Abstract
Tankyrases are novel poly(ADP-ribose) polymerases that have SAM and ankyrin protein-interaction domains. They are found at telomeres, centrosomes, nuclear pores, and Golgi vesicles and have been shown to participate in telomere length regulation. Their other function(s) are unknown, and it has been difficult to envision a common role at such diverse cellular locations. We have shown that tankyrase 1 polymerizes through its sterile alpha motif (SAM) domain to assemble large protein complexes. In vitro polymerization is reversible and still allows interaction with ankyrin-domain binding proteins. Polymerization can also occur in vivo, with SAM-dependent association of overexpressed tankyrase leading to formation of large tankyrase-containing vesicles, disruption of Golgi structure, and inhibition of apical secretion. Finally, tankyrase polymers are dissociated efficiently by poly(ADP-ribosy)lation. This disassembly is prevented by mutation of the PARP domain. Our findings indicate that tankyrase 1 has the unique capacity to promote both assembly and disassembly of large protein complexes. Thus, tankyrases appear to be master scaffolding proteins that regulate the formation of dynamic protein networks at different cellular locations. This implies a common scaffolding function for tankyrases at each location, with specific tankyrase interaction partners conferring location-specific roles to each network, e.g., telomere compaction or regulation of vesicle trafficking.
Tankyrase 1 and 2 are homologous multidomain proteins that contain ∼20 ankyrin repeats, a SAM (sterile alpha motif) domain, and a PARP (poly(ADP-ribose) polymerase) domain (31). The PARP domain catalyses the addition of poly(ADP-ribose) onto acceptor proteins with NAD+ as a substrate (34). This posttranslational modification confers a large negative charge to the modified protein and hence has the ability to alter protein function and interactions (9, 31). The ankyrin and SAM domains both mediate protein-protein interactions. The SAM domain enables tankyrase to self-associate, while the ankyrin repeats interact with a number of unrelated proteins such as TRF1, NuMA, IRAP, and Grb 14 (7, 12, 21, 25, 34). These tankyrase binding proteins participate in a wide array of cellular processes such as telomere and spindle organization, Golgi dynamics, and apoptosis. Their various functions are reflected in the diverse array of cellular locations at which tankyrases are found; these include telomeres, centrosomes, nuclear pores, and Golgi-associated vesicles (7, 32).
Tankyrase function is best understood at telomeres, where it appears to regulate telomere length by altering the organization of the telomeric DNA-protein complex. Poly(ADP-ribosyl)ation of the telomere protein TRF1 by tankyrase leads to release of TRF1 from the telomere (6, 8, 34). This loss of TRF1 gives telomerase increased access to the DNA terminus (37), thus allowing telomere elongation. Recent studies indicate that tankyrase has an additional telomeric function during mitosis, as the telomeres of sister chromatids require tankyrase 1 to separate (13).
The function of tankyrase at centrosomes and nuclear pores is unknown, but its association with Golgi vesicles is thought to be important for vesicle trafficking (7, 21, 26). Tankyrase 1 and 2 purify with vesicle fractions, and both proteins interact with the cytoplasmic tail of IRAP, a transmembrane protein found on GLUT4 and a variety of other secretory vesicles (7). GLUT4 vesicles are present in adipocytes, where they translocate to the membrane upon insulin stimulation and mediate glucose uptake (5). Interestingly, insulin increases tankyrase activity through the mitogen-activated protein kinase pathway (7), and tankyrase 2 is known to interact with Grb14, an adapter protein that dampens insulin signaling (21). These findings connect tankyrases to insulin signaling, but their exact role in this pathway remains unclear. A further indication that tankyrase is involved in signal transduction comes from the finding that both tankyrases interact with members of the Mcl-1 protein family (1). Mcl-1 proteins are regulators of apoptosis, and overexpression of tankyrase 1 antagonized their apoptotic function. These connections to signal transduction, together with the known capacity of poly(ADP-ribosyl)ation to modify protein function, suggest that tankyrases may act as the effector molecules for various signaling pathways.
To learn more about the general role of tankyrase at different cellular locations, we have focused on interactions mediated by the tankyrase SAM domain. SAM domains are versatile interaction modules that commonly mediate homo- and heterotypic protein-protein interactions (18). They exist as compact five helical bundles and have been found in >1,000 different proteins. While most SAM domains are thought to mediate dimerization, the SAM domains of the ephrin B2 receptor (EphB2) and the transcriptional repressors TEL (translocation Ets leukemia) and polyhomeotic (PH) have two separate interaction surfaces and can promote formation of oligomers or polymers (19, 20, 35). EphB2 oligomerization is quite weak (30), but self-association is thought to occur when ligand binding causes receptor clustering. This may create a new interaction surface for recruiting proteins that act downstream in the signaling pathway. In contrast, polymerization of both TEL and PH is robust and results in the formation of long filaments. These are thought to promote transcriptional repression by promoting spreading of silent chromatin (19, 20).
Our group has previously shown that the SAM domains from tankyrase 1 and 2 can self-associate to form multiprotein complexes when incubated with chemical cross-linkers (12). We now show that full-length tankyrase 1 resembles TEL and PH in that it can also undergo SAM-mediated polymerization. We further show that these tankyrase polymers can be disrupted by auto-poly(ADP-ribosyl)ation. This unique ability to mediate both complex assembly and disassembly suggests that tankyrases are master scaffolding proteins which regulate the formation of large protein networks.
MATERIALS AND METHODS
Cell lines.
LMH cells (ATCC CRL-2117) were maintained in RPMI medium supplemented with 10% fetal calf serum, 1% chicken serum, 100 U of penicillin/liter, 50 μg of streptomycin/ml, 2 mM glutamine, and 0.1 mM β-mercapto-ethanol. Sf21 insect cells were grown in SF-900 medium with 10% fetal calf serum and 50 U of penicillin/liter-25 μg of streptomycin/ml.
Antibodies.
Antibody to the SAM domain of chicken tankyrase 1 was raised in rabbits as described previously (12). Other antibodies used were as follows: anti-poly(ADP-ribose) (clone 10H; Pharmingen); mouse anti-MYC (clone 9E10; ATTC CRL-1729); rabbit anti-MYC (polyclonal ab6; Neomarkers); Anti-FTCD (clone 58K-9; Sigma); anti-mouse and anti-rabbit horseradish peroxidase conjugates (Pierce); RRX anti-mouse and Cy2 anti-mouse (Jackson Immuno Labs); Cy2 anti-rabbit (Rockland); and normal goat serum (Jackson Immuno Labs).
Expression constructs.
Sections of chicken tankyrase 1 and 2 (12) were cloned into pcDNA3 with N-terminal Myc or Flag tags. Tankyrase1-ΔSAM was constructed by overlap PCR using primers that deleted nucleotides 2884 to 3120. Catalytically inactive tankyrase 1 (tankyrase1-PARP†) was created by mutating H1122→A and E1229→A with a QuikChange site-directed mutagenesis kit (Stratagene). The Flag-SAM construct encoded amino acids 891 to 1089 of tankyrase 1. Glycosylphosphatidylinositol-anchored green fluorescent protein (GPI-GFP) and vesicular stomatitis virus glycoprotein 3 fused to GFP (VSVG3-GFP) were expressed from plasmids GFP-GL-GPI and VSVG3-SP-GFP, respectively, as described previously (16).
