Abstract
Complex and hybrid N-glycans contain sugar residues that have been implicated in fertilization, compaction of the embryo, and implantation. Inactivation of the Mgat1 gene responsible for their synthesis is embryonic lethal, but homozygous mutant blastocysts are phenotypically normal due to the presence of maternal Mgat1 gene transcripts. To identify roles for complex and hybrid N-glycans in oogenesis and preimplantation development, the Mgat1 gene in oocytes was deleted by using a ZP3Cre recombinase transgene. All mutant oocytes had an altered zona pellucida (ZP) that was thinner than the control ZP, and they did not possess complex N-glycans but contained ZP1, ZP2, and ZP3 glycoproteins. Mutant eggs were fertilized, all embryos implanted, and heterozygotes developed to birth. However, mutant females had decreased fertility, yielded fewer eggs after stimulation with gonadotropins, and produced a reduced number of preimplantation embryos and less progeny than controls. About 25% of embryonic day 3.5 (E3.5) embryos derived from mutant eggs were severely retarded in development, even when they were heterozygous and expressed complex N-glycans. Thus, a proportion of Mgat1−/− oocytes were developmentally compromised. Surprisingly, mutant eggs also gave rise to Mgat1−/− embryos that developed normally, implanted, and progressed to E9.5. Therefore, complex or hybrid N-glycans are required at some stage of oogenesis for the generation of a developmentally competent oocyte, but fertilization, blastogenesis, and implantation may proceed in their absence.
During oogenesis, the oocyte becomes surrounded with the zona pellucida (ZP), an extracellular matrix comprised of three major glycoproteins termed ZP1, ZP2, and ZP3 (8). Sperm binding occurs at the outer layer of the ZP and is thought to be mediated in the mouse by ZP3 (7, 64) or a determinant formed by the supramolecular structure of the ZP (14, 46). Sugars on ZP glycoproteins have been implicated in sperm recognition (59, 64). Oocyte glycosyltransferases dictate the glycosylation content of ZP glycoproteins, as shown by the fact that human ZP3 made in mouse oocytes has essentially the same O-glycan complement as mouse ZP3 (15). O-glycans on ZP3 were originally proposed to carry the sperm recognition determinant (23); subsequently, Ser332 and Ser334 in mouse ZP3 were identified as critical O-glycan sites (11). However, a ZP3 transgene mutated at these sites did not reduce sperm binding (35), and mass spectrometry of mouse ZP3 failed to find O-glycans at Ser332 or Ser334 (9). Treatment of ZP3 with α-galactosidase inactivates its ability to inhibit sperm binding (6). However, eggs from mice lacking α3-galactosyltransferase, which do not bind a lectin specific for α3Gal residues, are fertilized in vivo (61). Terminal GlcNAc on ZP3 has also been proposed as a sperm receptor (36, 39, 55), and oligosaccharides terminating in α3Gal, or in α3Fuc in the LewisX determinant, inhibit sperm binding (29). Importantly, female mice that express both human ZP3 and human ZP2 in place of mouse ZP2 and ZP3 are fertile, and their eggs do not bind human sperm (46). This failure of humanized mouse ZP to bind human sperm could be structurally based, as reflected by the fact that human ZP2 cleavage does not occur during the cortical reaction in the mouse (14, 46). The combined data, however, do not preclude a direct or indirect role for sugars in sperm-egg recognition (46).
Complex N-glycans are present on mouse ZP glycoproteins ZP1, ZP2, and ZP3 (4, 9, 19, 40, 62). In addition, cell adhesion molecules and other membrane glycoproteins required for compaction and preimplantation development, such as E-cadherin, carry N-glycans (49, 65). N-glycan synthesis is increased at the blastocyst stage (2), and treatment of preimplantation mouse embryos with tunicamycin, an inhibitor of N-glycan synthesis, prevents blastocyst development (3, 58). However, tunicamycin removes all N-glycans, resulting in empty glycosylation sites with severe structural consequences. Removal of just the complex and hybrid classes of N-glycans will not affect glycoprotein folding and exit from the endoplasmic reticulum (20) and may be achieved by mutation of the Mgat1 gene. The Mgat1 gene encodes N-acetylglucosaminyltransferase I (GlcNAc-TI), which initiates complex and hybrid N-glycan synthesis (31, 52). In the absence of GlcNAc-TI, N-glycans of mature glycoproteins will be solely oligomannosyl and will lack all branch antennae that contain GlcNAc, Gal, Fuc, and sialic acid (50, 53) (Fig. 1).
FIG. 1.
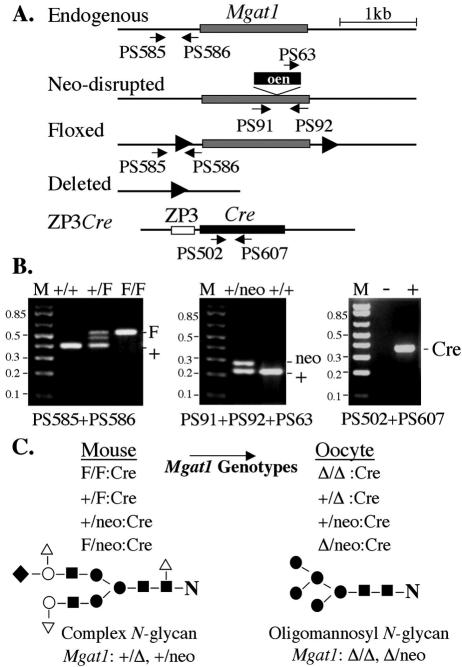
Oocyte-specific deletion of the Mgat1 gene coding exon. (A) Diagram of wild-type, floxed, and neo-disrupted alleles of the Mgat1 gene and the ZP3Cre transgene with locations of primers used for PCR genotyping. The Mgat1 and Cre recombinase coding exons are shaded, and the ZP3 promoter region is an open box. (B) PCR genotyping identified the various alleles. The intermediate band in +/F heterozygotes was due to hybrid formation during PCR. Lane M, 1-kb plus markers. (C) Mgat1 genotypes of females and their respective oocytes after deletion (Δ) of the Mgat1 gene by Cre recombinase. The Mgat1 gene deletion abolishes the synthesis of complex N-glycans so that mature glycoproteins express the oligomannosyl substrate (Man5GlcNAc2Asn) of GlcNAc-TI. ▪, GlcNAc; •, Man; ○, Gal; ▿, Fuc; and ⧫, sialic acid.
Inactivation of the mouse Mgat1 gene leads to embryonic lethality at approximately embryonic day 9.5 (∼E9.5) (27, 38). Mutant embryos are developmentally retarded, have defects of the vasculature and neural tube, and exhibit situs inversus. A cell-type-specific defect in lung was identified by tracking Mgat1−/− embryonic stem (ES) cells in chimeric embryos (26). Mutant ES cells fail to contribute to the organized layer of bronchial epithelium. A role for hybrid N-glycans in maintaining brain structure and function was recently revealed by synapsinCre-mediated deletion of the Mgat1 gene in neuronal cells (66). However, Mgat1−/− preimplantation blastocysts develop normally (27, 38), because the Mgat1−/− genotype is phenotypically overridden during fertilization, blastogenesis, and implantation (10, 25) due to the presence of wild-type Mgat1 gene transcripts of maternal origin (25). Maternal rescue of mutant blastocysts also occurs for the glycosyltransferase that initiates glycosylphosphatidylinositol anchor synthesis (1) and probably for most glycosyltransferase gene-inactivating mutations. Transcripts of the Fut4 and Fut9 genes responsible for transferring the α3Fuc residue implicated in sperm-egg recognition (29) are present in eggs (32). Mouse eggs also contain α3-galactosyltransferase gene transcripts (28). A comprehensive microarray analysis has detected many other glycosyltransferase gene transcripts in mouse eggs (57). The persistence of low amounts of maternal transcripts after fertilization may also explain discordant data on roles for sugars in blastogenesis. Thus, inhibition studies of preimplantation development indicated functional roles for α3Fuc in the stage-specific embryonic antigen 1 or LewisX determinant during compaction (5, 22), but Fut9−/− embryos express little or no stage-specific embryonic antigen 1 at the late eight-cell stage and develop normally (32). In another case, antibodies to the H blood group inhibit blastocyst implantation (63, 67) but mice with an inactivated Fut1 or Fut2 gene are fertile (16).
