Immortalized cell lines established from B cells of XLA patients manifest marked impairment of BCR-induced IP3 production and Ca2+ signaling, whereas the extent of BCR-induced tyrosine phosphorylation of PLC-γ2 in these cells was indistinguishable from that in wild-type B cells (10). These results can now be explained by our finding that the Tyr residues essential for PLC-γ2 activation are minor sites of phosphorylation. The sum of pY753 and pY759 thus constituted only ∼40% of total phosphorylated Tyr residues in PLC-γ2 in stimulated Ramos cells, and this percentage was further decreased to ∼25% in cells in which Btk function was fully inhibited. Moreover, given that phosphorylation of Y753 and of Y759 decayed faster than did that of Y1217, the percentage would be even smaller if the samples were not prepared at the peak time. It is thus likely that the level of BCR-induced tyrosine phosphorylation of PLC-γ2 in XLA B cells could be less than that in normal human B cells but that the difference would not be sufficiently large to be detected with antibodies to tyrosine phosphate.
Feedback inhibition of PLC-γ2 through Btk-dependent phosphorylation.
The activity of Btk is inhibited as a result of phosphorylation of the enzyme by PKC (25), suggesting the existence of a link between PKC activation and PLC-γ2 phosphorylation on Y753 and Y759. Indeed, activation of PKC by PMA attenuated the BCR-induced phosphorylation of these two residues of PLC-γ2 without affecting that of Y1217. Conversely, inhibition of PKC by BIM augmented the BCR-induced phosphorylation of Y753 and Y759 without altering that of Y1217. Consistent with these observations, the BCR-induced production of inositol phosphates was also increased in cells exposed to BIM. Given that the activation of PKC occurs immediately downstream of that of PLC, inhibition of Btk activity and the consequent down-regulation of PLC-γ2 activity induced by PKC activation constitute a negative feedback loop in BCR signaling.
Similarity and dissimilarity between the phosphorylation and activation of PLC-γ2 and those of PLC-γ1.
PLC-γ1 is phosphorylated on Y783 and Y1253 in response to stimulation of cells with PDGF or EGF, and the level of Y783 phosphorylation is 1.5- to 2-fold greater than that of Y1253 phosphorylation. Unlike PLC-γ2 phosphorylation in B cells, a single kinase, the receptor PTK, appears to phosphorylate both Y783 and Y1253 of PLC-γ1, mostly on the same molecules. Similar to PLC-γ2, however, phosphorylation of the linker region residue (Y783), but not that of the COOH-terminal residue (Y1253), is required for activation of the lipase function of PLC-γ1. Also similar to PLC-γ2, phosphorylation of the activating residue (Y783) appears to occur independently of cell type, whereas that of the nonactivating site (Y1253) was not detected in Ramos and Jurkat cells stimulated via their immune receptors (39).
Activation of both PLC-γ1 and PLC-γ2 is enhanced by PIP3, but the corresponding modes of action of this lipid might be cell type dependent. Whereas in immunoreceptor signaling PIP3 promotes the activation of PLC-γ by enhancing both its tyrosine phosphorylation by Tec family PTKs (38, 46) and the catalytic activity of the resulting phosphorylated enzyme (this study), the lipid promotion of PLC-γ activation in growth factor signaling is limited to the effect on the phosphorylated enzyme (39).
Acknowledgments
Y.S.K. is a recipient of the Brain Korea 21 fellowship from the Ministry of Education, South Korea.