Protein expression.
MBP fusion proteins were expressed in Escherichia coli and purified as described previously (12). His-tagged chicken tankyrase 1, tankyrase1-ΔSAM, and human TRF1 were cloned into pFastbac1 and expressed using a Bac-to-Bac baculovirus expression system (Invitrogen). Sf21 cells were infected at a multiplicity of infection (MOI) of 10 and harvested after 47 h (hTRF1) or 72 h (full-length tankyrase 1 [FL-tankyrase1] and tankyrase1-ΔSAM). To purify TRF1 and tankyrase1-ΔSAM, cells were lysed with buffer A (500 mM NaCl, 20 mM Tris-HCl [pH 7.9], 5 mM imidazole, 1 mM phenylmethylsulfonyl fluoride [PMSF]) and yeast protease inhibitor cocktail (6 μg of chymostatin/ml, 1 μg of E64/ml, 2 μg of aprotinin/ml, 0.5 μg of phosphoramidon/ml, 1 μg of pepstatin A/ml, 5 μg of leupeptin/ml, 5 μg of antipain/ml, 0.1 mM benzamidine). The cells were sonicated briefly, extracted for 30 min on ice, and centrifuged to remove debris. The supernatant was applied to Ni2+-charged chelating Sepharose (Amersham Pharmacia) and incubated for 1 h at 4°C with rocking. The beads were washed extensively with buffer B (500 mM NaCl, 20 mM Tris-HCl [pH 7.9], 80 mM imidazole). Protein was eluted in three fractions with buffer C (500 mM NaCl, 20 mM Tris-HCl [pH 7.9], 500 mM imidazole) and dialyzed against buffer D (300 mM KCl, 20 mM HEPES [pH 7.5], 3 mM MgCl2, 20% glycerol, 1 mM dithiothreitol [DTT]). hTRF1 was tested for DNA binding activity by a mobility shift assay using double-stranded DNA containing five T2AG3 repeats (34). Cells expressing FL-tankyrase1 were washed with phosphate-buffered saline (PBS) and lysed with modified buffer A (500 mM NaCl, 20 mM Tris-HCl [pH 7.9], 3 mM MgCl2, 5 mM imidazole, 0.05% Triton X-100, 1 mM PMSF, yeast protease inhibitor cocktail). After sonication, additional modified buffer A was added to achieve a final volume of one-half of the culture volume. Cells were extracted for 30 min at 30°C and centrifuged to pellet debris. Subsequent purification steps were performed essentially as described for hTRF1 except that buffer D was modified to 300 mM NaCl-20 mM HEPES (pH 7.5)-3 mM MgCl2-10% glycerol-5 mM β-mercapto-ethanol.
Gel filtration.
Samples were separated on a Superose 6 column (Amersham Pharmacia) at 4°C, samples were centrifuged at 100,000 × g before loading, and 350-μl fractions were collected and analyzed by Western blotting with tankyrase antibody. For analysis of purified MBP-SAM1, the column was equilibrated with 20 mM HEPES (pH 7)-150 mM NaCl-1 mM DTT. For analysis of endogenous tankyrase, ∼7 × 106 LMH cells were extracted for 30 min on ice with buffer E (150 mM NaCl, 50 mM Tris [pH 8.0], 0.2 mM EDTA, 5 mM MgCl2, 1% NP-40, 0.5 mM PMSF, yeast protease inhibitor cocktail) supplemented with 100 μg of α-2-macroglobulin/ml. The column was equilibrated with buffer E supplemented with 100 μg of α-2-macroglobulin/ml but without the protease inhibitors. One-fifth of the extract was loaded on the column, and fractions were trichloroacetic acid precipitated prior to Western blotting. For analysis of overexpressed Myc-tankyrase 1 and Myc-tankyrase1-ΔSAM, 5 × 106 LMH cells were transfected with 24 μg of DNA and 72 μl of TransIT-LT1 (Mirus) according to the manufacturer's instructions. Cells were harvested 24 h posttransfection and extracted for 30 min at 25°C with buffer E plus 1 mM DTT. The column was equilibrated with buffer E plus 1 mM DTT.
Electron microscopy (EM).
Purified MBP-SAM1 was adsorbed for 3 min to Formvar-coated 200-mesh copper grids (EMS), and grids were then negatively stained with filtered 1% uranyl acetate for 3 min. Digital images were taken at 80 kV on a JEOL JEM-1230 transmission electron microscope equipped with an AMT Advantage Plus 2K by 2K digital camera. The lengths of 40 MBP-SAM1 rods were determined from digital electron micrographs of negatively stained MBP-SAM1. The rods were then grouped into the length categories shown in Fig. 1D. For proteinase K treatment, MBP-SAM1 was incubated with 200 μg of proteinase K (Invitrogen)/ml at 42°C for 3.5 h in the presence of 5 mM CaCl2.
FIG. 1.
Self-association of the tankyrase 1 SAM domain. (A) Superose 6 fractionation of MBP-SAM1. Peak fractions containing molecular weight markers are indicated: 2,000 kDa, dextran blue; 669 kDa, thyroglobulin; 232 kDa, catalase. Fraction numbers are shown below. (B and C) Transmission electron microscopy of uranyl acetate-stained MBP-SAM1 polymers. Arrows indicate SAM polymers. Samples shown in panel C were treated with proteinase K prior to deposition on the EM grid. Bar, 100 nm. (D) Histogram showing the length distribution of MBP-SAM1 rods. Mean (37 nm) and median (36 nm) lengths were calculated for 40 rods, but the shortest rods were probably underrepresented because they were difficult to distinguish from background.
Pelleting and dilution assays.
A total of 20 μl of a 12.5 μg/ml solution of purified protein was centrifuged at 18,000 × g for 5 min. Pellets were separated from supernatants, and the entire sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. For the dilution experiment, purified His-tankyrase1 (50 μg/ml) was diluted 3×, 9×, 27×, 81×, and 243× with dilution buffer (300 mM NaCl, 20 mM HEPES [pH 7.5], 3 mM MgCl2, 10% glycerol, 1 mM DTT, 0.001% NP-40). After 1 h at 30°C, 20 μl (or 2 × 20 μl) of each sample was centrifuged for 15 min at 18,000 × g and the following sample volumes were analyzed by SDS-PAGE: for the 3× dilution, 9 μl; for the 9× dilution, 27 μl; for the 27× dilution, 3 μl; for the 81× dilution, 9 μl; and for the 243× dilution, 27 μl. Tankyrase was detected by Western blotting using West-Pico chemiluminescent substrate (Pierce) for the 3× and 9× dilutions and West-Femto substrate (Pierce) for the remaining dilutions. To determine whether TRF1 interacts with polymerized tankyrase, His-tagged TRF1 was incubated with or without purified His-tagged tankyrase 1 or tankyrase1-ΔSAM for 1 h at 37°C in modified buffer D and then centrifuged for 15 min at 18,000 × g. To pellet overexpressed protein, LMH cells were transiently transfected with 2 μg of FL-tankyrase1, tankyrase1-ΔSAM, and tankyrase1-PARP† by use of TransIT-LT1 (Mirus). Extracts were prepared at 4 and 25°C 24 h after transfection and analyzed as described above. To study the effect of poly(ADP-ribosyl)ation, purified protein or cell extracts were incubated with 1 mM NAD+ and/or 10 mM 3-amino-benzamide prior to centrifugation.
