Abstract
The CCAAT/enhancer binding proteins C/EBPα and C/EBPβ are related transcription factors that are important for the function of various organs in the postnatal mouse. Gene replacement and tissue culture experiments have suggested partial redundancy of both transcription factors. Here we show that mouse embryos deficient of both C/EBPα and C/EBPβ (C/EBPαβ−/−) die between embryonic day 10 (E10) and E11 and display defective placentas. In situ hybridization revealed that C/EBPα and C/EBPβ are coexpressed in the chorionic plate at E9.5 and later in the trophoblasts of the labyrinthine layer. In C/EBPαβ−/− placentas, allantoic blood vessels invaded the chorion; however, vessel expansion and development of the labyrinthine layer was impaired. Furthermore, a single copy of either C/EBPα in the absence of C/EBPβ or C/EBPβ in the absence of C/EBPα is sufficient to complete development, suggesting complementation of these C/EBPs during embryogenesis. A single copy of C/EBPα in the absence of C/EBPβ, however, fails to rescue survival after birth, suggesting haploinsufficiency of C/EBPα in newborns. Our data thus reveal novel essential, redundant, and dosage dependent functions of C/EBPs.
The family of CCAAT/enhancer binding proteins (C/EBPs) entails gene regulatory proteins that control proliferation, differentiation, and apoptosis in various cell types. Gene targeting studies in mice demonstrated the importance of C/EBP proteins in the development and function of hematopoietic cells, keratinocytes, hepatocytes, and intestinal and mammary epithelial cells, as well as in the development of the ovary and the lung (31).
The four most related members of the C/EBP family (α, β, δ, and ɛ) display extensive homology in their carboxyl-terminal basic and leucine zipper domains, which mediate DNA binding and dimerization, respectively. Toward the N terminus, C/EBPs are more divergent and homology is restricted to distinct peptide motifs that are involved in transactivation or regulation of the activity of C/EBPs (17, 19, 46). Although data obtained from mouse molecular genetics and expression profiling studies suggest that the functions of C/EBPα and C/EBPβ are rather distinct, it has also been shown that C/EBPβ can partially replace C/EBPα functions when expressed from the C/EBPα locus (8, 16). It is not clear, however, to what extent regulation of C/EBPα and C/EBPβ gene expression or structural differences of the transcription factors account for redundant or divergent functions. Tissue specificity also appears to result from combinatorial interactions with unrelated transcription factors, such as c-Myb, to specify hematopoietic gene regulation (25, 27), or peroxisome proliferator activated receptor γ (PPARγ) to specify adipogenic gene regulation (13, 34). Accordingly, an important issue in understanding C/EBP biology and physiology is to determine the degree of functional redundancy or specificity of individual C/EBPs.
Here we report novel sites of expression of C/EBPα and C/EBPβ during mouse embryogenesis. We show that at embryonic day 9.5 (E9.5) C/EBPβ is expressed in the ectoplacental cone and in the chorionic plate, whereas C/EBPα expression is restricted to the chorionic plate. At E10.5 C/EBPα is coexpressed with C/EBPβ in the labyrinthine layer of the chorioallantoic placenta. C/EBPβ is found in all of the trophoblast cell lineage of the placenta. Deletion of all C/EBPα and -β alleles (C/EBPαβ−/−) results in embryonic developmental arrest at around E10 to E11 that is associated with a gross failure of the placenta to undergo proper morphogenesis. We also show that a single copy of either C/EBPα in the absence of C/EBPβ, or C/EBPβ in the absence of C/EBPα could rescue embryogenesis. Our data suggest essential and redundant functions of both transcription factors during early embryonic development. Furthermore, C/EBPα is haploinsufficient for survival of the newborns after birth in the absence of C/EBPβ.
MATERIALS AND METHODS
Generation of C/EBPαβ−/− embryos.
The C/EBPα and C/EBPβ knockout strains were obtained from Gretchen Darlington (Baylor College of Medicine, Houston, Tex.) and Esta Sterneck (NCI, Frederick, Md.), respectively, and have been described elsewhere (42, 43). Since the C/EBPβ−/− females are sterile and the C/EBPα−/− newborns die of hypoglycemia 8 h after birth (18, 42, 43), double C/EBP heterozygous mice (C/EBPαβ+/−) were generated and intercrossed to obtain double knockout C/EBPαβ−/− embryos. Genotyping was routinely performed by PCR analysis with two upper primers located either in the pGK promoter of the neomycin cassette (5′-ACGAGACTAGTGAGACGTGCTAC-3′) or in the 3′-untranslated region for C/EBPα (5′-GGAGGAAGATACAGGAAGTGAGAT-3′) or in the coding region for C/EBPβ (5′-GCTTCGAACCCGCGGACTGCAA-3′) and a common lower primer located in the 3′-untranslated region of the gene of interest (C/EBPα, 5′-TGGTTTAGCATAGACGTGCACACT-3′; C/EBPβ, 5′-CATCTTTAAGGTGATTACTCAGGGC-3′). DNA was obtained from tail biopsies or, in the case of embryos, from yolk sacs. PCR was performed in 50-μl reaction mixtures. The cycling conditions consisted of an initial 5-min denaturating step at 95°C, followed by 40 cycles of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C. For C/EBPα, reaction products of 542 and 305 bp represent wild-type and mutant alleles, respectively. For C/EBPβ, reaction products of 771 and 530 bp represent wild-type and mutant alleles, respectively.