REFERENCES
- 1.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 2.Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, N. Engels, J. Wienands, L. Simeoni, J. Osterreicher, E. Aguado, M. Malissen, B. Schraven, and V. Horejsi. 2002. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhl, A. M., and J. C. Cambier. 1999. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton's tyrosine kinase. J. Immunol. 162:4438-4446. [PubMed] [Google Scholar]
- 4.Carpenter, G., and Q. Ji. 1999. Phospholipase C-γ as a signal-transducing element. Exp. Cell Res. 253:15-24. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R. H., D. A. Tuveson, D. J. Park, S. G. Rhee, and D. T. Fearon. 1991. The CD19 complex of B lymphocytes. Activation of phospholipase C by a protein tyrosine kinase-dependent pathway that can be enhanced by the membrane IgM complex. J. Immunol. 147:3663-3671. [PubMed] [Google Scholar]
- 6.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu, C. W., M. Dalton, M. Ishiai, T. Kurosaki, and A. C. Chan. 2002. BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins. EMBO J. 21:6461-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton, E., G. Bandi, S. E. Bell, D. Chantry, C. P. Downs, A. Gray, L. A. Humphries, D. Rawlings, H. Raynolds, E. Vigorito, and M. Turner. 2002. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J. Exp. Med. 196:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsam, R. T., S. Kim, J. Jin, and S. P. Kunapuli. 2002. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J. Biol. Chem. 277:47588-47595. [DOI] [PubMed] [Google Scholar]
- 10.Fluckiger, A.-C., Z. Li, R. M. Kato, M. I. Wahl, H. D. Ochs, R. Longnecker, J.-P. Kinet, O. N. Witte, A. M. Scharenberg, and D. J. Rawlings. 1998. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 17:1973-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruman, D. A., and L. C. Cantley. 2002. Phosphoinositide 3-kinase in immunological systems. Semin. Immunol. 14:7-18. [DOI] [PubMed] [Google Scholar]
- 12.Fruman, D. A., A. B. Satterthwaite, and O. N. Witte. 2000. Xid-like phenotypes: a B cell signalosome takes shape. Immunity 13:1-3. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto, M., J. C. Poe, A. B. Satterthwaite, M. I. Wahl, O. N. Witte, and T. F. Tedder. 2002. Complementary roles for CD19 and Bruton's tyrosine kinase in B lymphocyte signal transduction. J. Immunol. 168:5465-5476. [DOI] [PubMed] [Google Scholar]
- 14.Funamoto, S., R. Meili, S. Lee, L. Parry, and R. A. Firtel. 2002. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109:611-623. [DOI] [PubMed] [Google Scholar]
- 15.Haugh, J. M., F. Codazzi, M. Teruel, and T. Meyer. 2000. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J. Cell Biol. 151:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinonen, J. E., C. I. Smith, and B. F. Nore. 2002. Silencing of Bruton's tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA). FEBS Lett. 527:274-278. [DOI] [PubMed] [Google Scholar]
- 17.Huang, P. S., L. Davis, H. Huber, P. J. Goodhart, R. E. Wegrzyn, A. Oliff, and D. C. Heimbrook. 1995. An SH3 domain is required for the mitogenic activity of microinjected phospholipase C-γ1. FEBS Lett. 358:287-292. [DOI] [PubMed] [Google Scholar]
- 18.Iijima, M., and P. Devreotes. 2002. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109:599-610. [DOI] [PubMed] [Google Scholar]
- 19.Irvin, B. J., B. L. Williams, A. E. Nilson, H. O. Maynor, and R. T. Abraham. 2000. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20:9149-9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishiai, M., H. Sugawara, M. Kurosaki, and T. Kurosaki. 1999. Association of phospholipase C-γ2 Src homology 2 domains with BLNK is critical for B cell antigen receptor signaling. J. Immunol. 163:1746-1749. [PubMed] [Google Scholar]
- 21.Janssen, E., M. Zhu, W. Zhang, S. Koonpaew, and W. Zhang. 2003. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 4:117-123. [DOI] [PubMed] [Google Scholar]
- 22.Ji, Q. S., G. E. Winnier, K. D. Niswender, D. Horstman, R. Wisdom, M. A. Magnuson, and G. Carpenter. 1997. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development. Proc. Natl. Acad. Sci. USA 94:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan, M. S., A. L. Singer, and G. A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110-116. [DOI] [PubMed] [Google Scholar]
- 24.Jou, S.-T., N. Carpino, Y. Takahashi, R. Piekorz, J.-R. Chao, N. Carpino, D. Wang, and J. N. Ihle. 2002. Essential, nonredundant role for the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22:8580-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, S. W., M. I. Wahl, J. Chu, J. Kitaura, Y. Kawakami, R. M. Kato, R. Tabuchi, A. Tarakhovsky, T. Kawakami, C. W. Turck, O. N. Witte, and D. J. Rawlings. 2001. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20:5692-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein, G., B. Giovanella, A. Westman, J. S. Stehlin, and D. Mumford. 1975. An EBV-genome-negative cell line established from an American Burkitt lymphoma: receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology 5:319-334. [DOI] [PubMed] [Google Scholar]