Tankyrase activity assay.
Activity assays were performed using 150 mM NaCl-20 mM HEPES (pH 7.0). A total of 0.02 μg of purified His-tankyrase1 or His-tankyrase1-ΔSAM was incubated with combinations of the following: 1 mM NAD+, 10 mM 3AB, and 0.05 μg of TRF1. After 1 h at 37°C, reactions were stopped by addition of SDS-PAGE loading buffer and one-half of each reaction mixture was used for analysis by Western blotting with anti-poly(ADP-ribose) antibody.
Immunofluorescence.
Cells were grown on collagen-coated glass coverslips (Becton Dickinson), washed three times in PBS, fixed with 4% formaldehyde, washed twice in PBS, permeabilized with 0.1% Triton X-100, washed twice with PBS, blocked for 30 min with either 1% bovine serum albumin (BSA) in PBS (for transfected cells) or 5% normal goat serum in PBS (for detection of endogenous tankyrase), incubated with primary antibodies for 90 min, washed five times with 1% BSA in PBS, incubated with secondary antibodies for 45 min, stained with DAPI (4′,6′-diamidino-2-phenylindole) (2.5 μg/ml), washed five times with 1% BSA in PBS, mounted with antifading reagent (gel/mount; Biomeda), and visualized on a Nikon E600 epifluorescence microscope with a 100× oil immersion objective.
RESULTS
Polymerization of the tankyrase SAM domain.
Although our previous studies demonstrated that the SAM domains of tankyrase 1 and 2 can be cross-linked into large homotypic complexes (12), chemical cross-linkers can trap transient interactions and then drive oligomerization. Thus, this approach did not yield information about the stability of the SAM-domain association or the natural size of the SAM domain complexes. We therefore sought to determine the extent to which the tankyrase SAM domain can self-associate in the absence of cross-linker. In initial experiments, we analyzed the size of purified SAM domain by gel filtration. The SAM domain of tankyrase 1 was produced in E. coli as a maltose binding protein fusion (MBP-SAM1) and purified by amylose affinity chromatography. When the 65-kDa fusion protein was applied to a Superose 6 column, it showed a broad elution profile (Fig. 1A). The peak fraction corresponded to a mass of ∼930 kDa or complexes of ∼14 MBP-SAM1 molecules, but a significant amount (>2,000 kDa) of protein fractionated close to the void volume of the column and hence must have contained complexes of >30 MBP-SAM1 molecules. Since our previous experiments demonstrated that MBP does not mediate self-association (12), this result indicates that the tankyrase 1 SAM domain can polymerize in absence of cross-linker. Moreover, as the polymers remain assembled during gel filtration, the interactions mediating the SAM-SAM association must be quite stable.
To further examine the tankyrase SAM-domain complexes, we performed transmission electron microscopy on purified MBP-SAM1 preparations that had been negatively stained with uranyl acetate. As shown in Fig. 1B and D, we observed small rod-shaped structures that varied in length from ∼18 to 80 nm, with a mean of ∼37 nm. They were only observed in the presence of purified MBP-SAM1 and were destroyed by treatment with proteinase K (Fig. 1C), confirming that they were composed of the MBP-SAM1 protein. Thus, the tankyrase 1 SAM domain resembles the SAM domains of the TEL and PH repressors, which also polymerize into ordered rod or fiber-like structures that can be observed by EM (19, 20, 36).
Self-association of full-length tankyrase.
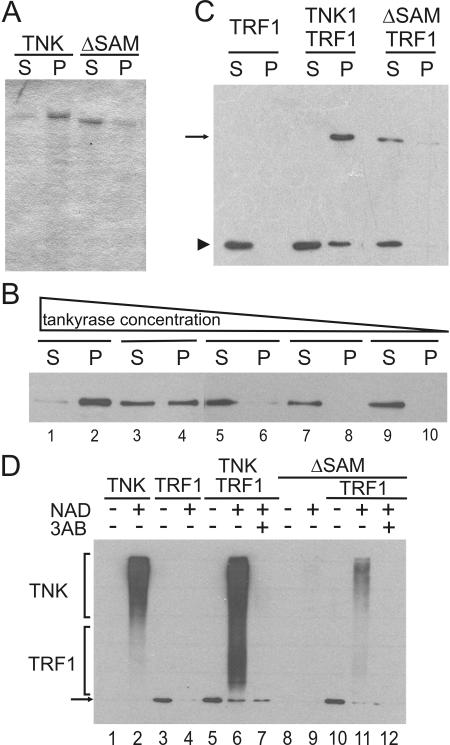
We next sought to determine whether full-length tankyrase also undergoes SAM-dependent polymerization. His-tagged FL-tankyrase1 and His-tagged tankyrase 1 lacking the SAM domain (tankyrase1-ΔSAM) were produced in Sf21 insect cells by use of a baculovirus expression vector and purified by Ni2+-affinity chromatography. After elution with imidazole and dialysis to reduce the imidazole concentration, both protein preparations were subjected to centrifugation (5 min at 18,000 × g) to remove any insoluble material. Interestingly, nearly all the FL-tankyrase1 was pelleted, whereas most of the tankyrase1-ΔSAM remained in the supernatant (Fig. 2A). Since the FL-tankyrase1 insolubility could reflect protein misfolding and subsequent aggregation rather than SAM-dependent polymerization, we next tested whether the solubility was concentration dependent, as might be expected for a specific protein-protein interaction.
FIG. 2.
Solubility and catalytic activity of full-length tankyrase 1. (A) Solubility of recombinant tankyrase. Purified full-length tankyrase 1 (TNK) or tankyrase1-ΔSAM (ΔSAM) was centrifuged, and protein in the supernatant (S) or pellet (P) was detected by SDS-PAGE and Coomassie staining. (B) Increase in tankyrase 1 solubility with increasing dilution. Purified tankyrase was diluted to 16.7, 5.6, 1.9, 0.62, or 0.2 μg/ml prior to centrifugation, and protein in the supernatant (S) or pellet (P) was detected by Western blotting with tankyrase antibody. (C) TRF1 binding to tankyrase polymers. His-tagged TRF1 was incubated with or without purified His-tagged tankyrase1 (TNK) or tankyrase1-ΔSAM (ΔSAM) and then centrifuged. Soluble and pelleted fractions were analyzed by Western blotting with His antibody. The arrow points to tankyrase 1 or tankyrase1-ΔSAM; the arrowhead indicates TRF1. (D) PARP activity of recombinant tankyrase 1. Tankyrase (upper bracket) or TRF1 (lower bracket) poly(ADP-ribosyl)ation was detected by Western blotting with antibody to poly(ADP-ribose). The arrow marks the input TRF1, which started with a low level of modification. This was probably caused by a copurifying PARP activity. The proteins present in each reaction mixture are indicated at the top; the presence (+) or absence (−) of 1 mM NAD and 10 mM 3-aminobenzamide (3AB) is indicated.