Histology, isolectin B4 staining, and in situ hybridization.
Entire conceptuses and placentas were isolated from E9.5 to E14.5 from staged pregnancies. The plug day was designated day 0.5, although the embryos were staged according to standard morphological landmarks.
For histological analyses, placentas were fixed overnight at 4°C in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections of 8 μm were stained with eosin and hematoxylin (H&E) according to the standard procedure. Placenta sections were stained with isolectin B4 as described elsewhere (28).
Whole-mount in situ hybridization on Vibratome slices of the placenta (70 μm) was performed as described previously (37) with antisense RNA probes for C/EBPα (corresponding to the fragment from nucleotides 1451 to 2099 of mouse genomic DNA), C/EBPβ (40), C/EBPδ (41), distal-less 3 gene (Dlx3) (24), fms-like tyrosine kinase receptor 1 (Flt-1) (23), glial cell missing 1 transcription factor (Gcm1) (3), placental lactogen-1 (PL-1) (9), PPARγ (5), and the vascular endothelial growth factor (VEGF) (44).
RESULTS
Embryonic lethality of mice lacking both C/EBPα and C/EBPβ.
Genotype analysis (from E9.5 to birth) of 822 progeny from intercrosses of C/EBPαβ+/− yielded the expected Mendelian ratios of all genotypes at E10 (Table 1) . As expected, loss of both copies of C/EBPα resulted in death shortly after birth (22, 43). Interestingly, loss of one copy of C/EBPα in a C/EBPβ−/− background also resulted in death of the newborns few hours after birth, whereas C/EBPβ−/− animals that retain both copies of C/EBPα were born alive and survived as long as the wild-type mice. This result shows that, in the absence of C/EBPβ, C/EBPα is haploinsufficient for survival of the newborns after birth.
TABLE 1.
Embryonic lethality of C/EBP αβ−/− micea
| Litter
|
Mendelian ratio (%) obtained with genotype (expected ratio)a:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryonic stage | No. of animals | No. of embryos | +/+ (6.25) | α+/− (12.5) | β+/− (12.5) | α+/− β+/− (25) | α−/− (6.25) | β−/− (6.25) | α−/− β−/− (6.25) | α−/− β+/− (12.5) | β−/− α+/− (12.5) |
| E9.5 to E10.25 | 20 | 154 | 8.44 | 11.69 | 11.69 | 26.62 | 5.84 | 6.49 | 6.49 | 10.39 | 12.34 |
| E10.25 to E11 | 16 | 132 | 9.09 | 15.91 | 16.67 | 16.67 | 6.06 | 6.82 | 4.55 (2)* | 9.85 | 14.39 |
| E11.5 | 13 | 71 | 8.45 | 11.27 | 22.54 | 29.58 | 7.04 | 7.04 | 0 (3)* | 5.63 | 8.45 |
| E12.5 | 18 | 136 | 8.82 | 11.76 | 12.5 | 26.47 | 5.15 | 5.88 | 2.21 (1)* | 14.71 | 12.5 |
| E13.5 | 10 | 69 | 2.9 | 21.74 | 15.94 | 28.99 | 2.9 | 5.8 | 0 (1)* | 10.14 | 11.59 |
| E14.5b | 2 | 13 | 0 | 15.38 | 15.38 | 38.46 | 0 | 7.69 | 0 | 7.69 | 15.38 |
| At birth | 49 | 247 | 8.91 | 15.4 | 21.9 | 45.3 | 0 | 7.29 | 0 | 0 | 1.21c |
The different genotypes obtained from intercrosses of C/EBPαβ+/− mice are indicated in the column headings. The expected Mendelian ratios are given in parentheses in the column heading. Only live embryos were included in the statistics; the numbers in parentheses (*) indicate the numbers of dead C/EBPαβ−/− embryos. The stage from E9.5 to E10.25 includes embryos with 20 to 30 somites, and the stage from E10.25 to E11 includes embryos with 30 to 38 somites. The number of resorbed embryos increased from E11.5 onward, but DNA was not recovered for genotyping.
The given values at E14.5 are not statistically significant.
Three newborns died a few days after birth, and the remains were not available for analysis.
At E9.5 to E10.25, C/EBPαβ−/− embryos were recovered at the expected Mendelian ratio of ca. 6%. The embryos were viable and externally indistinguishable from their littermates in both size and gross morphology. At E10.25 to E11, C/EBPαβ−/− embryos were recovered at a lower frequency (4,55%). Few C/EBPαβ−/− embryos (3 of 136 [2%]) survived until E12.5. No live C/EBPαβ−/− embryos, however, were found at E13.5 or beyond (Table 1). These data show that a single copy of either C/EBPα in the absence of C/EBPβ or C/EBPβ in the absence of C/EBPα rescues early lethality that occurs around E10 to E11 in the absence of both factors, suggesting redundant functions of C/EBPα and C/EBPβ in early embryo survival.
Expression of C/EBPα and C/EBPβ in early placental development.