In order to identify roles for complex or hybrid N-glycans in oocyte production and function and preimplantation development, we have deleted the Mgat1 gene specifically in oocytes. Here, we show that despite the absence of complex N-glycans, mutant eggs are fertilized and Mgat1−/− embryos can develop normally, implant, and progress in utero to midgestation. However, females with mutant oocytes produce fewer eggs after stimulation with gonadotropins and less progeny, and a proportion of mutant eggs give rise to severely underdeveloped preimplantation embryos, revealing a role(s) for complex N-glycans in the generation of a developmentally competent oocyte.
MATERIALS AND METHODS
Mice and cells.
Mice carrying the Mgat1 gene coding region flanked by loxP sites (floxed) have been described (66). Mice heterozygous for the Mgat1neo null mutation (27) and mice carrying the ZP3Cre transgene were also previously described (1, 41, 54). C57BL/6 mice were obtained from the Jackson Labs, Bar Harbor, Maine. Chinese hamster ovary (CHO) cells Pro−5 and the Pro−Lec1.3C mutant (12, 56) were grown in suspension culture in complete α medium (Invitrogen, Carlsbad, Calif.) containing 10% fetal calf serum (Gemini, Calabasas, Calif.).
PCR genotyping.
To distinguish floxed, neo, and wild-type Mgat1 alleles, separate PCRs were performed on genomic DNA (Fig. 1). Primers PS585 (5′-TGCAAGCCAACACTTGTCTC-3′) and PS586 (5′-GAGACCTGCTTACTGCAGCC-3′) detected floxed Mgat1 (561 bp) and wild-type Mgat1 (421 bp) alleles. Primer PS63 (5′-GGTGGATGTGGAATGTGTGC-3′) in the pgk promoter and primers PS91 (5′-CCAGGGTTACTACAAGATTGC-3′) and PS92 (5′-CTCAGGTTTGCTTGAGTCTAC-3′) in the Mgat1 gene were used together. PS63 and PS92 detect the neo-disrupted Mgat1 allele (280 bp) and primers PS91 and PS92 detect the wild-type Mgat1 allele (231 bp). Primers PS502 (5′-GGACATGTTCAGGGATCGCCAGGCG-3′) and PS607 (5′-CCATGAGTGAACGAACCTGG-3′) detected the Cre recombinase gene coding region (364 bp). PCRs (25 μl) contained PCR buffer, 1.5 μl of 25 mM MgCl2, 0.5 μl of 10 mM dNTPs, 0.5 μl of 10 μM concentrations of the primers, 1.25 U of Taq polymerase (Perkin Elmer, Boston, Mass.), and 1 μl of DNA. After preheating (94°C, 2 min), 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min were performed, followed by one cycle of 72°C for 5 min.
Cytochemistry of ovarian sections.
Ovaries were fixed in 10% buffered formalin (Sigma, St. Louis, Mo.) for 8 h at room temperature (RT), washed three times in 70% ethanol (5 min each time) and paraffin embedded, and sections of 5 μm were made. Before cytochemistry, sections were dewaxed with Histoclear (Sigma) and rehydrated. For binding of fluorescein isothiocyanate-labeled Phaseolus vulgaris leukoagglutinin (FITC-L-PHA; Vector Labs, Burlingame, Calif.), or rhodamine-concanavalin A (Rh-ConA; Vector Labs) unstained or hematoxylin-stained sections were preincubated for 1 h at RT in phosphate-buffered saline (PBS) containing 1 mM MgCl2 and 1 mM CaCl2 (PBS) and 2% bovine serum albumin (BSA) (Fraction V; Sigma), rinsed, and incubated with 20 to 30 μg of FITC-L-PHA/ml or 2.5 μg of Rh-ConA/ml in PBS-2% BSA. After 1 h at RT, sections were washed with PBS-2% BSA and photographed.
Monoclonal antibodies to ZP1 (48), ZP2 (17), and ZP3 (18) were prepared from hybridoma cells obtained from the American Type Culture Collection (Bethesda, Md.) cultured in Dulbecco minimum essential medium (Invitrogen) containing 3.7 g of sodium bicarbonate/liter, 20% fetal bovine serum, 10 mM HEPES buffer, 100 mM sodium pyruvate, and 5 mM β-mercaptoethanol. Ovarian sections were incubated in methanol containing 0.3% hydrogen peroxide (30 min, RT), washed with water and Tris-buffered saline (TBS; 0.1 M Tris [pH 7.5] and 0.3 M NaCl) with 0.05% Tween 20 (TBST) for 3 min, and incubated in TBS containing 15% normal rabbit serum (Vectastain Elite ABC) for 30 min at RT in a humidified chamber. Sections were incubated with hybridoma medium diluted 1:250 in TBS containing 15% normal rabbit serum at 4°C overnight or for 2 h at RT. After washing three times with TBST, sections were incubated with rabbit anti-rat immunoglobulin G biotinylated secondary antibody (Vectastain Elite ABC kit; ∼50 μl in 10 ml of TBS) for 30 min at RT, washed, and incubated with ABC solution for 30 min at RT. After three washes with PBS-0.05% Tween 20, sections were stained by using a DAB kit (Vector Labs) and counterstained with hematoxylin before dehydration and mounting.
Toluidine blue staining and follicle staging.
Female mice were perfused with PBS followed by fixative (2.5% glutaraldehyde, 1.2% acrolein in Sym-Sollidine buffer). Ovaries stored in this fixative at 4°C were postfixed in 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through a graded series of ethanol, and embedded in LX112 resin (LADD Research Industries, Burlington, Vt.). Sections of 1 μm were stained with 1% toluidine blue in 1% sodium borate and imaged on a Zeiss Axiophot microscope.
For follicle staging, females of 7 to 9 weeks of age were given 5 IU of pregnant mare's serum gonadotropins (PMSG; Calbiochem, La Jolla, Calif.), and ovaries were removed after 48 h, fixed in 10% buffered formalin for 6 to 7 h at RT, and transferred into 70% ethanol. After embedding, serial sections of 8 μm were stained with hematoxylin. Antral follicles in all sections (∼110 per ovary) were staged as described previously (42).
Egg collection and cytochemistry.
Females of 4 to 14 weeks of age were superovulated by intraperitoneal injection of 5 IU of PMSG followed 46 h later by 5 IU of human chorionic gonadotropin (hCG; Sigma). The following day, oviducts were removed into a drop of M2 medium (Specialty Media, Phillipsburg, N.J.), the cumulus mass was extracted, and cumulus cells were dissociated from eggs in M2 medium containing hyaluronidase (Specialty Media). As soon as cumulus cells had detached, eggs were washed through 3 drops of M2.
For lectin binding, eggs were washed in 3 sequential drops of PBS-2% BSA and fixed in 2% formaldehyde in PBS-2% BSA (1 h, RT). After incubation in PBS-2% BSA (1 h, RT), the eggs were transferred to 20 μg of fluorescein-L-PHA (Vector Labs)/ml in PBS-2% BSA. After 1 h at RT, the eggs were washed through 3 drops of PBS-2% BSA and incubated with 2.5 μg of Rh-ConA/ml in PBS-2% BSA for 1 h at RT. Eggs were transferred through 3 drops of PBS-2% BSA and photographed.