To test for solubility, we made serial dilutions of FL-tankyrase1 and centrifuged identical volumes of each dilution. The protein was then detected in the supernatant or pellet by Western blotting with tankyrase antibody. As shown in Fig. 2B, decreasing the tankyrase concentration led to a dramatic change in the fraction of protein that was soluble; essentially all the protein in a ∼120 nM solution appeared in the pellet, whereas most of the protein in a ∼15 nM solution remained in the supernatant. Thus, the insolubility of full-length tankyrase appeared to be caused by specific SAM domain-mediated polymerization into a high-molecular-weight form. This behavior is similar to what was previously observed for the purified TEL repressor, with which SAM-mediated polymerization leads to the formation of large, insoluble complexes (20, 36). We were also able to cycle full-length tankyrase between soluble and insoluble forms by the addition and removal of imidazole but not by adding salt (NaCl) and detergent (Triton X-100) (data not shown). Purified FL-tankyrase1 was soluble when first eluted from the Ni+ column with imidazole; it became insoluble when the imidazole was removed by dialysis but returned to the soluble form upon readdition of imidazole. The reversible nature of the tankyrase insolubility again indicates that solubility is determined by a specific polymerization-depolymerization reaction.
To further assess the properties of the polymerized tankyrase, we next determined whether it retained the capacity to interact with TRF1, a known ankyrin domain binding protein, and whether it was catalytically active. To test for interaction with TRF1, we incubated FL-tankyrase1 or tankyrase1-ΔSAM with purified TRF1 and then centrifuged the sample to monitor TRF1 solubility. As shown in Fig. 2C, all the TRF1 was soluble when incubated without tankyrase or with tankyrase1-ΔSAM, but much of it became insoluble in the presence of FL-tankyrase1, indicating that TRF1 can interact with tankyrase polymers.
To examine the catalytic activity of the tankyrase polymers we assayed for autoribosylation and poly(ADP-ribosyl)ation of TRF1. Purified tankyrase was incubated with its substrate, NAD+, in the presence or absence of purified TRF1 and the PARP inhibitor 3-amino-benzamide (3AB). PARP activity was then assessed by Western blot analysis using an antibody to poly(ADP-ribose) (Fig. 2D). FL-tankyrase1 showed robust autoribosylation (Fig. 2D, lane 2) and poly(ADP-ribosyl)ation of TRF1 (lane 6). The activity was dependent on the presence of NAD+ and inhibited by 3AB. Interestingly, the tankyrase1-ΔSAM was much less active than FL-tankyrase1 (lanes 9 and 11). This decrease in PARP activity suggests that the SAM domain is required for optimal catalytic activity, perhaps because dimerization-oligomerization promotes catalysis, as has been observed for the related enzyme PARP-1 (22, 23). Overall, our results show that full-length, catalytically competent tankyrase 1 molecules can undergo a reversible SAM-mediated association that results in assembly of large high-molecular-weight complexes. As the isolated SAM domain of tankyrase 2 can also self-associate (12), we would expect tankyrase 2 to also oligomerize in a SAM-dependent manner.
In vivo formation of tankyrase complexes.
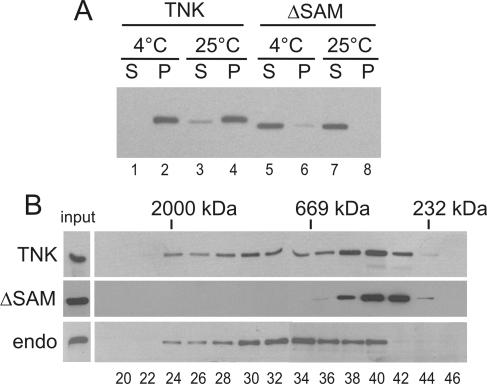
To evaluate whether tankyrases can also polymerize in vivo, we assayed for tankyrase complexes in cells that overexpressed tankyrase 1. Initially we carried out a simple pelleting experiment using extracts from chicken LMH cells that had been transiently transfected with Myc-tagged FL-tankyrase1 or tankyrase1-ΔSAM. The cells were lysed with NP-40 24 h after transfection, and the cell lysates were centrifuged to assess the solubility of the tagged protein. As shown in Fig. 3A, essentially all the FL-tankyrase1 was pelleted when extracts were prepared at 4°C and only a small fraction was soluble at 25°C. In contrast, the tankyrase1-ΔSAM was fully soluble at both temperatures. The difference in solubility between the full-length protein and tankyrase1-ΔSAM in cell extracts is strikingly similar to what was observed with the purified protein, suggesting that tankyrase can also undergo SAM-mediated polymerization in vivo. While the SAM-dependent insolubility could be caused by the SAM domain mediating interactions with other insoluble proteins (e.g., components of the cytoskeleton), the high level of tankyrase expression would have been expected to saturate such interactions, leaving some protein free in solution.
FIG. 3.
In vivo complex formation. (A) Solubility of overexpressed tankyrase 1 in LMH cell lysates. Cells expressing FL-tankyrase1 (TNK) or tankyrase1-ΔSAM (ΔSAM) were lysed at 4 or 25°C and centrifuged, and tankyrase was detected in the supernatant (S) or pellet (P) by Western blotting with tankyrase antibody. (B) Superose 6 fractionation of soluble tankyrase extracted from cells expressing FL-tankyrase1 (TNK) or tankyrase1-ΔSAM (ΔSAM) or from untransfected cells (endo). The total amount of protein loaded on the column was ∼250 μg from TNK- and ΔSAM-expressing cells and ∼650 μg from wild-type cells. The bands corresponding to the input material are shown in the lane to the left; the amounts loaded were 1% of the total for TNK, 2% for SAM, and 10% for wild-type extracts. The peak fractions containing marker proteins are shown at the top, and fraction numbers are shown below. Detection was by Western blotting with tankyrase antibody.
The small amount of soluble tankyrase 1 obtained after room temperature extraction suggested that tankyrase polymers tend to disassemble at higher temperatures. To assess the size of the soluble FL-tankyrase1 complexes and the tankyrase1-ΔSAM, we fractionated extracts from tankyrase-transfected LMH cells by gel filtration on a Superose 6 column. Although most proteins in the extracts from FL-tankyrase-expressing cells eluted as a well defined peak, tankyrase exhibited a broad elution profile, with the largest complexes fractionating close to the void volume (>2,000 kDa) and the smallest showing a mass of 250 to 350 kDa. In contrast, tankyrase1-ΔSAM eluted in a sharp peak, with an apparent mass of 350 kDa. Thus, even under conditions that appear to cause partial dissociation of tankyrase polymers, much of the full-length tankyrase still exists in large complexes that are dependent on the SAM domain. It is unclear whether the tankyrase1-ΔSAM exists as a monomer or as a small complex, as the deviation from the expected mass of 130 kDa for the monomer could result from the elongated shape of the ankyrin repeat domain (27).
To compare the size of the complexes containing overexpressed tankyrase to those formed by endogenous tankyrase, we also performed gel filtration with extracts from untransfected LMH cells. The solubility of endogenous tankyrase depended on the volume of buffer used to extract the cells (data not shown); thus, for these experiments we used sufficient buffer to render most of the tankyrase soluble. When fractionated on a Superose 6 column, endogenous tankyrase 1 displayed an elution profile similar to that of the overexpressed FL-tankyrase1 (Fig. 3B). Thus, like the overexpressed tankyrase, the endogenous protein also exists in very large complexes. However, we would expect these complexes to contain a higher ratio of tankyrase interaction partners than complexes from cells that overexpress tankyrase, because the level of overexpressed tankyrase is likely to greatly exceed that of the interaction partners. In both cases, the complexes that can be extracted from cells are likely to be considerably smaller than the full-sized complexes that exist in vivo.