- 27.Kurosaki, T. 1997. Molecular mechanisms in B cell antigen receptor signaling. Curr. Opin. Immunol. 9:309-318. [DOI] [PubMed] [Google Scholar]
- 28.Kurosaki, T., and S. Tsukada. 2000. BLNK: connecting Syk and Btk to calcium signals. Immunity 12:1-5. [DOI] [PubMed] [Google Scholar]
- 29.Liao, F., H. S. Shin, and S. G. Rhee. 1993. In vitro tyrosine phosphorylation of PLC-γ1 and PLC-γ2 by src-family protein tyrosine kinases. Biochem. Biophys. Res. Commun. 191:1028-1033. [DOI] [PubMed] [Google Scholar]
- 30.Mahajan, S., S. Ghosh, E. A. Sudbeck, Y. Zheng, S. Downs, M. Hupke, and F. M. Uckun. 1999. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [α-cyano-β-hydroxy-β-methyl-N-(2, 5-dibromophenyl)propenamide]. J. Biol. Chem. 274:9587-9599. [DOI] [PubMed] [Google Scholar]
- 31.Noh, D. Y., S. H. Shin, and S. G. Rhee. 1995. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim. Biophys. Acta 1242:99-113. [DOI] [PubMed] [Google Scholar]
- 32.Okkenhaug, K., and B. Vanhaesebroeck. 2003. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 3:317-330. [DOI] [PubMed] [Google Scholar]
- 33.Patterson, R. L., D. B. van Rossum, D. L. Ford, K. J. Hurt, S. S. Bae, P.-G. Suh, T. Kurosaki, S. H. Snyder, and D. L. Gill. 2002. Phospholipase C-γ is required for agonist-induced Ca2+ entry. Cell 111:529-541. [DOI] [PubMed] [Google Scholar]
- 34.Rhee, S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez, R., M. Matsuda, O. Perisic, J. Bravo, A. Paul, N. P. Jones, Y. Light, K. Swann, R. L. Williams, and M. Katan. 2001. Tyrosine residues in phospholipase C γ2 essential for the enzyme function in B-cell signaling. J. Biol. Chem. 276:47982-47992. [DOI] [PubMed] [Google Scholar]
- 36.Samelson, L. E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371-394. [DOI] [PubMed] [Google Scholar]
- 37.Scharenberg, A. M., O. El-Hillal, D. A. Fruman, L. O. Beitz, Z. Li, S. Lin, I. Gout, L. C. Cantley, D. J. Rawlings, and J. P. Kinet. 1998. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 17:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharenberg, A. M., and J. P. Kinet. 1998. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell 94:5-8. [DOI] [PubMed] [Google Scholar]
- 39.Sekiya, F., B. Poulin, Y. J. Kim, and S. G. Rhee. 2004. Mechanism of tyrosine phosphorylation and activation of phospholipase C-γ1: tyrosine-783 phosphorylation is not sufficient for lipase activation. J. Biol. Chem. 279:32181-32190. [DOI] [PubMed] [Google Scholar]
- 40.Smith, M., Y. Liu, N. Matthews, S. G. Rhee, W. Sung, and H. Kung. 1994. Phospholipase C-γ1 can induce DNA synthesis by a mechanism independent of its lipase activity. Proc. Natl. Acad. Sci. USA 91:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh, P. G., S. H. Ryu, W. C. Choi, K. Y. Lee, and S. G. Rhee. 1988. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J. Biol. Chem. 263:14497-14504. [PubMed] [Google Scholar]
- 42.Sun, T., M. Campbell, W. Gordon, and R. B. Arlinghaus. 2001. Preparation and application of antibodies to phosphoamino acid sequences. Biopolymers 60:61-75. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, H., S. Matsuda, Y. Terauchi, M. Fujiwara, T. Ohteki, T. Asano, T. W. Behrens, T. Kouro, K. Takatsu, T. Kadowaki, and S. Koyasu. 2003. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat. Immunol. 4:280-286. [DOI] [PubMed] [Google Scholar]
- 44.Takata, M., and T. Kurosaki. 1996. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med. 184:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takata, M., H. Sabe, A. Hata, T. Inazu, Y. Homma, T. Nukada, H. Yamamura, and T. Kurosaki. 1994. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 13:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takesono, A., L. D. Finkelstein, and P. L. Schwartzberg. 2002. Beyond calcium: new signaling pathways for Tec family kinases. J. Cell Sci. 115:3039-3048. [DOI] [PubMed] [Google Scholar]
- 47.Tuveson, D. A., R. H. Carter, S. P. Soltoff, and D. T. Fearon. 1993. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science 260:986-989. [DOI] [PubMed] [Google Scholar]
- 48.Wang, D., J. Feng, R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase C γ2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe, D., S. Hashimoto, M. Ishiai, M. Matsushita, Y. Baba, T. Kishimoto, T. Kurosaki, and S. Tsukada. 2001. Four tyrosine residues in phospholipase C-γ2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J. Biol. Chem. 276:38595-38601. [DOI] [PubMed] [Google Scholar]
- 50.Wilde, J. I., and S. P. Watson. 2001. Regulation of phospholipase C γ isoforms in haematopoietic cells: why one, not the other? Cell. Signal. 13:691-701. [DOI] [PubMed] [Google Scholar]
- 51.Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. Mitchison, and H. R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114:201-214. [DOI] [PubMed] [Google Scholar]
- 52.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-γ1 (PLC-γ1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-γ1 and NFAT. Mol. Cell. Biol. 21:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]