For antibody binding, eggs recovered after stimulation with gonadotropins were fixed in 2% formaldehyde in TBS-3% BSA for 1 h at RT and incubated in TBS-3% BSA for 1 h at RT. Eggs were incubated with ZP hybridoma medium at 1:250 in TBS-3% BSA at 4°C overnight, washed three times in TBS-3% BSA, and incubated for 1 h at RT with FITC-conjugated anti-rat secondary antibody (Jackson Labs) diluted 1:100 in TBS-3% BSA, washed in TBS-3% BSA, and photographed.
Blastocyst collection and analysis.
Blastocysts were flushed from the uterus at E3.5 with M2 and transferred to M16 medium (Specialty Media) under light mineral oil and incubated at 37°C. Phase contrast photographs were taken at various times. To obtain inner cell mass outgrowth, embryos were transferred to 0.1 ml of ES medium (27) in a 96-well gelatinized plate and incubated at 37°C for 5 days. For L-PHA binding, cell outgrowths were rinsed with PBS and incubated in PBS-2% BSA (1 h, 4°C) followed by 30 μg of FITC-L-PHA/ml in PBS-2% BSA (1 h, 4°C). Photographs were taken after rinsing with PBS. To obtain genomic DNA for genotyping, 25 μl of proteinase K (0.1 mg/ml in PCR buffer containing MgCl2) was added to cell outgrowths, and the plate was incubated at 55°C overnight. The digest was heated at 98°C for 5 min before 5 μl was used for PCR genotyping with primers PS585 and PS586 or PS91, PS92, and PS63 (Fig. 1).
ZP3 from Lec1 CHO cells.
Pro−5 CHO and Pro−Lec1.3C CHO (termed Lec1) were cotransfected with ZP3 cDNA (from Jurrien Dean, National Institutes of Health) subcloned into pcDNA3.1+ (Invitrogen) and pEGFP-N1 (BD Biosciences Clontech, Palo Alto, Calif.) by using Lipofectamine 2000 (Invitrogen). After 48 h, cell pellets were prepared and stored at −20°C. Frozen cells (∼4 × 10−6) were resuspended in 100 μl of 40 mM Tris, 150 mM NaCl, complete protease inhibitors (Roche, Indianapolis, Ind.), 0.1% sodium dodecyl sulfate, and 5% β-mercaptoethanol, and 8 μl was used for glycosidase digestion. Ovaries were homogenized in the same buffer except that β-mercaptoethanol was excluded for ZP2 analysis.
Glycosidase digestions and Western analysis.
Digestion of 10 ovulated eggs or extract of ovary (∼10 to 15 μg of protein) or CHO (∼50 μg of protein) was performed in denaturing buffer containing protease inhibitors (Roche), 1 mM EDTA, and 15 μM pepstatin. Samples were boiled for 10 min in digestion buffer before adding 2 μl of NP-40 followed by 1,000 U of PNGase F (New England Biolabs, Beverley, Mass.) or 0.01 U of endoglycosidase H (Endo H; Roche) without NP-40. Samples were incubated at 37°C for ∼12 h with additional enzyme added after 6 h. For electrophoresis, 20-μl samples were boiled for 10 min in loading buffer containing β-mercaptoethanol (except for ZP2) and electrophoresed on a Tris-HCl 4 to 20% gradient gel. Proteins were transferred to membrane in Tris-glycine buffer with 5% methanol and incubated in 5% nonfat milk-TBST for 2 h. Anti-ZP hybridoma medium was used at 1:250 in 1% nonfat milk-TBST for 90 min. The membrane was washed in TBST for 30 min and incubated in 1:2000 rabbit anti-rat immunoglobulin G (H+L) conjugated to horseradish peroxidase (Zymed, South Francisco, Calif.) for 90 min. Signal was detected by using ECL+ (Amersham, Piscataway, N.J.). CHO blots were reprobed with anti-green fluorescent protein (GFP) antibody conjugated to horseradish peroxidase (Santa Cruz, Santa Cruz, Calif.).
RESULTS
Oocyte-specific deletion of the Mgat1 gene.
The Mgat1 gene coding region is contained in a single exon (33, 44). To obtain a conditional Mgat1 allele, the coding exon was flanked by loxP sites in the targeting vector pFlox and introduced into the Mgat1 gene locus by homologous recombination to generate the Mgat1F allele (Fig. 1) (66). To obtain oocyte-specific deletion, Mgat1F/F females were crossed to males with the ZP3Cre transgene. The portion of the ZP3 promoter that drives Cre recombinase in this transgene is active solely in the oocyte (1, 41, 54). Use of males with a different ZP3Cre transgene (34) never produced Mgat1F/F:ZP3Cre females, presumably due to the deletion of the Mgat1 gene in embryos. Females carrying one copy of the Mgat1F allele and one copy of the null Mgat1neo allele were also used (Fig. 1) (27). In the latter females, only one Mgat1F gene had to be deleted by Cre recombinase. Test female genotypes included Mgat1F/F or F/neo:ZP3Cre mutants, and controls or heterozygotes carrying the ZP3Cre transgene, or the same genotypes lacking ZP3Cre. The identification of each allele was determined by PCR of genomic DNA (Fig. 1).
Mgat1−/− oocytes and ZP lack complex N glycans
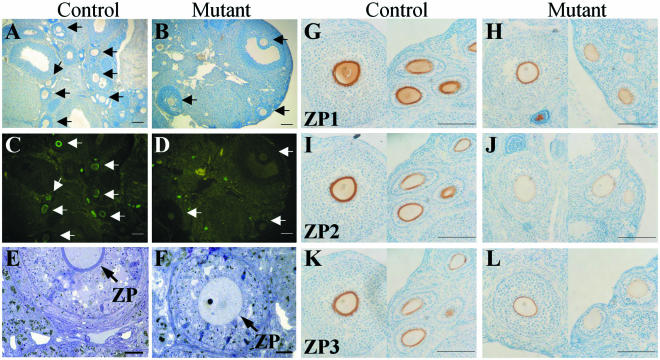
Most ovaries from Mgat1F/F or F/neo:ZP3Cre and control females did not differ morphologically or by weight, and hematoxylin staining revealed oocytes at all stages of maturation in both groups (Fig. 2A and B). The presence of complex N-glycans was investigated by lectin cytochemistry. Complex N-glycans bind the leukoagglutinin L-PHA, but oligomannosyl N-glycans do not (13, 24). In control ovaries (n = 12), all cells and oocytes bound L-PHA and ZP bound L-PHA very strongly, producing a bright green ring (Fig. 2C). By contrast, in ovaries from Mgat1F/F or F/neo:ZP3Cre mutants (n = 21), there was no staining of the ZP surrounding oocytes, nor of oocytes themselves, regardless of size (Fig. 2D). Binding of L-PHA to non-oocytes in mutant and control ovaries was equivalent. Therefore, oocyte-specific knockout of the Mgat1 gene was achieved efficiently. The ZP surrounding mutant oocytes was thinner than the ZP from control oocytes (Fig. 2E and 2F). However, ZP1, ZP2, and ZP3 glycoproteins were present in the ZP of mature mutant oocytes (Fig. 2H, J, and L, left sides) and in mutant oocytes at earlier stages of maturation (Fig. 2H, J, and L, right sides). It is clear that mutant oocytes synthesized all three ZP glycoproteins.
FIG. 2.