Overexpressed tankyrase localizes to vesicle-like structures.
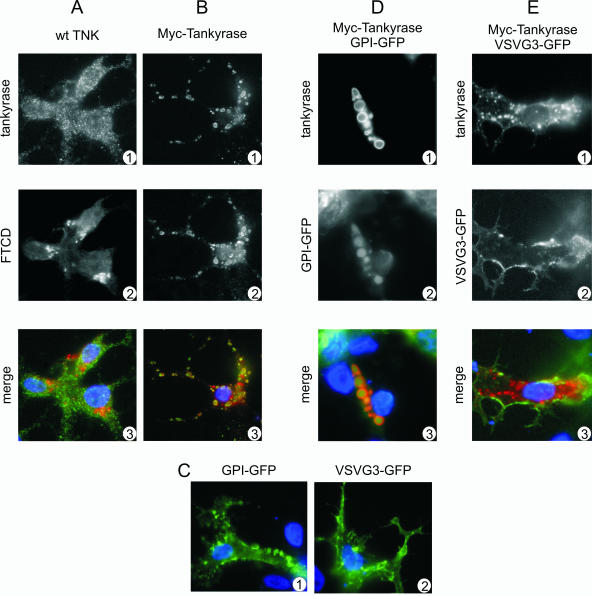
To analyze the nature of the tankyrase complexes formed in vivo, we used indirect immunofluorescence to examine the location of tankyrase in LMH cells transiently transfected with Myc-tagged FL-tankyrase1 or tankyrase1-ΔSAM and with Flag-tagged SAM domain from tankyrase 1. The FL-tankyrase1 showed an unexpected localization pattern, with many cells exhibiting staining of large vesicle-like structures rather than the punctate staining pattern observed with the endogenous protein (compare Fig. 4A, panel 1, to Fig. 4B). The SAM domain was required for this localization, as cells expressing tankyrase1-ΔSAM instead showed a diffuse cytoplasmic staining. This finding suggests that the vesicle-like structures resulted from SAM-mediated association between tankyrase molecules. Similar large vesicle-like structures were seen when chicken tankyrase 1 was expressed in HeLa cells and when chicken tankyrase 2 was expressed in LMH cells (data not shown).
FIG. 4.
In vivo tankyrase localization. (A) LMH cells expressing Myc-tagged FL-tankyrase1 (Myc-Tankyrase), Myc-tagged tankyrase1-ΔSAM (Myc-ΔSAM), or Flag-tagged SAM domain (Flag-SAM) were fixed and incubated with antibody to the Myc or Flag tags. Nuclei were counterstained with DAPI. (B) Untransfected cells were stained with antibody to the tankyrase SAM domain (panel 1), tankyrase antibody that had been preincubated with purified SAM domain (panel 2), or secondary antibody (panel 3). (C) Cells coexpressing Myc-tagged FL-tankyrase1 and Flag-tagged SAM domain. Panel 1, staining with Myc antibody; panel 2, staining with Flag antibody; panel 3, combination of the Myc (green) and Flag (red) signals. (D) Examples of the different size of vesicle-like structures observed in FL-tankyrase1-expressing cells. Detection was with the Myc antibody.
Examination of cells overexpressing Flag-tagged SAM domain revealed a diffuse cytoplasmic staining similar to that observed with tankyrase1-ΔSAM, indicating that the isolated domain was not sufficient to form the vesicle-like structures (Fig. 4A, panels 2 and 3). However, when the SAM domain was coexpressed with FL-tankyrase1, the distribution of the isolated SAM domain changed and it also became localized to the vesicle-like structures (Fig. 4C). These observations indicate that tankyrase SAM-SAM interactions take place in vivo as well as in vitro. They also show that regions outside the SAM domain are required for formation of the “tankyrase vesicle” structures. One possibility is that the ankyrin domain mediates recruitment of tankyrase molecules by a membrane-associated protein (e.g., IRAP), with SAM-mediated polymerization then leading to the formation of tankyrase vesicles.
Although the tankyrase vesicles were larger than normal vesicles, optical sectioning by confocal microscopy revealed that they had the same typical spherical structure with a central lumen (data not shown). The actual sizes of the vesicles differed considerably between cells (Fig. 4D). In some cells the FL-tankyrase1 showed a punctate cytoplasmic distribution that was similar to the pattern obtained with endogenous tankyrase (compare panels 1 in Fig. 4B and D); some other cells had intermediate size vesicles with a clear lumen, while others had very large vesicles (Fig. 4D, panels 2 and 3). The size of the structures in any one cell appeared to be determined by the amount of tankyrase DNA taken up by that cell, because transfection of cells with increasing amounts of tankyrase DNA led to an increase in the fraction of cells with large or very large vesicles (data not shown). In time-course experiments conducted to look at the initial size and shape of the vesicles, FL-tankyrase1 could be detected within 4 h of transfection (data not shown). At this stage, most cells showed a punctate staining, but some already contained vesicles with a visible lumen. At subsequent time points the fraction of cells with large vesicles gradually increased, suggesting that the vesicles grow over time. Because the tankyrase vesicles result from abnormally high levels of tankyrase expression, their physiological relevance is unclear. However, their dependence on the SAM domain, together with their capacity to expand as tankyrase levels increase, provides strong support for SAM-mediated oligomerization of tankyrase in vivo.
Involvement of tankyrase in protein secretion.
The established cofractionation of tankyrase with low-density microsomes (7, 21) prompted us to investigate whether the tankyrase vesicles might affect the secretory pathway. We did this by looking for tankyrase colocalization with established secretory pathway and Golgi markers. Previous studies had indicated that the extent to which endogenous tankyrase colocalizes with the Golgi marker FTCD is dependent on the cell type. In fibroblasts, tankyrase and FTCD show an almost complete overlap in their distribution (7), while in cells of epithelial origin there is almost no overlap (21). When we examined the distribution of endogenous tankyrase and FTCD in LMH hepatocytes, we also found little colocalization between the two proteins. However, the opposite proved true for cells overexpressing FL-tankyrase1. In these cells, most of the normal Golgi staining was disrupted and much of the FTCD now colocalized with the tankyrase vesicles (Fig. 5A and B).
FIG. 5.
Effects of tankyrase overexpression on the secretory pathway. (A and B) Colocalization of tankyrase vesicles with FTCD. Control LMH cells (A) or LMH cells expressing Myc-tagged FL-tankyrase1 (B) were fixed and incubated with tankyrase antibody (A1), Myc antibody (B1), or FTCD antibody (A2 and B2). Panels A3 and B3 show an overlay of the tankyrase (green) and FTCD (red) signals. Cells were counterstained with DAPI. wt, wild type. (C and D) Effect of Myc-FL-tankyrase1 on secretion of marker proteins. LMH cells were transfected with GPI-GFP or VSVG3-GFP and analyzed by fluorescence microscopy after 30 h at 37°C (GPI-GFP) or 32°C (the permissive temperature for temperature-sensitive VSVG3-GFP). (C) Localization of GPI-GFP and VSVG3-GFP in wild-type cells. (D and E) Localization of GPI-GFP and VSVG3-GFP after tankyrase overexpression. Cells were transfected with Myc-tagged FL-tankyrase1 and GPI-GFP or VSVG3-GFP. (D1 and E1) Exogenous tankyrase detected with Myc antibody; (D2 and E2) GPI-GFP and VSVG3-GFP staining; (D3 and E3) overlay of panels 1 and 2. Red, tankyrase; green, GPI-GFP and VSVG3-GFP.