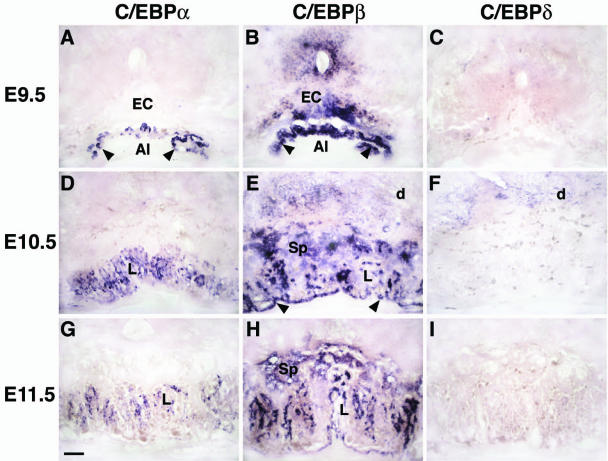
The possibility that C/EBPα and C/EBPβ are important in early placental development (E10 to E11) was unanticipated, since previous reports failed to detect expression of these genes in embryonic tissues earlier than E13.5 (6, 7, 20, 47). However, placental abnormalities were clearly evident in C/EBPαβ−/− progeny at E10. Accordingly, we reinvestigated placental C/EBP expression by in situ hybridization. At E9.5, C/EBPα mRNA is expressed in the chorionic plate (Fig. 1A), whereas C/EBPβ mRNA is present not only in the chorionic plate but also in the ectoplacental cone (Fig. 1B). At E10.5, C/EBPα and C/EBPβ mRNA are coexpressed in the trophoblasts of the labyrinthine layer (Fig. 1D and E). In addition, C/EBPβ mRNA is found in the trophoblast cells of the three layers of the placenta (Fig. 1E). No C/EBPδ mRNA expression has been found in placental cells from E9.5 to E11.5 (Fig. 1C, F, and I), whereas C/EBPα and C/EBPβ mRNA are present in mouse placenta at least until E11.5 (Fig. 1G and H). Notably, neither C/EBPα nor C/EBPβ mRNA are expressed in the fetal endothelium permeating the presumptive labyrinth (Fig. 1A, B, D, E, G, and H) and in the yolk sac (data not shown).
FIG. 1.
Expression of C/EBPα, C/EBPβ, and C/EBPδ in wild-type placenta at E9.5 (A to C), E10.5 (D to F) and E11.5 (G to I). (A to C) C/EBPα expression is restricted to the chorionic plate (arrowheads) (A), whereas C/EBPβ is strongly expressed in the ectoplacental cone (EC) and the chorionic plate (arrowheads) (B). In panels D to I, C/EBPα and C/EBPβ are coexpressed in the labyrinthine layer (L). C/EBPβ mRNA is also found in spongiotrophoblasts (Sp). Note the absence of C/EBPδ expression in the placenta at all stages (C, F, and I). Al, allantoic cavity; d, maternal decidua. Scale bar, 10 μm.
Abnormal formation of the labyrinthine layer in C/EBPαβ−/− placenta.
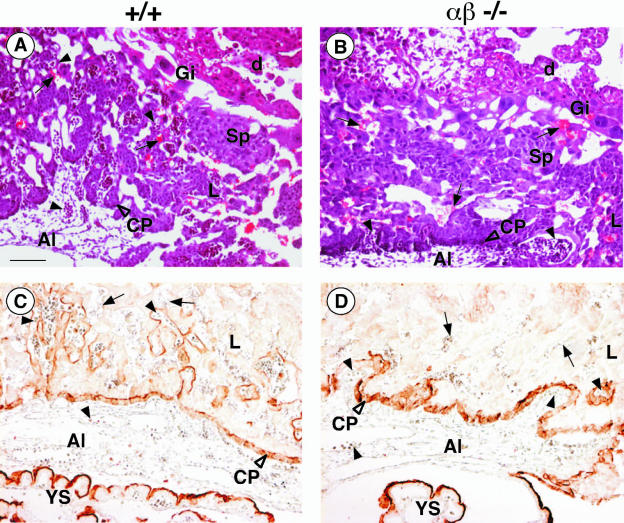
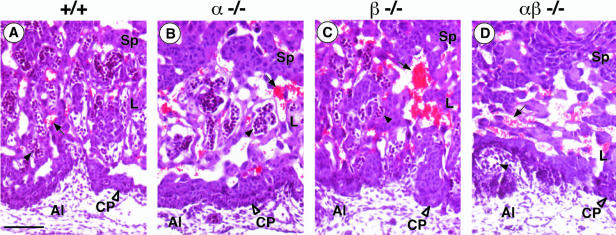
At E10, the placentas of wild-type, C/EBPα−/−, and C/EBPβ−/− embryos exhibit the characteristic three-layered appearance with the labyrinthine layer as the inner layer, the spongiotrophoblast in the middle, and the trophoblast giant cells forming the outer layer that interfaces with the maternal decidua (see Fig. 3A and data not shown) (35). In C/EBPαβ−/− placentas, the spongiotrophoblast layer and the giant cells appeared normal. The labyrinthine layer, however, was drastically reduced in thickness (compare Fig. 2D with 2A to C). The chorionic trophoblasts in C/EBPαβ−/− were found densely packed at the base of the placentas (Fig. 2D and 3B), whereas chorionic trophoblasts in age-matched wild-type and single-knockout placentas had already started to spread inward and participate in the formation of the labyrinthine layer, together with the angiogenic allantoic mesenchyme (Fig. 2A to C and 3A). In the C/EBPαβ−/−, fetal blood vessels, identified by luminal nucleated embryonic blood cells, were detected either outside of the placenta or (when in the placenta) had formed exclusively at the base where the surrounding trophoblast tissue was very compact (Fig. 2D and 3B). In contrast, in wild-type and single-knockout placentas an intricate network of fetal blood vessels had developed in juxtaposition to maternal blood sinuses (compare Fig. 2A to C and compare 3A to 2D and 3B). The elongated differentiated trophoblasts that form the hemotrichorial barrier around the fetal blood vessels were undetectable in the C/EBPαβ−/− placentas (compare Fig. 2A to C and D). The tight apposition of fetal blood vessels and maternal blood sinuses seen in the normal placenta is, however, a key prerequisite for the function of the labyrinth in the exchange of gas and nutrients between fetal and maternal blood.