Mgat1−/− oocytes do not bind L-PHA but contain ZP1, ZP2, and ZP3. (A to D) Ovarian sections from Mgat1F/F or F/neo:ZP3Cre (Mutant) and Mgat1+/F or +/neo:ZP3Cre (Control) ovaries were stained with hematoxylin (A and B) or treated with FITC-L-PHA (C and D). Arrows point to oocytes. FITC-L-PHA bound to stromal cells equivalently in control and mutant ovaries. Bar = 100 μm. (E and F) Toluidine blue staining of mutant and control ovary sections. Arrows point to ZP. Bar = 20 μm. (G to L) Monoclonal antibodies to ZP1, ZP2, and ZP3 were detected by diaminobenzidine precipitation and hematoxylin staining of ovary sections. Control, Mgat1+/F:ZP3Cre ovary. Mutant, Mgat1F/F:ZP3Cre ovary. The left sides of each panel show a mature oocyte; the right sides of each panel show oocytes at earlier stages of oogenesis. Bar = 100 μm.
The ZP from mutant oocytes was also examined following superovulation. All ovulated eggs from Mgat1F/F or F/neo:ZP3Cre females had a ZP that was less rigid and more loosely attached to the egg than the ZP of control or heterozygous eggs (Fig. 3A and B). Cumulus cells bound to the surface of the ZP were readily released from control eggs by a brief (<5 min) hyaluronidase treatment, but even a 20-min treatment did not usually remove all cumulus cells from mutant eggs (Fig. 3B).
FIG. 3.
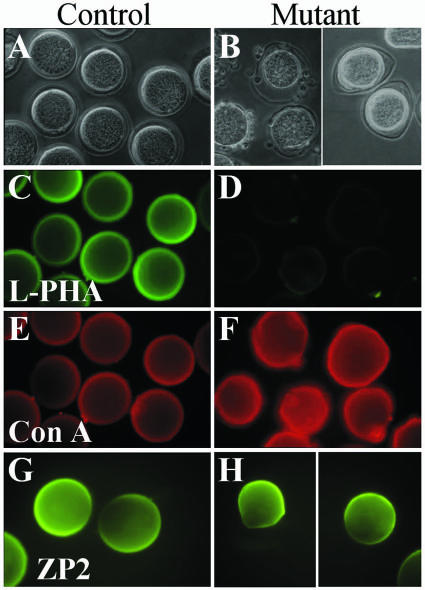
Ovulated eggs lacking complex and hybrid N-glycans do not bind L-PHA. Eggs recovered after superovulation of Mgat1F/F (Control) or Mgat1F/F or F/neo:ZP3Cre (Mutant) females. (A and B) Phase contrast microscopy shows that ZP of mutant eggs is less rigid than that of the control. Cumulus cells remaining after 20 min of hyaluronidase treatment were all on the external surface of mutant ZP. (C and D) FITC-L-PHA bound to control but not mutant eggs. (E and F) Rh-ConA bound to control eggs, and binding was enhanced for mutant eggs. (G and H) ZP2 antibody binding to eggs from Mgat1F/neo (Control) and Mgat1F/neo:ZP3Cre (Mutant). Results for ZP1 and ZP3 antibodies were identical.
Ovulated eggs from control and heterozygous females bound FITC-L-PHA, whereas those from mutant females did not, signifying their lack of complex N-glycans (Fig. 3C and D). Some bright spots on mutant eggs probably reflect the stickiness of their ZP. Both mutant and control eggs bound Rh-ConA (Fig. 3E and F). However, mutant eggs were significantly brighter than controls, consistent with the increased expression of oligomannosyl N-glycans in the absence of GlcNAc-TI (see Fig. 1). Antibodies to ZP1, ZP2, and ZP3 (Fig. 3G and H and data not shown) bound to the ZP of mutant eggs. The latter appeared smaller than the ZP of control eggs, reflecting the fact that mutant ZP was thinner.
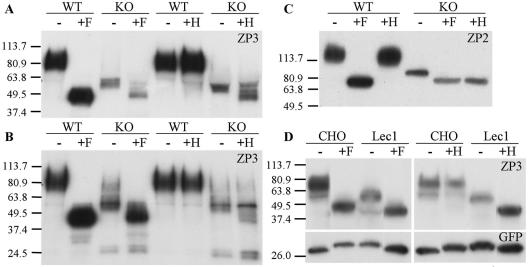
Further evidence that the ZP of ovulated mutant eggs lacked complex N-glycans was obtained by endoglycosidase digestion of ZP2 and ZP3. Peptide N-glycanase F (PNGase F) removes all N-glycans by cleaving the GlcNAc-Asn bond (see Fig. 1C). Endo H removes only oligomannosyl N-glycans by cleaving between the two core GlcNAc residues (see Fig. 1C), because complex N-glycans are resistant to Endo H (reviewed in reference 60). Panels A and C of Fig. 4 show that the amount of ZP3 or ZP2 from 10 mutant eggs was less than that from 10 control eggs, consistent with a thin mutant ZP (Fig. 2 and 3). ZP1 levels were also lower in the mutant compared to the control (data not shown). The molecular weight of ZP3 and ZP2 from mutant eggs was markedly reduced compared to that from control eggs, consistent with the absence of complex N-glycans in mutant ZP (Fig. 4A and C). A similar result was obtained for ZP3 from the ovary (Fig. 4B), although a small fraction of ZP3 in mutant ovary had the molecular weight of control ZP3. In addition, low-molecular-weight ZP3 fragments were present in ZP3 from mutant ovary (Fig. 4B) but were not found in ZP3 from ovulated mutant eggs (not shown). The ZP3 fragments probably arise from proteolysis of mutant ZP that is devoid of complex and hybrid N-glycans.
FIG. 4.
Western blot analysis of ZP2 and ZP3. (A) Each lane contains 10 eggs from knockout (KO; mutant Mgat1F/F:ZP3Cre) or wild-type (WT; control Mgat1+/F:ZP3Cre) females before or after digestion with PNGase F (F) or Endo H (H). The blot was probed with anti-ZP3 monoclonal antibody. (B) Lanes are as for panel A except that each lane contains ∼10 to 15 μg of ovary protein. (C) Lanes are as for panel A except that the blots were probed with anti-ZP2 monoclonal antibody. (D) Lec1 cell lysate (∼50 μg protein) following transient transfection of ZP3 and GFP cDNAs. The blot was probed with anti-ZP3 monoclonal antibody, stripped, and reprobed with anti-GFP antibody.
Digestion with PNGase F to remove all N-glycans reduced ZP2 and ZP3 from both mutant and control eggs to the same respective molecular weights (Fig. 4A to C). ZP2 and ZP3 from control eggs were unaffected by treatment with Endo H, indicating that all N-glycans on mature ZP2 and ZP3 are complex, in agreement with mass spectrometric analysis (9). By contrast, ZP2 and ZP3 from mutant eggs were largely susceptible to digestion with Endo H, as expected for oligomannosyl N-glycans. Ovarian ZP3 had small amounts of a species resistant to Endo H but sensitive to PNGase F, indicating the presence of some complex N-glycans (Fig. 4B). Because the ovary contains oocytes at all stages of oogenesis, the presence of a small proportion of ZP3 with complex N-glycans in mutant ovary may reflect the window of time required to completely remove GlcNAc-TI activity during oogenesis. This Endo H-resistant species was essentially gone from ZP3 in ovulated eggs (data not shown). This finding may be due to a dilution effect caused by the long half-life of the ZP.