To study whether protein secretion was affected by this apparent disruption in Golgi integrity, we performed colocalization experiments with two secretory pathway markers, GPI-anchored GFP for the apical secretion pathway and VSVG3-GFP for the basolateral secretory pathway (16). When either marker construct was transfected into LMH cells in the absence of FL-tankyrase1, it showed the expected membrane localization pattern (Fig. 5C). Likewise, when VSV3-GFP and FL-tankyrase1 were cotransfected into the cells the VSV3-GFP distribution remained normal. However, cotransfection of GPI-GFP and FL-tankyrase1 caused the GPI-GFP to redistribute to the tankyrase vesicles (Fig. 5D and E). Confocal microscopy showed that the GPI-GFP colocalized with tankyrase on the periphery of the vesicles, suggesting that they are surrounded by a lipid membrane (data not shown). Moreover, no GPI-GFP could be detected at the plasma membrane, indicating that normal trafficking of GPI-GFP was disrupted. Thus, although the vesicles are clearly abnormal structures caused by tankyrase overexpression, their properties fit with previous studies linking tankyrase to Golgi dynamics (7, 26) and suggest that tankyrase may play a role in the apical secretion pathway.
Disruption of tankyrase polymers by poly-(ADP-ribosylation).
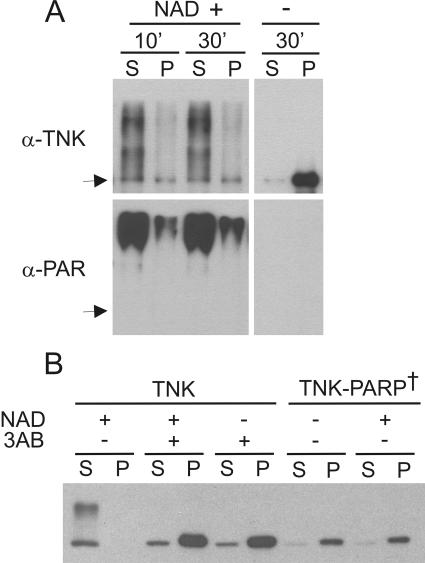
As poly(ADP-ribosyl)ation confers a large negative charge to modified proteins, it seemed likely that the charge buildup during tankyrase autoribosylation might cause tankyrase polymers to dissociate. To test this possibility, we examined the effect of autoribosylation on tankyrase solubility. Purified tankyrase was incubated with NAD+ for various periods of time; the reactions were then centrifuged, and the soluble and insoluble fractions were monitored for reaction products by Western blot analysis using antibodies to tankyrase and poly(ADP-ribose). As shown in Fig. 6A, incubation of tankyrase with NAD+ for only 10 min resulted in substantial autoribosylation and a dramatic change in tankyrase solubility. In the absence of NAD+ the tankyrase was largely insoluble, whereas after addition of NAD+ most of it became soluble and remained in the supernatant. Thus, tankyrase polymers are indeed dissociated by auto-poly(ADP-ribosyl)ation.
FIG. 6.
Dissociation of tankyrase complexes by tankyrase autoribosylation. (A) Solubility of purified full-length tankyrase 1 after incubation with NAD+. Samples were incubated with (+) or without (−) 1 mM NAD+ for 10 or 30 min at 25°C and centrifuged to separate soluble (S) from insoluble (P) material. Tankyrase was detected by Western blotting with antibody to tankyrase (α-TNK) or poly(ADP-ribose) (α-PAR). Arrow, position of unmodified tankyrase. (B) Solubility of overexpressed tankyrase 1 in LMH cell extracts. Cells expressing FL-tankyrase1 (TNK) or catalytically inactive FL-tankyrase1 (TNK-PARP†) were lysed at 25°C in the presence (+) or absence (−) of 1 mM NAD+ and/or 10 mM 3AB and centrifuged. Tankyrase was detected in the supernatant (S) or pellet (P) by Western blotting with tankyrase antibody.
Given that tankyrase overexpression causes PARP-dependent release of TRF1 from the telomere (8, 33), we expected that interactions between tankyrase and its various binding partners would also be disrupted by poly(ADP-ribosyl)ation. We therefore formed complexes between tankyrase and TRF1 and looked for an increase in TRF1 solubility after addition of NAD+ (data not shown). While TRF1 solubility did increase with time, the increase was slower than that observed with tankyrase homopolymers. However, further analysis suggested that TRF1, like TRF2, can bind to poly(ADP-ribose) chains (10). Thus, following dissociation from tankyrase, TRF1 appears to form secondary complexes with the poly(ADP-ribose) chains. Such complexes would probably be unstable in vivo, as they would be degraded by PARG (11).
To test whether poly(ADP-ribosyl)ation disrupts tankyrase complexes formed in vivo, we next examined the solubility of overexpressed tankyrase in cell extracts made in the presence of NAD+. As before, LMH cells were transiently transfected with Myc-tagged FL-tankyrase1, the cells were lysed 24 h later, and the lysate was centrifuged to separate insoluble from soluble material. In contrast to what had been observed in previous experiments where most of the FL-tankyrase1 was insoluble, addition of NAD+ to the extraction buffer caused all of the tankyrase to become soluble (Fig. 6B). The NAD+ also caused the tankyrase to run as a smear in an SDS gel, indicating that it had been poly(ADP-ribosyl)ated. Addition of 3AB to the extraction buffer prevented both poly(ADP-ribosyl)ation and conversion to the soluble form. We therefore conclude that poly(ADP-ribosyl)ation results in dissociation of tankyrase complexes that have been formed in vivo.
To ensure that the ADP-ribosylation had been carried out by tankyrase rather than another PARP activity in the cell lysate, we performed the same experiment with catalytically inactive tankyrase. Mutation of two residues in the PARP domain of human tankyrase 1 has been shown to completely inhibit its activity (8). Since the PARP domains of human and chicken tankyrase 1 are 99% identical, we mutated the same residues to obtain an inactive chicken tankyrase 1. The mutant gene was transfected into LMH cells, and cells were processed as before. As expected, the inactive tankyrase fractionated to the insoluble fraction after extraction in absence of NAD+. However, the protein also remained insoluble when NAD+ was added to the extraction buffer, indicating that dissociation of tankyrase complexes containing catalytically active tankyrase had been caused by auto-poly(ADP-ribosyl)ation.