FIG. 3.
Placental abnormalities in C/EBPαβ−/− conceptus at E10. (A and B) H&E-stained sagittal sections of +/+ (A) and C/EBPαβ−/− (B) placentas. Giant cells (Gi) are indistinguishable between C/EBPαβ−/− and wild-type placentas. Fetal blood vessels (arrowhead) were rarely found in the labyrinthine layer (L) of the C/EBPαβ−/− placenta compared to the wild-type placenta. Maternal blood sinuses (arrow) were present in normal numbers but were never close to embryonal vessels as observed for the wild type. (C and D) Isolectin B4-stained sagittal sections of +/+ (C) and C/EBPαβ−/− (D) placentas at E10. Isolectin B4 labeled the matrix surrounding the fetal blood vessels. Note the poor labeling of the labyrinthine layer (L) in C/EBPαβ−/− placentas compared to the wild-type placentas. The staining of the yolk sac (YS) and of the chorionic plate (CP) were indistinguishable between C/EBPαβ−/− and wild-type placentas. Al, allantoic cavity; d, maternal decidua; Sp, spongiotrophoblast; arrow, maternal erythrocyte sinus. Scale bar, 10 μm.
FIG. 2.
Histological analysis of controls and C/EBPαβ−/− placentas at E10. H&E-stained sagittal sections of the labyrinthine layer of +/+ (A), C/EBPα−/− (B), C/EBPβ−/− (C), and C/EBPαβ−/− (D) placentas. In panel D, note the abnormal thickening of the C/EBPαβ−/− chorionic plate (CP) and poor impregnation of the C/EBPαβ−/− presumptive labyrinth (L) by fetal vessels (arrowhead, containing blue-stained nucleated fetal erythrocytes). Al, allantoic cavity; arrow, maternal erythrocyte sinus; Sp, spongiotrophoblast; L, labyrinthine layer. Scale bar, 10 μm.
Vascular abnormalities in C/EBPαβ−/− concepti.
All C/EBPαβ−/− placentas that were analyzed at E10 were associated with live embryos, as indicated by beating hearts. Their yolk sac appeared histologically normal and well vascularized. No obvious vascularization defects were observed in these embryos. The formation of their vitellin vessels and their major arteries was similar to those of wild-type animals (data not shown). However, in the labyrinthine layer of C/EBPαβ−/− placentas, loss of isolectin B4 activity, a marker for the matrix surrounding the fetal vessels, was observed (Fig. 3D), whereas wild-type placentas showed strong staining in their labyrinthine layer (Fig. 3C). The chorionic plate and the yolk sac were similarly stained by isolectin B4 in both genotypes (Fig. 3C and D). Taken together, these data suggest that the vascularization of the labyrinthine layer of the placenta was impaired in C/EBPαβ−/− conceptuses.
Analysis of marker gene expression in C/EBPαβ−/− placenta.
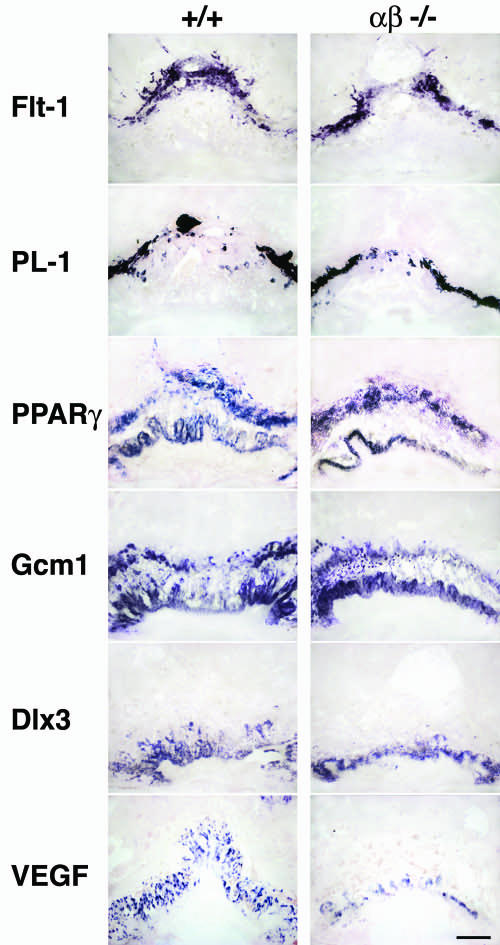
For a more detailed characterization of the placental defect, the expression of several trophoblast markers in C/EBPαβ−/− and control placentas was compared. Since the expression patterns of trophoblast marker mRNAs in wild-type, C/EBPα−/−/β+/−, and C/EBPα+/−/β−/− placentas were similar (data not shown), results obtained with C/EBPαβ−/− placentas are compared only to wild-type placentas in Fig. 4. In situ hybridization of placental sections of C/EBPαβ−/− at E10 showed no difference in the expression of the spongiotrophoblast marker Flt-1 (a receptor tyrosine kinase for VEGF and placental growth factor [PlGF]) (12, 23) and of the trophoblast giant cell marker PL-1 (9) compared to the wild-type placenta. Alkaline phosphatase activity in secondary giant cells and in the chorionic plate was identical in wild-type and C/EBPαβ−/− placentas (data not shown). Thus, although the morphology was highly abnormal in C/EBPαβ−/− placentas, cytodifferentiation of giant cell and spongiotrophoblast placental cell types and expression of their specific markers still occurred. Gcm1, a marker for the branching initiation of the chorioallantoic placenta (3, 37), was slightly less expressed in C/EBPαβ−/− placentas, probably because of the dramatic reduction in size of the labyrinthine layer. However, the presence of Gcm1 indicates that the branching initiation of the morphogenesis could still occur.