Endo H effectively removed the N-glycans from mutant ZP2 (Fig. 4C). However, Endo H treatment gave rise to partially digested species of mutant ZP3, even when digestion times were prolonged and enzyme amounts were increased (Fig. 4A and B). This result may be due to inaccessibility or modification of the Man5GlcNAc2Asn on mutant ZP3, such as removal of one or more Man residues by an α-mannosidase, or modification of a core GlcNAc residue to produce Endo H-resistant species. Complete removal of N-glycans by both enzymes was readily achieved when mouse ZP3 was expressed in Lec1 CHO cells in the absence of ZP1 and ZP2 (Fig. 4D). Lec1 CHO cells lack GlcNAc-TI due to a null mutation in the Mgat1 gene (12).
Eggs lacking complex and hybrid N-glycans are fertilized.
Mgat1F/F or F/neo:ZP3Cre females of 2.5 to 3.5 months of age were mated with Mgat1+/+ C57BL/6 males. As expected, heterozygous Mgat1+/Δ (n = 12) or Mgat1+/neo (n = 11) pups were obtained. No pups carried a floxed Mgat1 allele, showing that the ZP3Cre recombinase worked efficiently. Therefore, eggs with a ZP that lacks complex and hybrid N-glycans are fertilized, heterozygous blastocysts implant, and embryos develop normally with GlcNAc-TI provided solely from sperm. However, litter size was significantly reduced compared to matings of Mgat1F/F or F/neo females lacking Cre recombinase with C57BL/6 males. Whereas control females (n = 10) had an average of 7.1 pups per litter, females with oocytes lacking GlcNAc-TI (n = 6) produced only 3.8 pups per litter. Subsequent experiments showed that this result was not an effect of the Cre recombinase (see below).
Blastocysts lacking GlcNAc-TI implant and develop to mid-gestation.
Mgat1−/− embryos that possess maternal Mgat1 gene transcripts during blastogenesis die at mid-gestation (27, 38). To determine whether embryos lacking GlcNAc-TI during preimplantation development would implant and progress, progeny were examined at E8.5 and E9.5. Crosses of Mgat1F/F or F/neo:ZP3Cre mutant females with heterozygous Mgat1+/neo males gave rise to both mutant and heterozygous embryos (Table 1). The Mgat1−/− embryo phenotype was the same as that described previously (27, 38). Therefore, eggs lacking GlcNAc-TI give rise to Mgat1−/− embryos that implant and develop. However, both Mgat1F/F or F/neo:ZP3Cre mutant females had ∼25% empty deciduae due to embryos that implanted but did not develop (Table 1). By contrast, empty deciduae were rare in control females (Table 1). The average number of deciduae per mutant female was 4.6 (n = 17), similar to the average number of preimplantation embryos per mutant female (4.6; n = 14) obtained from crosses with Mgat1+/neo males (Table 2). This result suggests that all embryos implanted. However, only about 75% of them progressed to mid-gestation.
TABLE 1.
Embryos at E8.5 or E9.5 from crosses of Mgat1F/F or F/neo:ZP3Cre females by Mgat1+/neo males
| Female genotype | No. of:
|
|||||
|---|---|---|---|---|---|---|
| Females | Deciduae | Embryos | Embryos genotypedb | Mgat1+/− embryos | Mgat1−/− embryos | |
| Mgat1F/F:ZP3Cre | 9 | 40 | 26 | 24 | 14 | 10 |
| Mgat1F/neo:ZP3Cre | 8 | 38 | 28 | 19 | 11 | 8 |
| Controla | 22 | 170 | 160 | NDc | ||
Control females included Mgat1F/F or F/+ females lacking the ZP3Cre transgene or Mgat1F/+ females carrying the ZP3Cre transgene and mated to Mgat1+/+ males.
Contamination with maternal tissue occurred with resorbing embryos and precluded genotyping in several cases.
ND, not determined because all progeny were Mgat1+/− or +/+.
TABLE 2.
Embryos collected at E3.5 from crosses of Mgat1F/F or F/neo:ZP3Cre mutant females by Mgat1+/neo males
| Genotype | No. of females | No. of embryos
|
Total no. of embryos | Avg no. of embryos/ female | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 cellb | 2 cell | 4 cell | 8 to 16 cell | Compacted abnormalc | Normal blastocyst | ||||
| Mutant | 14 | 4 | 2 | 4 | 5 | 17 | 33 | 65 | 4.6 |
| Controla | 23 | 0 | 0 | 0 | 0 | 0 | 152 | 152 | 6.6 |
Control mice lacked the ZP3Cre transgene or were heterozygous females with a ZP3Cre transgene. Data include blastocysts from crosses with Mgat1+/neo and Mgat1+/+ C57BL/6 males.
Two appeared to be unfertilized eggs.
Compacted but abnormal morphology.
Defective oogenesis in Mgat1−/− oocytes.
Careful examination of serial sections through five regions of both ovaries of 12 mutant females showed that all oocytes with a ZP failed to bind L-PHA, consistent with the elimination of the Mgat1 gene by ZP3Cre early in oocyte expansion. A mosaic ovary was seen in 1 of 21 mutant females examined in this study. To examine follicular development, 6-week-old mutant and control females were treated with PMSG, and 48 h later both ovaries were fixed and serially sectioned. Follicle development was examined in all eight micron sections. The numbers of antral follicles at each of the stages (6, 7, and 8) were equivalent in mutant and control females at this age (Table 3). Therefore, oocyte maturation and folliculogenesis did not appear to be compromised in the absence of GlcNAc-TI. However, when ovulation was stimulated by PMSG and hCG treatment, the number of eggs obtained from Mgat1F/F or F/neo:ZP3Cre females was less than 50% of that obtained from control females (Table 3). A number of mutant females (7 of 18) produced no eggs after superovulation, compared to only 1 of the 18 control females. In addition, mutant females had reduced fertility. Of 65 mutant females that mated within 5 days of being placed for the first time with a male, 38 gave rise to embryos but 27 (41%) had no evidence of embryo formation (Table 3). By contrast, of 51 control females, 45 gave rise to embryos (Table 3). Ten control females carrying the Cre recombinase transgene produced 70 embryos (7 per female), showing that the reduced fertility of mutant females was not due to the presence of Cre recombinase.
TABLE 3.
Reduced ovulation in Mgat1F/F or F/neo:ZP3Cre mutant femalese
| Female genotypea | Antral follicle countsb
|
Ovulated eggsc | Embryos per femaled | ||
|---|---|---|---|---|---|
| Stage 6 | Stage 7 | Stage 8 | |||
| Mutant | 34.2 ± 8.3 (n = 5) | 12.2 ± 2.5 (n = 5) | 9.2 ± 3.8 (n = 5) | 10.5 ± 2 (n = 18) | 4.4 ± 0.4 (n = 38) |
| Control | 43.3 ± 8.6 (n = 4) | 9.5 ± 2.1 (n = 4) | 10.8 ± 0.9 (n = 4) | 27.0 ± 2 (n = 18) | 7.2 ± 0.3 (n = 45) |
Mutant females were Mgat1F/F or F/neo:ZP3Cre, and control females either lacked the ZP3Cre transgene or were heterozygous females with a ZP3Cre transgene. n, number of females.
Follicles at stages 6, 7, and 8 were counted in all sections prepared from both ovaries 48 h after PMSG treatment of 6-week-old females.
Females were superovulated with PMSG followed by hCG as described in Materials and Methods, and recovered eggs were counted.
Data include pre- and postimplantation embryos from crosses with Mgat1+/neo and Mgat1+/+ males.
Values are means ± standard errors of the means.
