Abstract
A prerequisite for cellular immortalization in human cells is the elongation of telomeres through the upregulation of telomerase or by the alternative lengthening of telomeres (ALT) pathway. In this study, telomere structure in multiple ALT cell lines was examined by electron microscopy. Nuclei were isolated from GM847, GM847-Tert, and WI-38 VA13 ALT cells, psoralen photo-cross-linked in situ, and the telomere restriction fragments were purified by gel filtration chromatography. Examination of telomere-enriched fractions revealed frequent extrachromosomal circles, ranging from 0.7 to 56.8 kb. t-loops were also observed, with the loop portion ranging from 0.5 to 70.2 kb. The total length of the loop plus tail of the t-loops corresponded to the telomere restriction fragment length from the ALT cell lines as determined by pulsed-field gel electrophoresis. The presence of extrachromosomal circles containing telomeric DNA was confirmed by two-dimensional pulsed-field gel electrophoresis. These results show that extrachromosomal telomeric DNA circles are present in ALT nuclei and suggest a roll-and-spread mechanism of telomere elongation similar to that seen in previous observations of multiple yeast species. Results presented here also indicate that expression of telomerase in GM847 cells does not affect t-loop or extrachromosomal circle formation.
Telomeres are the protein-DNA structures at chromosome termini in eukaryotic cells that protect chromosomes from end-to-end fusion and likely function as a trigger for proliferative arrest in mammalian cells. In mammals, telomeric DNA consists of a tandem array of the 6-nucleotide (nt) repeat 5′TTAGGG3′/3′CCCTAA5′ (26), approximately 4 to 14 kb long in humans (10), terminating in a 150- to 200-nt 3′ single-strand DNA overhang of the G-rich strand (23, 46). A structural solution to chromosome end protection is provided by sequestering the 3′ telomeric overhang in a displacement loop within the intratelomeric duplex DNA. We identified this structure, termed a telomere loop (t-loop), following electron microscopic (EM) examination of telomeric DNA isolated from in situ photo-cross-linked genomic DNA from human and mouse nuclei (13). t-loops have also been observed in plant (7), avian (31), and multiple protist species (27, 28), suggesting that t-loops are a common mechanism for chromosome protection among eukaryotes.
Due to the end replication problem (33, 45), oxidative damage (44), and possible nucleolytic processing (23), telomeres in human cells erode by approximately 100 bp with each cell division (14). In human somatic tissue, little or no telomerase activity is present, resulting in telomere-induced cellular senescence after many population doublings (14, 15, 24). If senescence is bypassed by mutations or expression of viral oncogenes, the cell will continue to divide until crisis and eventual cell death (12). However, human cells can stave off induction of senescence or escape crisis by regenerating the telomeric DNA (4, 9, 17). This occurs most commonly, in approximately 90 to 95% of human cancers and 60 to 70% of immortalized human cell lines, through the upregulation of telomerase (19, 37). However, in the remaining 30 to 40% of immortalized and 5 to 10% of cancerous human cells, this occurs through a less understood mechanism termed alternative lengthening of telomeres (ALT) (5, 6, 16).
A human ALT cell is characterized as a cell possessing an indefinite replication capacity that maintains telomere length in the absence of telomerase. Physical markers of ALT cells include long and heterogeneous telomeres, ranging from 2 to greater than 20 kb within an individual cell, and ALT-associated promyelocytic leukemia bodies (APBs) (5, 6, 47). APBs are nuclear bodies present in approximately 5% of ALT cells that stain with antibodies to several DNA metabolic and telomere binding proteins (16, 47). Extrachromosomal telomeric repeat (ECTR) DNA molecules are also present in ALT cells, though little is known about the ECTR molecules other than that they are commonly, but not exclusively, associated with the APBs (32, 41, 47).
The exact mechanism by which ALT cells maintain their telomere length is unknown, though several lines of evidence suggest that homologous recombination plays a significant role. In Saccharomyces cerevisiae two distinct classes of survivors (the equivalents of mammalian ALT) have been identified (21, 40). Type I survivors have short telomeres but exhibit amplification within the subtelomeric regions, whereas type II survivors exhibit significant telomere lengthening and heterogeneity similar to that of the human ALT condition. Both classes are dependent on Rad52, implicating homologous recombination as a requirement for telomere maintenance. Survivors from the yeast species Kluyveromyces lactis are also dependent on Rad52 and resemble type II S. cerevisiae survivors with long heterogeneous telomeres (25). Genetic data from K. lactis further suggests that maintenance of telomeric DNA in survivors from this species is mediated by a roll-and-spread mechanism, where small ECTR circles are utilized as a template for rolling circle replication (rcr) (29, 30). This results in the generation of long tracts of telomeric DNA that can be incorporated into the genomic telomeres via homologous recombination, thus increasing their length. A similar mechanism utilizing rcr on small telomeric repeat circles is also believed to maintain mitochondrial telomeres in the yeast species Candida parapsilosis, which has a linear mitochondrial genome but lacks a mitochondria telomere-specific telomerase (42, 43). Evidence supporting the roll-and-spread mechanism includes the observation by EM of small ECTR circles and DNA molecules resembling rcr intermediates from K. lactis genomic (C. Groff-Vindman, S. Natarajan, S. Iyer, A. J. Cesare, J. D. Griffith, and M. McEachern, unpublished data) and C. parapsilosis mitochondrial DNA preparations (J. Nosek, A. Rycovska, A. M. Makhov, J. D. Griffith, and L. Tomaska, submitted for publication).