FIG. 4.
Expression of cell-type-specific markers analyzed by in situ hybridization of wild-type (+/+) and C/EBPαβ−/− placentas at E10. Sagittal sections (70 μm) of paraformaldehyde-fixed placentas were hybridized with antisense probes for Flt-1 (spongiotrophoblast cells); PL-1 (trophoblast giant cells); PPARγ (diploid trophoblasts); and Gcm1, Dlx3, and VEGF (labyrinthine layer). Note that the expression of Gcm1, Dlx3, and VEGF is lower in C/EBPαβ−/− placentas than in +/+ placentas. Scale bar, 15 μm.
C/EBP proteins collaborate with or regulate the expression of transcription factors which have been shown to be essential for mouse placental development such as PPARγ and Dlx3 (5, 24). In addition, C/EBP proteins are implicated in the regulation of angiogenic factors such as VEGF (29). We therefore examined the expression of Dlx3, PPARγ, and VEGF as potential downstream C/EBP target genes in placental development. As shown in Fig. 4, at E10 PPARγ mRNA expression is similar in all layers of C/EBPαβ−/− and wild-type placentas, except in the labyrinthine layer of C/EBPαβ−/− placentas due to its reduction or absence (compare the labyrinthine layer of C/EBPαβ−/− placentas to the wild-type placentas). Dlx3 and VEGF expression is restricted to the chorionic plate in C/EBPαβ−/− placentas, whereas both are expressed in the labyrinthine layer of wild-type placentas (Fig. 4). VEGF production by trophoblast giant cells is not affected in C/EBPαβ−/− placentas compared to wild-type placentas (data not shown). These data indicate that the structural abnormalities observed in C/EBPαβ−/− placentas are associated with altered expression of specific markers of the labyrinthine layer. These results also show that C/EBPα and C/EBPβ are involved in placental development either independently of or downstream of PPARγ.
DISCUSSION
Analyses of C/EBPαβ−/− conceptuses reveal a novel function of C/EBPα and C/EBPβ transcription factors in mouse placental development. C/EBPαβ−/− embryos die at ca. E10 to E11 when the placenta starts functioning as the site of gas and nutrient exchange between fetal and maternal blood. In situ hybridization data showed that the spatial and temporal distribution of C/EBPα and C/EBPβ mRNA supports a functional role of both transcription factors in placenta. They are coexpressed at E9.5 in the chorionic plate and at E10.5 in the labyrinthine trophoblast cells. The placenta-restricted expression of C/EBPα and C/EBPβ and the early lethal effect resulting from the absence of both factors in C/EBPαβ−/− embryos strongly suggests an essential role of C/EBPs in placentogenesis.
Lethality during the postimplantation period can be the result of either defects in yolk sac or chorioallantoic placentas (10). Because C/EBPα and C/EBPβ are not expressed in the yolk sac (data not shown) and C/EBPαβ−/− yolk sacs are histologically normal and because mouse mutants displaying similar phenotypes die at ca. E10.5 from placental deficit (15), our data suggest that the lethality of C/EBPαβ−/− embryos is probably due to placental defects.
Evidence for redundant functions of C/EBPα and C/EBPβ in the liver and in hematopoiesis was previously reported in studies using a gene replacement strategy (8, 16). Our data confirm and extend these observations by showing that, in the absence of C/EBPβ, C/EBPα is haplosufficient for placentogenesis but haploinsufficient for survival of the newborns after birth. Furthermore, in the absence of C/EBPα, C/EBPβ is haplosufficient for placentogenesis.