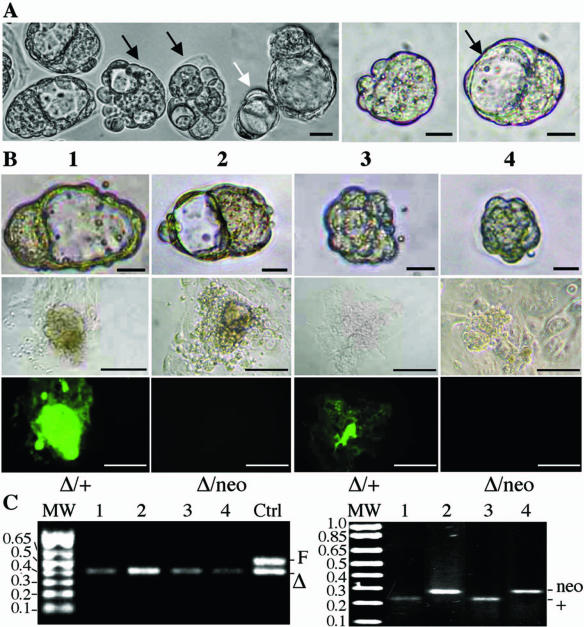
To investigate the developmental competence of ovulated mutant eggs, E3.5 embryos from Mgat1F/F:ZP3Cre females that had been mated with Mgat1+/neo heterozygous males were analyzed. A spectrum of underdeveloped embryos was obtained, whereas control crosses gave rise to normal blastocysts only (Table 2). Embryos of ≤16 cells with no blastocoel and in which the cells were not symmetric were quite common (Table 2) (Fig. 5A). Culturing of these embryos often led to blastocoel development, even from embryos that had only four cells at E3.5 (Fig. 5A). Surprisingly, the genotyping of cellular outgrowths revealed that embryos with normal morphology could be homozygous mutant (n = 5), while poorly developed embryos could be heterozygous (n = 4). Thus, cells from underdeveloped embryo 3 bound L-PHA and were heterozygous, while cells from normal blastocyst 2 did not bind L-PHA and were mutant (Fig. 5B). Importantly, the same mixture of normal and underdeveloped embryos was obtained when all progeny were heterozygous following crosses with Mgat1+/+ males. Of 15 Mgat1+/Δ embryos that all expressed complex N-glycans, 6 were normal whereas 9 had no blastocoel and were aberrant. About half of the abnormal embryos were clearly able to develop after implantation (Tables 1 to 3). Therefore, there appear to be two general populations of mutant eggs. One is developmentally compromised and gives rise to the ∼25% of embryos that do not develop after implantation, while the other is developmentally competent, even when fertilized by an Mgat1− sperm. Thus, complex or hybrid N-glycans appear to be required at some stage of oogenesis for the production of fully functional eggs.
FIG. 5.
Two populations of ovulated Mgat1−/− eggs. (A) Morphology of E3.5 embryos from a cross between a Mgat1F/F:ZP3Cre female and a Mgat1+/neo male. Most embryos had no ZP at E3.5. About half of the embryos were developmentally retarded or abnormal (black arrows). The white arrow points to a four-cell embryo with a formed cavity. The middle panel shows a compacted embryo. The right panel shows cavity formation in this embryo after culture in M16 medium for 1 h (bar = 25 μm). (B) The top row shows the morphology of normal and aberrant E3.5 embryos that were cultured and genotyped (bar = 25 μm). The next row shows phase contrast microscopy of embryo outgrowths after culture in ES cell medium for 5 days (bar = 100 μm). The third row shows outgrowths after binding of FITC-L-PHA (bar = 100 μm). (C) PCR genotyping of cultured outgrowths. The left gel shows that a floxed Mgat1 allele was absent from each embryo and detects the deleted (Δ) allele with primers PS585 and PS586, which also detect the Mgat1+ allele. The right gel shows whether Mgat1+ or Mgat1neo sperm fertilized the mutant oocyte with primers PS91, PS92, and PS63 (see Fig. 1) to detect the Mgat1+ and Mgat1neo alleles.
DISCUSSION
Efficient deletion of the Mgat1 gene in oocytes was achieved by the ZP3Cre recombinase transgene. No progeny with a floxed Mgat1 allele were obtained. The ZP of developing oocytes and ovulated eggs from Mgat1F/F or F/neo:ZP3Cre females did not bind L-PHA. The N-glycans of ZP3 in mutant oocytes and eggs also reflected the loss of GlcNAc-TI. However, Mgat1−/− oocytes devoid of complex and hybrid N-glycans had varying developmental competence. Whereas normal numbers of antral follicles were generated in mutant ovaries, it seems that ∼30% of Mgat1−/− oocytes did not ovulate, based on the low numbers of preimplantation embryos obtained from mutant females and consistent with the reduced recovery of eggs after stimulation of mutant females with gonadotropins. Unfertilized mutant eggs were very rare. However, absorption of some eggs soon after ovulation, or of a few early embryos soon after fertilization, cannot be excluded. Of the Mgat1−/− oocytes that ovulated, about 25% gave rise to severely underdeveloped embryos. By contrast, ∼75% of mutant eggs were functional and, despite having a thin ZP, generated normal blastocysts after fertilization. These numbers were the same for two genetic backgrounds and do not appear to be due to the action of a modifying gene. The data reveal new roles for complex or hybrid N-glycans in oocyte maturation and ovulation and show, unexpectedly, that Mgat1−/− embryos can develop normally, implant, and progress to E9.5.
The two populations of ovulated Mgat1−/− eggs may arise because complex or hybrid N-glycans are essential on one or a subset of glycoproteins active early in oogenesis. A likely role for complex N-glycans is in the communication between oocyte and granulosa cells, which is essential for an oocyte to develop with full competence (47). The ZP3 promoter becomes active when primordial oocytes begin to develop (21), and so ZP3Cre recombinase should be present from the beginning of oocyte expansion. However, the loss of GlcNAc-TI will depend not only on when the Mgat1 coding region is excised but also on the half-life of preexisting Mgat1 mRNA and GlcNAc-TI protein. This window of GlcNAc-TI activity is a potential reason for the observed variation in mutant oocyte and egg phenotypes. Indeed, a very small proportion of ZP3 from mutant ovaries did have complex N-glycans (Fig. 4B). Perhaps oocytes that lose their complex N-glycans early in oocyte expansion develop but do not ovulate; those that lose them a little later ovulate but are incompetent for blastocyst formation; and those that lose them latest support normal preimplantation development. This model implicates oocyte glycoproteins that must require their complex or hybrid N-glycans to function. Alternatively, the origin of different populations of Mgat1−/− oocytes might be due to stochastic effects of an altered ZP on oocyte development. For example, oocytes lacking ZP1 generate a thin ZP, and few oocytes ovulate and those that do are developmentally compromised after ovulation (45).
While the generation of competent oocytes appears to require complex or hybrid N-glycans, our data show that fertilization, blastocyst formation, and implantation may proceed in their absence. Ovulated eggs lacking complex N-glycans have a thin ZP that nevertheless permits fertilization in vivo. Mgat1−/− embryos develop normally, implant, and progress to ∼E9.5. There are no apparent differences between phenotypically normal Mgat1−/− blastocysts that possess maternal Mgat1 transcripts (27, 38) and Mgat1−/− blastocysts that have none (this study). This is a surprising result because the phenotype of Mgat1−/− embryos at ∼E9.5 reflects retarded growth and development suggestive of altered cell-cell interactions (27, 38). Since adhesion between cells of the developing blastocyst is critical to normal progression through blastogenesis (49), it was expected that complex N-glycans would be important for blastocyst formation. It was also possible that complex or hybrid N-glycans would be important for sperm binding or implantation of the blastocyst. However, our data show that fertilization occurs in the absence of complex or hybrid N-glycans synthesized by the oocyte. Thus, a ZP with no complex N-glycans is functional; this may be because O-glycans provide critical determinants or because the mutant ZP we generated functions by an alternative new mechanism. Fertilization might also occur via a glycoprotein that becomes associated with the ZP after synthesis in the oviduct and functions as a sperm receptor (51).