DISCUSSION
Tankyrase was first identified as a telomere protein, and its role in telomere length regulation is now well established (8, 33, 34). Thus, the discovery that tankyrase 1 and 2 reside at diverse cellular locations and have multiple interaction partners has raised considerable debate over their nontelomeric function(s) (7, 32). Because telomeres, Golgi bodies, and centrosomes have very different functions, it has been unclear how tankyrases could play a common role at each location. We now show that tankyrase 1 can polymerize through its SAM domains both in vivo and in vitro to form very large protein complexes; the complexes formed in vitro contain >25 monomers. We also show that tankyrase can use its PARP activity to efficiently disrupt these complexes by autoribosylation. Thus, tankyrase 1 has a unique capacity to promote both formation and dissociation of large protein complexes. This property is likely to be shared by tankyrase 2, as this protein can also polymerize in vitro (12) and in vivo (data not shown) and has PARP activity (8). We therefore propose that tankyrases are master scaffolding molecules that use their SAM domains and PARP activity to first assemble and then disassemble large protein lattices at different locations in the cell (Fig. 7). These lattices are likely to perform different functions at each cellular location. At telomeres they probably help regulate opening and closing of the telomeric complex, whereas at the centrosome they may promote spindle assembly or disassembly and at Golgi vesicles they may regulate Golgi dynamics and vesicle trafficking. Different signals may activate or repress tankyrase PARP activity at specific cellular locations, thus causing a location-specific response.
FIG. 7.
Model for tankyrase lattice assembly and disassembly. Lattice assembly occurs through SAM domain-mediated self-association of tankyrase molecules and binding of tankyrase interaction partners to the individual tankyrase ankyrin repeat modules. Tankyrase autoribosylation and poly(ADP-ribosyl)ation of interaction partners causes lattice disassembly. Subsequent removal of the poly(ADP-ribose) chains by PARG would then allow lattice reassembly.
Tankyrase as a scaffolding molecule.
The ability to bring proteins together into large complexes is a characteristic that is shared by many different scaffolding proteins. These proteins are important for defining cellular structures (2, 15), and they function in signal transduction pathways, where they bring together groups of signaling molecules or anchor them at specific locations within a cell (4, 14, 24). Because tankyrases have five separate ankyrin repeat clusters that each interact independently with tankyrase binding proteins, they had previously been proposed to act as scaffolding molecules (28, 29). However, our findings add a new dimension to the proposed scaffolding function. First, we have shown that the complexes are much larger than previously thought and may actually take the form of a lattice-like structure with tankyrase polymers linked together by interaction partners that form dimers or have multiple tankyrase binding sites (e.g., TRF1). Second, our findings indicate that tankyrases differ from other scaffolding proteins in that they have a catalytic effector function that they can use to disassemble these large lattices. It is this unique combination of scaffolding plus effector activity that leads us to suggest that their in vivo function is to act as a regulated tether that controls the formation and dissociation of large protein lattices in response to cellular signals. The precise composition and hence role of each lattice would depend on its location in the cell.
Although tankyrases are unique in that they have both scaffolding and effector activities combined within one molecule, they share some traits with other scaffolding proteins such as the Shank proteins, Sans, and the AKAP (A-kinase anchoring proteins) family (4, 17, 24). The Shank and Sans proteins resemble tankyrases in that they contain both ankyrin repeats and a SAM domain and are thought to oligomerize through their SAM domain. Shank proteins are found at excitatory synapses, where they organize the postsynaptic density by linking postsynaptic receptors to the cytoskeleton (4). Sans is critical for formation of the protein networks that organize hair bundles in the inner ear, and deletion of the SAM domain leads to disorganised hair bundles and deafness (17). The finding that multiple scaffolding proteins contain both ankyrin repeats and a SAM suggests that this arrangement of domains is well suited for assembling large protein networks, because it allows the scaffolding protein to both self-associate and interact with other proteins.
Although AKAPs differ in structure from tankyrases, they also target a general effector enzyme activity to a specific location in a cell. AKAPs themselves do not have a catalytic activity, but each AKAP binds protein kinase A (PKA) and anchors it at a different site within the cell. This compartmentalization of PKA provides a mechanism for the cell to respond to a localized cyclic AMP gradient by activating PKA at that site, thus ensuring a directed response to a general signal. It seems likely that tankyrase PARP activity, and hence complex dissociation, is regulated in a similar location-specific manner. The localization of each AKAP-PKA complex is achieved through a targeting domain on the AKAP that interacts with molecules at the desired location. In some ways this parallels the mechanism for tankyrase localization, where factors such as TRF1 or the centrosomal protein NuMA bind the ankyrin repeats, thus tethering the tankyrase scaffold at a specific location.
Functions of tankyrase scaffolds.
The ability of tankyrases to assemble and disassemble protein networks indicates that tankyrases are unlikely to form permanent structural scaffolds but rather form more dynamic structures that are associated with processes that require rapid changes in cellular organization. The intracellular distribution of tankyrase fits well with this hypothesis, as telomeres, mitotic spindles, and the Golgi network are all structures that can undergo dramatic changes during the cell cycle. Telomeres are large DNA-protein complexes that are thought to cycle between a closed compact structure that protects the DNA terminus and a more open structure that makes the terminus accessible to telomerase during S phase (3). As the telomere protein TRF1 is an important architectural component of the telomeric complex (37), its removal by tankyrase would on its own be expected to cause some decompaction of the telomeric complex. However, our finding that tankyrase forms protein scaffolds that could effectively cross-link distant regions of the telomeric tract suggests that disruption of the whole tankyrase-TRF1 protein lattice may have a much more profound effect on telomere opening.
The finding that tankyrase colocalizes with Golgi and secretory vesicles has led to the suggestion that tankyrase is somehow involved in vesicle trafficking (7, 21, 26). Again, vesicle trafficking is a dynamic process that requires coordinated budding, storage, release, transport, and fusion with the plasma membrane, so it is fairly easy to envision a role for tankyrase scaffolds in this process. In insulin-starved adipocytes tankyrase 1 colocalizes with GLUT4 vesicles, but after insulin stimulation tankyrase remains in the cytoplasm whereas the GLUT4 vesicles translocate to the cell membrane (7). This loss of colocalization suggests that tankyrase scaffolds may serve as the tether that prevents vesicle translocation. Tankyrase binds to the N terminus of IRAP, the region that protrudes from the GLUT4 vesicle; thus, a tankyrase scaffold could anchor the vesicle until the insulin response activates tankyrase PARP activity and hence scaffold dissociation.
Our studies with overexpressed tankyrase also support a role for tankyrase in secretion, because increasing the extent of SAM-mediated scaffold formation by protein overexpression led to redistribution of a Golgi marker and inhibition of the apical but not the basolateral secretory pathway. While the mechanism underlying these effects is unclear, they might reflect a role for tankyrase scaffolds in vesicle budding from the trans Golgi stacks or in the translocation or fusion of specific vesicle types. Regulation of protein secretion is critical for a wide array of key cellular events, but many aspects of the various secretory pathways are still poorly understood. Thus, our finding that tankyrase scaffolds may underlie the mechanism that regulates some of these pathways is exciting and may provide novel ways to study this complex area.
Acknowledgments
We thank Christina Bennett-Stamper for assistance with electron and confocal microscopy and K. Simons for the GPI and VSVG3 constructs. We are grateful to Cathy Chia, Linda Parysek, and members of the Price lab for helpful comments.
The work was supported by National Institutes of Health grant AG17212 to C.M.P. and a fellowship from the Albert J. Ryan Foundation to M.D.R.