The epithelial compartment of the placenta is derived from the trophoblast cell lineage, whereas the stromal and vascular compartments are derived from extraembryonic mesoderm, specifically the allantoic mesoderm in the mouse. We show that C/EBPβ mRNA is found in the three trophoblast cell types—giant cells, spongiotrophoblasts, and trophoblasts of the labyrinthine layer—and that C/EBPα is found in trophoblasts of the labyrinthine layer. In addition, their mRNAs were also expressed in the trophoblast stem cells of the chorionic plate and in the trophoblast stem cell line (TS) kept under undifferentiated conditions (data not shown). In humans, C/EBPα and C/EBPβ are also localized in the trophoblast cells during pregnancy (4). Thus, these data strongly suggest a role of C/EBPα and C/EBPβ in trophoblast cell lineage. The absence of both C/EBPα and C/EBPβ in early embryogenesis results in a defective placental development. Histological analyses of C/EBPαβ−/− placentas show the absence of extensive villous branching that creates the labyrinth. This abnormal phenotype was completely penetrant, since all of the E10 C/EBPαβ−/− placentas examined, but none of the wild-type and other genotype controls, exhibited the same cellular anomalies. In C/EBPαβ−/− placentas, a defect in trophoblast cell growth and maturation could account for the defective labyrinthine layer formation. C/EBPα and C/EBPβ have already been implicated in branching morphogenesis of mammary epithelial cells (32, 39). It is tempting to propose a similar role for C/EBPα and C/EBPβ in the branching morphogenesis of the placenta. Gcm1, the marker for branching morphogenesis initiation (3, 37), is still detectable in C/EBPαβ−/− placentas, indicating that C/EBPα and C/EBPβ are not required for the initiation of branching morphogenesis but probably for its progression. Branching morphogenesis and progression (also called chorioallantoic morphogenesis) are prerequisites for proper vascularization of the placenta (1, 11). Thus, the absence of vascularization of the labyrinth of the C/EBPαβ−/− placentas, visualized by the lack of isolectin B4 activity, is most likely a consequence of the block in the progression of chorioallantoic morphogenesis. This hypothesis is supported by the observation that all other vascular processes in the C/EBPαβ−/− embryos appear to be normal, including the establishment of yolk sac circulation, the formation of vitellin vessels and major arteries, and chorioallantoic fusion. Therefore, placental vascularization appears to be dependent on both C/EBPα- and C/EBPβ-mediated trophoblast functions.
Since mice deficient in C/EBPα, C/EBPβ, C/EBPδ, C/EBPɛ, and the compound C/EBPβδ survive at least until birth (see reference 31 and references therein) and since C/EBPδ was absent in the placental tissue at the studied stages, our data also suggest that C/EBPα and C/EBPβ are the major C/EBPs in placental morphogenesis, in particular in the formation of the labyrinthine layer. Moreover, placental morphogenesis occurred normally in mice that retained one allele of either C/EBPα in the absence of C/EBPβ, or C/EBPβ in the absence of C/EBPα, but not after removal of both transcription factors. These results clearly show that C/EBPs are indispensable during embryogenesis and that C/EBPα and C/EBPβ are redundant during placental development. How C/EBPα and C/EBPβ repress or activate gene expression in placentogenesis still remains to be determined. Our data imply that C/EBPα or C/EBPβ homo- or heterodimers are required or, alternatively, that C/EBPα or C/EBPβ are equivalent components of other gene regulatory protein complexes during placentogenesis. AP-1 family members JunB or FRA1 are potential candidates since they may heterodimerize with C/EBP proteins (26) and they are required for proper placental morphogenesis (36, 38).
Several other transcription factors have been found to be critical for the development of the placental labyrinthine layer after initiation of morphogenesis (11, 35). Among these transcription factors, the nuclear hormone receptor PPARγ was an interesting potential C/EBP target because it is activated by C/EBPα and/or C/EBPβ at the onset of adipogenic differentiation (33, 34). In addition, PPARγ, C/EBPα, and C/EBPβ are coexpressed in the placenta. The expression level of PPARγ, however, was found to be similar in both C/EBPαβ−/− and control placentas, indicating that activation of PPARγ does not rely on C/EBP functions during early placentogenesis. This possibility is supported by the fact that PPARγ1 is the only isoform expressed in the placenta, whereas PPARγ2, initiated from an alternative promoter, is the effector of C/EBPs in adipogenesis (5).
Recent studies suggested that C/EBPα and C/EBPβ regulate basal Dlx3 promoter activity in placental cells (14). Moreover, Dlx3-deficient embryos die around E10 due to a defect in labyrinthine layer development (24). The data presented here, however, show that Dlx3 is still expressed in C/EBPαβ−/− placentas but only in the chorionic plate. Consistently, all of the markers used to label the labyrinthine layer (Gcm1, Dlx3, and VEGF) showed reduced expression that is most likely a secondary effect of defective formation of the labyrinthine layer.
The molecular mechanism underlying the prevention of placental labyrinth formation in C/EBPαβ−/− mice await further studies. In this context, it is important to note that C/EBPβ has been identified as an important target of cyclin D1 in many human tumors (21), suggesting a proliferation-inducing function in addition to the differentiation-inducing activity of C/EBPs. Moreover, it has been shown that C/EBPα and C/EBPβ regulate the production of growth factors and cytokines that sustain proliferation (2, 30, 45). The placenta is a major source of growth factors, cytokines, and hormones that affect both the mother and the fetus. We therefore speculate that the absence of both C/EBPα and C/EBPβ might have several additional effects on embryogenesis once the placental dysfunction could be rescued. However, the low frequency of double-knockout generation (ca. 6%) currently precludes the efficient analysis of chimeric embryos that would be obtained by aggregating C/EBPαβ−/− embryonic stem cells derived from C/EBPαβ+/− intercrosses with wild-type tetraploid embryos (35). Thus, the question of molecular mechanisms and additional embryonic C/EBP functions will therefore have to await the generation of conditional knockout mice.
In conclusion, we report here the earliest developmental role of C/EBPs in the mouse. A concerted function of C/EBPα and C/EBPβ occurs at postimplantation stages during the generation of a functional chorioallantoic placenta. The death of C/EBPαβ−/− embryos is most likely due to the failure to establish a proper placental circulation. Recently, it has been reported that C/EBPα, C/EBPβ, and C/EBPδ are expressed in human placenta during pregnancy (4). Therefore, it would be important to determine whether dysregulation of C/EBP gene expression correlates with a pathological phenotype in human placentogenesis.