By the same argument, if a sugar residue or glycan epitope is necessary for compaction or implantation of mouse embryos (30, 43) it must not be carried solely on complex or hybrid N-glycans synthesized by the oocyte. Complex N-glycans can have up to seven branched antennae that may present a variety of sugars in a specific structural context. To date, no mouse mutants defective in the addition of a sugar residue have proved infertile (37). However, far from all candidate genes have been investigated, and oocyte-specific ablation of glycosyltransferases will probably be necessary to observe a preimplantation phenotype. The latter has been done for the Pig-a (1) and Ogt (41) GlcNAc-transferases that initiate glycosylphosphatidylinositol-anchor synthesis or the addition of O-GlcNAc to nucleocytoplasmic proteins, respectively. In both cases, oocyte-specific deletion caused females to be infertile (1, 41).
Acknowledgments
We thank Paula Cohen for critical comments on the manuscript and technical advice and Frank Macaluso for sectioning and microscopy.
This work was supported by grants RO1 CA 30645 to P.S. and RO1 DK 48257 to J.D.M. Partial support was provided by Albert Einstein Cancer Center grant PO1 13330.
REFERENCES
- 1.Alfieri, J. A., A. D. Martin, J. Takeda, G. Kondoh, D. G. Myles, and P. Primakoff. 2003. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J. Cell Sci. 116:2149-2155. [DOI] [PubMed] [Google Scholar]
- 2.Armant, D. R., H. A. Kaplan, and W. J. Lennarz. 1986. N-linked glycoprotein biosynthesis in the developing mouse embryo. Dev. Biol. 113:228-237. [DOI] [PubMed] [Google Scholar]
- 3.Atienza-Samols, S. B., P. R. Pine, and M. I. Sherman. 1980. Effects of tunicamycin upon glycoprotein synthesis and development of early mouse embryos. Dev. Biol. 79:19-32. [DOI] [PubMed] [Google Scholar]
- 4.Aviles, M., M. El-Mestrah, L. Jaber, M. T. Castells, J. Ballesta, and F. W. Kan. 2000. Cytochemical demonstration of modification of carbohydrates in the mouse zona pellucida during folliculogenesis. Histochem. Cell Biol. 113:207-219. [DOI] [PubMed] [Google Scholar]
- 5.Bird, J. M., and S. J. Kimber. 1984. Oligosaccharides containing fucose linked alpha(1-3) and alpha(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev. Biol. 104:449-460. [DOI] [PubMed] [Google Scholar]
- 6.Bleil, J. D., and P. M. Wassarman. 1988. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity Proc. Natl. Acad. Sci. USA 85:6778-6782. (Erratum, 85:9600.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleil, J. D., and P. M. Wassarman. 1980. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20:873-882. [DOI] [PubMed] [Google Scholar]
- 8.Bleil, J. D., and P. M. Wassarman. 1980. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev. Biol. 76:185-202. [DOI] [PubMed] [Google Scholar]
- 9.Boja, E. S., T. Hoodbhoy, H. M. Fales, and J. Dean. 2003. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 278:34189-34202. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, R. M., M. Metzler, M. Granovsky, J. W. Dennis, and J. D. Marth. 1995. Complex asparagine-linked oligosaccharides in Mgat1-null embryos. Glycobiology 5:535-543. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J., E. S. Litscher, and P. M. Wassarman. 1998. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc. Natl. Acad. Sci. USA 95:6193-61977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, W., and P. Stanley. 2003. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology 13:43-50. [DOI] [PubMed] [Google Scholar]
- 13.Cummings, R. D., and S. Kornfeld. 1982. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J. Biol. Chem. 257:11230-11234. [PubMed] [Google Scholar]
- 14.Dean, J. 2004. Reassessing the molecular biology of sperm-egg recognition with mouse genetics. Bioessays 26:29-38. [DOI] [PubMed] [Google Scholar]
- 15.Dell, A., S. Chalabi, R. L. Easton, S. M. Haslam, M. Sutton-Smith, M. S. Patankar, F. Lattanzio, M. Panico, H. R. Morris, and G. F. Clark. 2003. Murine and human zona pellucida 3 derived from mouse eggs express identical O-glycans. Proc. Natl. Acad. Sci. USA 100:15631-15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domino, S. E., L. Zhang, P. J. Gillespie, T. L. Saunders, and J. B. Lowe. 2001. Deficiency of reproductive tract α(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 α(1,2)fucosyltransferase locus. Mol. Cell. Biol. 21:8336-8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.East, I. J., and J. Dean. 1984. Monoclonal antibodies as probes of the distribution of ZP-2, the major sulfated glycoprotein of the murine zona pellucida. J. Cell Biol. 98:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.East, I. J., B. J. Gulyas, and J. Dean. 1985. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev. Biol. 109:268-273. [DOI] [PubMed] [Google Scholar]
- 19.Easton, R. L., M. S. Patankar, F. A. Lattanzio, T. H. Leaven, H. R. Morris, G. F. Clark, and A. Dell. 2000. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J. Biol. Chem. 275:7731-7742. [DOI] [PubMed] [Google Scholar]
- 20.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4:181-191. [DOI] [PubMed] [Google Scholar]
- 21.Epifano, O., L. F. Liang, M. Familari, M. C. Moos, Jr., and J. Dean. 1995. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 121:1947-1956. [DOI] [PubMed] [Google Scholar]
- 22.Fenderson, B. A., U. Zehavi, and S. Hakomori. 1984. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J. Exp. Med. 160:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florman, H. M., and P. M. Wassarman. 1985. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 41:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green, E. D., R. M. Brodbeck, and J. U. Baenziger. 1987. Lectin affinity high-performance liquid chromatography: interactions of N-glycanase-released oligosaccharides with leukoagglutinating phytohemagglutinin, concanavalin A, Datura stramonium agglutinin, and Vicia villosa agglutinin. Anal. Biochem. 167:62-75. [DOI] [PubMed] [Google Scholar]
- 25.Ioffe, E., Y. Liu, and P. Stanley. 1997. Complex N-glycans in Mgat1 null preimplantation embryos arise from maternal Mgat1 RNA. Glycobiology 7:913-919. [DOI] [PubMed] [Google Scholar]
- 26.Ioffe, E., Y. Liu, and P. Stanley. 1996. Essential role for complex N-glycans in forming an organized layer of bronchial epithelium. Proc. Natl. Acad. Sci. USA 93:11041-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioffe, E., and P. Stanley. 1994. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc. Natl. Acad. Sci. USA 91:728-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston, D. S., J. H. Shaper, N. L. Shaper, D. H. Joziasse, and W. W. Wright. 1995. The gene encoding murine alpha 1,3-galactosyltransferase is expressed in female germ cells but not in male germ cells. Dev. Biol. 171:224-232. [DOI] [PubMed] [Google Scholar]
- 29.Johnston, D. S., W. W. Wright, J. H. Shaper, C. H. Hokke, D. H. Van den Eijnden, and D. H. Joziasse. 1998. Murine sperm-zona binding, a fucosyl residue is required for a high affinity sperm-binding ligand. A second site on sperm binds a nonfucosylated, beta-galactosyl-capped oligosaccharide. J. Biol. Chem. 273:1888-1895. [DOI] [PubMed] [Google Scholar]
- 30.Kimber, S. J. 2000. Molecular interactions at the maternal-embryonic interface during the early phase of implantation. Semin. Reprod. Med. 18:237-253. [DOI] [PubMed] [Google Scholar]