REFERENCES
- 1.Bae, J., J. R. Donigian, and A. J. Hsueh. 2003. Tankyrase 1 interacts with Mcl-1 proteins and inhibits their regulation of apoptosis. J. Biol. Chem. 278:5195-5204. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, V., and A. J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353-1392. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. [DOI] [PubMed] [Google Scholar]
- 4.Boeckers, T. M., J. Bockmann, M. R. Kreutz, and E. D. Gundelfinger. 2002. ProSAP/Shank proteins-a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 81:903-910. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, N. J., R. Govers, and D. E. James. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3:267-277. [DOI] [PubMed] [Google Scholar]
- 6.Chang, W., J. N. Dynek, and S. Smith. 2003. TRF1 is degraded by ubiquitin-mediated proteolysis after release from telomeres. Genes Dev. 17:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi, N. W., and H. F. Lodish. 2000. Tankyrase is a Golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275:38437-38444. [DOI] [PubMed] [Google Scholar]
- 8.Cook, B. D., J. N. Dynek, W. Chang, G. Shostak, and S. Smith. 2002. Role for the related poly(ADP-ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol. Cell. Biol. 22:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amours, D., S. Desnoyers, I. D'Silva, and G. G. Poirier. 1999. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 342(Pt. 2):249-268. [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer, F., M. J. Giraud-Panis, I. Jaco, J. C. Ame, I. Schultz, M. Blasco, C. E. Koering, E. Gilson, J. Menissier-de Murcia, G. de Murcia, and V. Schreiber. 2004. Functional interaction between poly(ADP-ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol. Cell Biol. 24:1595-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidovic, L., M. Vodenicharov, E. B. Affar, and G. G. Poirier. 2001. Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism. Exp Cell Res. 268:7-13. [DOI] [PubMed] [Google Scholar]
- 12.De Rycker, M., R. N. Venkatesan, C. Wei, and C. M. Price. 2003. Vertebrate tankyrase domain structure and sterile alpha motif (SAM)-mediated multimerization. Biochem. J. 372:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dynek, J. N., and S. Smith. 2004. Resolution of sister telomere association is required for progression through mitosis. Science 304:97-100. [DOI] [PubMed] [Google Scholar]
- 14.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 15.Gascard, P., and N. Mohandas. 2000. New insights into functions of erythroid proteins in nonerythroid cells. Curr. Opin. Hematol. 7:123-129. [DOI] [PubMed] [Google Scholar]
- 16.Keller, P., D. Toomre, E. Diaz, J. White, and K. Simons. 2001. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat. Cell. Biol. 3:140-149. [DOI] [PubMed] [Google Scholar]
- 17.Kikkawa, Y., H. Shitara, S. Wakana, Y. Kohara, T. Takada, M. Okamoto, C. Taya, K. Kamiya, Y. Yoshikawa, H. Tokano, K. Kitamura, K. Shimizu, Y. Wakabayashi, T. Shiroishi, R. Kominami, and H. Yonekawa. 2003. Mutations in a new scaffold protein Sans cause deafness in Jackson shaker mice. Hum. Mol. Genet. 12:453-461. [DOI] [PubMed] [Google Scholar]
- 18.Kim, C. A., and J. U. Bowie. 2003. SAM domains: uniform structure, diversity of function. Trends Biochem. Sci. 28:625-628. [DOI] [PubMed] [Google Scholar]
- 19.Kim, C. A., M. Gingery, R. M. Pilpa, and J. U. Bowie. 2002. The SAM domain of polyhomeotic forms a helical polymer. Nat. Struct. Biol. 9:453-457. [DOI] [PubMed] [Google Scholar]
- 20.Kim, C. A., M. L. Phillips, W. Kim, M. Gingery, H. H. Tran, M. A. Robinson, S. Faham, and J. U. Bowie. 2001. Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression. EMBO J. 20:4173-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons, R. J., R. Deane, D. K. Lynch, Z. S. Ye, G. M. Sanderson, H. J. Eyre, G. R. Sutherland, and R. J. Daly. 2001. Identification of a novel human tankyrase through its interaction with the adaptor protein Grb14. J. Biol. Chem. 276:17172-17180. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza-Alvarez, H., and R. Alvarez-Gonzalez. 2004. The 40 kDa carboxy-terminal domain of poly(ADP-ribose) polymerase-1 forms catalytically competent homo- and heterodimers in the absence of DNA. J. Mol. Biol. 336:105-114. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza-Alvarez, H., and R. Alvarez-Gonzalez. 1999. Biochemical characterization of mono(ADP-ribosyl)ated poly(ADP-ribose) polymerase. Biochemistry 38:3948-3953. [DOI] [PubMed] [Google Scholar]
- 24.Michel, J. J., and J. D. Scott. 2002. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 42:235-257. [DOI] [PubMed] [Google Scholar]
- 25.Sbodio, J. I., and N. W. Chi. 2002. Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1. NuMA contains this RXXPDG motif and is a novel tankyrase partner. J. Biol. Chem. 277:31887-31892. [DOI] [PubMed] [Google Scholar]
- 26.Sbodio, J. I., H. F. Lodish, and N. W. Chi. 2002. Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1 (telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase). Biochem. J. 361:451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedgwick, S. G., and S. J. Smerdon. 1999. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem. Sci. 24:311-316. [DOI] [PubMed] [Google Scholar]
- 28.Seimiya, H., Y. Muramatsu, S. Smith, and T. Tsuruo. 2004. Functional subdomain in the ankyrin domain of tankyrase 1 required for poly(ADP-ribosyl)ation of TRF1 and telomere elongation. Mol. Cell. Biol. 24:1944-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seimiya, H., and S. Smith. 2002. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182). J. Biol. Chem. 277:14116-14126. [DOI] [PubMed] [Google Scholar]
- 30.Smalla, M., P. Schmieder, M. Kelly, A. Ter Laak, G. Krause, L. Ball, M. Wahl, P. Bork, and H. Oschkinat. 1999. Solution structure of the receptor tyrosine kinase EphB2 SAM domain and identification of two distinct homotypic interaction sites. Protein Sci. 8:1954-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, S. 2001. The world according to PARP. Trends Biochem. Sci. 26:174-179. [DOI] [PubMed] [Google Scholar]
- 32.Smith, S., and T. de Lange. 1999. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J. Cell Sci. 112(Pt. 21):3649-3656. [DOI] [PubMed] [Google Scholar]
- 33.Smith, S., and T. de Lange. 2000. Tankyrase promotes telomere elongation in human cells. Curr. Biol. 10:1299-1302. [DOI] [PubMed] [Google Scholar]
- 34.Smith, S., I. Giriat, A. Schmitt, and T. de Lange. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484-1487. [DOI] [PubMed] [Google Scholar]
- 35.Thanos, C. D., K. E. Goodwill, and J. U. Bowie. 1999. Oligomeric structure of the human EphB2 receptor SAM domain. Science 283:833-836. [DOI] [PubMed] [Google Scholar]
- 36.Tran, H. H., C. A. Kim, S. Faham, M. C. Siddall, and J. U. Bowie. 2002. Native interface of the SAM domain polymer of TEL. BMC Struct. Biol. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Steensel, B., and T. de Lange. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740-743. [DOI] [PubMed] [Google Scholar]