Acknowledgments
We are indebted to E. Sterneck (NCI, Frederick, Md.) for generously providing C/EBPβ+/− mice and G. Darlington (Baylor College of Medicine, Houston, Tex.) for generously providing C/EBPα+/− mice. We thank T. Müller and C. Birchmeier (Max Delbrueck Center for Molecular Medicine, Berlin, Germany) for valuable discussions and comments on the manuscript. We thank the members of Leutz lab for discussions. We are also grateful to E. Sterneck, U. Borgmeyer, D. Riethmacher, M. Soares, R. Evans, and E. E. Voest for providing cDNA probes and to J. Rossant for providing the TS cell line that served as a valuable control during the preparation of the manuscript.
REFERENCES
- 1.Adamson, S. L., Y. Lu, K. J. Whiteley, D. Holmyard, M. Hemberger, C. Pfarrer, and J. C. Cross. 2002. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250:358-373. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., A. Leutz, and T. Graf. 1984. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell 39:439-445. [DOI] [PubMed] [Google Scholar]
- 3.Anson-Cartwright, L., K. Dawson, D. Holmyard, S. J. Fisher, R. A. Lazzarini, and J. C. Cross. 2000. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 25:311-314. [DOI] [PubMed] [Google Scholar]
- 4.Bamberger, A. M., A. Makrigiannakis, M. Schroder, C. M. Bamberger, C. Relakis, B. Gellersen, K. Milde-Langosch, and T. Loning. 2004. Expression pattern of the CCAAT/enhancer-binding proteins C/EBP-α, C/EBP-β, and C/EBP-δ in the human placenta. Virchows Arch. 444:149-152. [DOI] [PubMed] [Google Scholar]
- 5.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 6.Birkenmeier, E. H., B. Gwynn, S. Howard, J. Jerry, J. I. Gordon, W. H. Landschulz, and S. L. McKnight. 1989. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 3:1146-1156. [DOI] [PubMed] [Google Scholar]
- 7.Cao, Z., R. M. Umek, and S. L. McKnight. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5:1538-1552. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S. S., J. F. Chen, P. F. Johnson, V. Muppala, and Y. H. Lee. 2000. C/EBPβ, when expressed from the C/ebpα gene locus, can functionally replace C/EBPα in liver but not in adipose tissue. Mol. Cell. Biol. 20:7292-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colosi, P., F. Talamantes, and D. I. Linzer. 1987. Molecular cloning and expression of mouse placental lactogen I complementary deoxyribonucleic acid. Mol. Endocrinol. 1:767-776. [DOI] [PubMed] [Google Scholar]
- 10.Copp, A. J. 1995. Death before birth: clues from gene knockouts and mutations. Trends Genet. 11:87-93. [DOI] [PubMed] [Google Scholar]
- 11.Cross, J. C., D. G. Simmons, and E. D. Watson. 2003. Chorioallantoic morphogenesis and formation of the placental villous tree. Ann. N. Y. Acad. Sci. 995:84-93. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara, N. 2001. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 280:C1358-C1366. [DOI] [PubMed] [Google Scholar]
- 13.Freytag, S. O., D. L. Paielli, and J. D. Gilbert. 1994. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 8:1654-1663. [DOI] [PubMed] [Google Scholar]
- 14.Holland, M. P., S. P. Bliss, K. A. Berghorn, and M. S. Roberson. 2004. A role for CCAAT/enhancer-binding protein β in the basal regulation of the distal-less 3 gene promoter in placental cells. Endocrinology 145:1096-1105. [DOI] [PubMed] [Google Scholar]
- 15.Ihle, J. N. 2000. The challenges of translating knockout phenotypes into gene function. Cell 102:131-134. [DOI] [PubMed] [Google Scholar]
- 16.Jones, L. C., M. L. Lin, S. S. Chen, U. Krug, W. K. Hofmann, S. Lee, Y. H. Lee, and H. P. Koeffler. 2002. Expression of C/EBPβ from the C/ebpα gene locus is sufficient for normal hematopoiesis in vivo. Blood 99:2032-2036. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J., C. A. Cantwell, P. F. Johnson, C. M. Pfarr, and S. C. Williams. 2002. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J. Biol. Chem. 277:38037-38044. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, T., V. M. Christoffels, S. Chowdhury, K. Iwase, H. Matsuzaki, M. Mori, W. H. Lamers, G. J. Darlington, and M. Takiguchi. 1998. Hypoglycemia-associated hyperammonemia caused by impaired expression of ornithine cycle enzyme genes in C/EBPα knockout mice. J. Biol. Chem. 273:27505-27510. [DOI] [PubMed] [Google Scholar]
- 19.Kowenz-Leutz, E., G. Twamley, S. Ansieau, and A. Leutz. 1994. Novel mechanism of C/EBP beta (NF-M) transcriptional control: activation through derepression. Genes Dev. 8:2781-2791. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, C. F., K. G. Xanthopoulos, and J. E. Darnell, Jr. 1990. Fetal and adult localization of C/EBP: evidence for combinatorial action of transcription factors in cell-specific gene expression. Development 109:473-481. [DOI] [PubMed] [Google Scholar]
- 21.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 22.Lee, Y. H., B. Sauer, P. F. Johnson, and F. J. Gonzalez. 1997. Disruption of the c/ebp alpha gene in adult mouse liver. Mol. Cell. Biol. 17:6014-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lescisin, K. R., S. Varmuza, and J. Rossant. 1988. Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 2:1639-1646. [DOI] [PubMed] [Google Scholar]