- 31.Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631-664. [DOI] [PubMed] [Google Scholar]
- 32.Kudo, T., M. Kaneko, H. Iwasaki, A. Togayachi, S. Nishihara, K. Abe, and H. Narimatsu. 2004. Normal embryonic and germ cell development in mice lacking alpha 1,3-fucosyltransferase IX (Fut9) which show disappearance of stage-specific embryonic antigen 1. Mol. Cell. Biol. 24:4221-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar, R., J. Yang, R. L. Eddy, M. G. Byers, T. B. Shows, and P. Stanley. 1992. Cloning and expression of the murine gene and chromosomal location of the human gene encoding N-acetylglucosaminyltransferase I Glycobiology 2:383-393. (Erratum, 9:ix, 1999.) [DOI] [PubMed] [Google Scholar]
- 34.Lewandoski, M., K. M. Wassarman, and G. R. Martin. 1997. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 7:148-151. [DOI] [PubMed] [Google Scholar]
- 35.Liu, C., E. S. Litscher, and P. M. Wassarman. 1995. Transgenic mice with reduced numbers of functional sperm receptors on their eggs reproduce normally. Mol. Biol. Cell 6:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez, L. C., E. M. Bayna, D. Litoff, N. L. Shaper, J. H. Shaper, and B. D. Shur. 1985. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J. Cell Biol. 101:1501-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe, J. B., and J. D. Marth. 2003. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72:643-691. [DOI] [PubMed] [Google Scholar]
- 38.Metzler, M., A. Gertz, M. Sarkar, H. Schachter, J. W. Schrader, and J. D. Marth. 1994. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 13:2056-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, D. J., M. B. Macek, and B. D. Shur. 1992. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature 357:589-593. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi, S., and M. Nakano. 1993. Structural characterization of the N-linked carbohydrate chains from mouse zona pellucida glycoproteins ZP2 and ZP3. Biochim. Biophys. Acta 1158:217-226. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell, N., N. E. Zachara, G. W. Hart, and J. D. Marth. 2004. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol. Cell. Biol. 24:1680-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen, T., and H. Peters. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 17:555-557. [DOI] [PubMed] [Google Scholar]
- 43.Poirier, F., and S. Kimber. 1997. Cell surface carbohydrates and lectins in early development. Mol. Hum. Reprod. 3:907-918. [DOI] [PubMed] [Google Scholar]
- 44.Pownall, S., C. A. Kozak, K. Schappert, M. Sarkar, E. Hull, H. Schachter, and J. D. Marth. 1992. Molecular cloning and characterization of the mouseUDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucos-aminyltransferase I gene. Genomics 12:699-704. [DOI] [PubMed] [Google Scholar]
- 45.Rankin, T., P. Talbot, E. Lee, and J. Dean. 1999. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 126:3847-3855. [DOI] [PubMed] [Google Scholar]
- 46.Rankin, T. L., J. S. Coleman, O. Epifano, T. Hoodbhoy, S. G. Turner, P. E. Castle, E. Lee, R. Gore-Langton, and J. Dean. 2003. Fertility and taxon-specific sperm binding persist after replacement of mouse sperm receptors with human homologs. Dev. Cell 5:33-43. [DOI] [PubMed] [Google Scholar]
- 47.Rankin, T. L., M. O'Brien, E. Lee, K. Wigglesworth, J. Eppig, and J. Dean. 2001. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 128:1119-1126. [DOI] [PubMed] [Google Scholar]
- 48.Rankin, T. L., Z. B. Tong, P. E. Castle, E. Lee, R. Gore-Langton, L. M. Nelson, and J. Dean. 1998. Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development 125:2415-2424. [DOI] [PubMed] [Google Scholar]
- 49.Riethmacher, D., V. Brinkmann, and C. Birchmeier. 1995. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 92:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson, M. A., J. R. Etchison, J. S. Robertson, D. F. Summers, and P. Stanley. 1978. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistant CHO cells. Cell 13:515-526. [DOI] [PubMed] [Google Scholar]
- 51.Rodeheffer, C., and B. D. Shur. 2004. Characterization of a novel ZP3-independent sperm-binding ligand that facilitates sperm adhesion to the egg coat. Development 131:503-512. [DOI] [PubMed] [Google Scholar]
- 52.Schachter, H. 2000. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj. J. 17:465-483. [DOI] [PubMed] [Google Scholar]
- 53.Schlesinger, S., C. Gottlieb, P. Feil, N. Gelb, and S. Kornfeld. 1975. Growth of enveloped RNA viruses in a line of Chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J. Virol. 17:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shafi, R., S. P. Iyer, L. G. Ellies, N. O'Donnell, K. W. Marek, D. Chui, G. W. Hart, and J. D. Marth. 2000. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. USA 97:5735-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shur, B. D., and N. G. Hall. 1982. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J. Cell Biol. 95:574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley, P., S. Narasimhan, L. Siminovitch, and H. Schachter. 1975. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase activity. Proc. Natl. Acad. Sci. USA 72:3323-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su, A. I., T. Wiltshire, S. Batalov, H. Lapp, K. A. Ching, D. Block, J. Zhang, R. Soden, M. Hayakawa, G. Kreiman, M. P. Cooke, J. R. Walker, and J. B. Hogenesch. 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101:6062-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surani, M. A. 1979. Glycoprotein synthesis and inhibition of glycosylation by tunicamycin in preimplantation mouse embryos: compaction and trophoblast adhesion. Cell 18:217-227. [DOI] [PubMed] [Google Scholar]
- 59.Talbot, P., B. D. Shur, and D. G. Myles. 2003. Cell adhesion and fertilization: steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion. Biol. Reprod. 68:1-9. [DOI] [PubMed] [Google Scholar]
- 60.Tarentino, A. L., R. B. Trimble, and T. H. Plummer, Jr. 1989. Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 32:111-139. [DOI] [PubMed] [Google Scholar]
- 61.Thall, A. D., P. Maly, and J. B. Lowe. 1995. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 270:21437-21440. [DOI] [PubMed] [Google Scholar]
- 62.Tulsiani, D. R., S. K. Nagdas, G. A. Cornwall, and M. C. Orgebin-Crist. 1992. Evidence for the presence of high-mannose/hybrid oligosaccharide chain(s) on the mouse ZP2 and ZP3. Biol. Reprod. 46:93-100. [DOI] [PubMed] [Google Scholar]
- 63.Wang, X. Q., Z. M. Zhu, B. A. Fenderson, G. Q. Zeng, Y. J. Cao, and G. T. Jiang. 1998. Effects of monoclonal antibody directed to LeY on implantation in the mouse. Mol. Hum. Reprod. 4:295-300. [DOI] [PubMed] [Google Scholar]
- 64.Wassarman, P. M. 1988. Zona pellucida glycoproteins. Annu. Rev. Biochem. 57:415-442. [DOI] [PubMed] [Google Scholar]
- 65.Watson, A. J., and L. C. Barcroft. 2001. Regulation of blastocyst formation. Front Biosci. 6:D708-730. [DOI] [PubMed] [Google Scholar]
- 66.Ye, Z., and J. D. Marth. 2004. N-glycan branching requirement in neuronal and post-natal viability. Glycobiology 14:547-558. [DOI] [PubMed] [Google Scholar]
- 67.Zhu, Z. M., N. Kojima, M. R. Stroud, S. I. Hakomori, and B. A. Fenderson. 1995. Monoclonal antibody directed to LeY oligosaccharide inhibits implantation in the mouse. Biol. Reprod. 52:903-912. [DOI] [PubMed] [Google Scholar]