- 24.Morasso, M. I., A. Grinberg, G. Robinson, T. D. Sargent, and K. A. Mahon. 1999. Placental failure in mice lacking the homeobox gene Dlx3. Proc. Natl. Acad. Sci. USA 96:162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ness, S. A., E. Kowenz-Leutz, T. Casini, T. Graf, and A. Leutz. 1993. Myb and NF-M: combinatorial activators of myeloid genes in heterologous cell types. Genes Dev. 7:749-759. [DOI] [PubMed] [Google Scholar]
- 26.Newman, J. R., and A. E. Keating. 2003. Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science 300:2097-2101. [DOI] [PubMed] [Google Scholar]
- 27.Oelgeschlager, M., I. Nuchprayoon, B. Luscher, and A. D. Friedman. 1996. C/EBP, c-Myb, and PU. 1 cooperate to regulate the neutrophil elastase promoter. Mol. Cell. Biol. 16:4717-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson, R., P. Falck, M. Hellstrom, P. Lindahl, H. Bostrom, G. Franklin, L. Ahrlund-Richter, J. Pollard, P. Soriano, and C. Betsholtz. 1999. PDGFB regulates the development of the labyrinthine layer of the mouse fetal placenta. Dev. Biol. 212:124-136. [DOI] [PubMed] [Google Scholar]
- 29.Omori, K., K. Naruishi, F. Nishimura, H. Yamada-Naruishi, and S. Takashiba. 2004. High glucose enhances interleukin-6-induced vascular endothelial growth factor 165 expression via activation of Gp130-mediated p44/42 MAPK-CCAAT/enhancer binding protein signaling in gingival fibroblasts. J. Biol. Chem. 279:6643-6649. [DOI] [PubMed] [Google Scholar]
- 30.Poli, V. 1998. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 273:29279-29282. [DOI] [PubMed] [Google Scholar]
- 31.Ramji, D. P., and P. Foka. 2002. CCAAT/enhancer-binding proteins: structure, function, and regulation. Biochem. J. 365:561-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, G. W., P. F. Johnson, L. Hennighausen, and E. Sterneck. 1998. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 12:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen, E. D., C. J. Walkey, P. Puigserver, and B. M. Spiegelman. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14:1293-1307. [PubMed] [Google Scholar]
- 35.Rossant, J., and J. C. Cross. 2001. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2:538-548. [DOI] [PubMed] [Google Scholar]
- 36.Schorpp-Kistner, M., Z. Q. Wang, P. Angel, and E. F. Wagner. 1999. JunB is essential for mammalian placentation. EMBO J. 18:934-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber, J., E. Riethmacher-Sonnenberg, D. Riethmacher, E. E. Tuerk, J. Enderich, M. R. Bosl, and M. Wegner. 2000. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol. Cell. Biol. 20:2466-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber, M., Z. Q. Wang, W. Jochum, I. Fetka, C. Elliott, and E. F. Wagner. 2000. Placental vascularization requires the AP-1 component fra1. Development 127:4937-4948. [DOI] [PubMed] [Google Scholar]
- 39.Seagroves, T. N., S. Krnacik, B. Raught, J. Gay, B. Burgess-Beusse, G. J. Darlington, and J. M. Rosen. 1998. C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 12:1917-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterneck, E., and P. F. Johnson. 1998. CCAAT/enhancer binding protein beta is a neuronal transcriptional regulator activated by nerve growth factor receptor signaling. J. Neurochem. 70:2424-2433. [DOI] [PubMed] [Google Scholar]
- 41.Sterneck, E., R. Paylor, V. Jackson-Lewis, M. Libbey, S. Przedborski, L. Tessarollo, J. N. Crawley, and P. F. Johnson. 1998. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc. Natl. Acad. Sci. USA 95:10908-10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterneck, E., L. Tessarollo, and P. F. Johnson. 1997. An essential role for C/EBPβ in female reproduction. Genes Dev. 11:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, N. D., M. J. Finegold, A. Bradley, C. N. Ou, S. V. Abdelsayed, M. D. Wilde, L. R. Taylor, D. R. Wilson, and G. J. Darlington. 1995. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269:1108-1112. [DOI] [PubMed] [Google Scholar]
- 44.Weindel, K., D. Marme, and H. A. Weich. 1992. AIDS-associated Kaposi's sarcoma cells in culture express vascular endothelial growth factor. Biochem. Biophys. Res. Commun. 183:1167-1174. [DOI] [PubMed] [Google Scholar]
- 45.Wessells J, S. S., S. Yakar, and P. F. Johnson. 2004. Critical prosurvival roles for C/EBPβ and insulin-like growth factor I in macrophage tumor cells. Mol. Cell. Biol. 24:3238-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, S. C., M. Baer, A. J. Dillner, and P. F. Johnson. 1995. CRP2 (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding, and cell specificity. EMBO J. 14:3170-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams, S. C., C. A. Cantwell, and P. F. Johnson. 1991. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 5:1553-1567. [DOI] [PubMed] [Google Scholar]