In human ALT cells, intertelomeric recombination was observed directly by inserting a unique sequence tag within an individual telomere in the GM847 cell line and observing its amplification and movement to other telomeres (11). Telomere sister chromatid exchange has also been observed in multiple human ALT cell lines, though it is extremely rare in both telomerase-positive and -negative non-ALT human cells (3, 20). Though recombination between telomeres in ALT cells is robust, no increase in genomic recombination has been observed, suggesting that the defect is telomere specific and does not affect the general recombination machinery (1, 2). One possibility that would allow intertelomeric recombination to occur in human ALT cells would be the improper maintenance of telomere structure. Many laboratory-derived ALT cell lines have been created by immortalization with the simian virus 40 (SV40) large T antigen, eliminating the p53 and Rb pathways shown previously to be vital in the cellular response to telomere uncapping (38). The lack of p53 and Rb function in ALT cells may therefore allow the normal constraints on telomere structure to be relaxed, increasing the probability of intertelomeric recombination.
To gain further understanding into the mechanisms of telomere maintenance in ALT cells, we investigated the physical structure of genomic and extrachromosomal telomeric DNA from multiple ALT cell lines. The telomeric DNA in nuclei isolated from GM847, GM847-Tert, and WI-38 VA13 (VA13) ALT cell lines were photo-cross-linked in situ, reduced to telomere restriction fragments (TRFs), isolated, and examined by EM. Our data show that t-loops are present in ALT cells, though loop size is generally small compared to total telomere length. ECTR circles were observed in all the ALT cell lines examined but not in a telomerase-positive HeLa cervical carcinoma or a telomerase-negative primary fibroblast cell line, suggesting that ECTR circles are unique to ALT cells. Lastly, exogenous expression of the telomerase catalytic subunit in the GM847 ALT cell line had no apparent effect on t-loop or ECTR circle formation. These data suggest that human ALT cells may maintain telomeric DNA through a roll-and-spread mechanism, similar to that of K. lactis survivors and the mitochondrial telomeres in C. parapsilosis.
MATERIALS AND METHODS
Cell culture.
GM847, GM847-Tert, and WI-38 VA13 cell lines were a gift from Shelia Stewart and Robert Weinberg at the Massachusetts Institute of Technology (Cambridge, Mass.). Tissue used to generate the primary foreskin fibroblast line (FSK) was from a healthy young donor. HeLa, FSK, and ALT cell lines were cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, Mo.) and 50 μg of gentamicin (Sigma)/ml. 293 cells were grown in MEM with Earle salts (Gibco), supplemented with 10% FBS, 100 nM nonessential amino acids (Gibco), 50 μg of gentamicin/ml, and 0.15% sodium bicarbonate.
TRAP assay.
Telomere repeat amplification protocol (TRAP) assays were done with the Serologicals (Norcross, Ga.) TRAPeze kit using the manufacturer's suggestions with the following modifications: 100 cell equivalents of extract were used per reaction, negative controls were treated with 200 ng of RNaseA/μl for 5 min at room temperature (RT) before addition of the radiolabeled oligonucleotide, and Taq polymerase was added at the first 95°C step in PCR amplification.
TRF isolation and Hirt precipitation for gel electrophoresis.
To generate TRFs, five 100-mm plates of cells at approximately 85% confluence were washed with 1× PBS (Gibco), trypsinized (Sigma), washed a second time with 1× PBS, and centrifuged at 180 × g in a Sorval RT7 Plus centrifuge for 5 min. The cell pellet was resuspended in 3 ml of 1× TNE (10 mM Tris [pH 7.6], 10 mM EDTA, 100 mM NaCl), lysed by addition of 3 ml of 1× TNES (1× TNE plus 1.0% sodium dodecyl sulfate), and incubated overnight with 250 μg of proteinase K/ml at 55°C. Genomic material was phenol-chloroform extracted, ethanol precipitated, resuspended in 10 mM Tris (pH 7.6), and digested with HinfI and HaeIII (New England Biolabs, Beverly, Mass.) at 3 U/μg of DNA and 200 ng of RNase A/μl for 6 h at 37°C. The material was ethanol precipitated and the DNA was resuspended in 10 mM Tris (pH 7.6). For Hirt precipitation, 107 cells were collected, washed twice in 1× PBS, resuspended in 5 ml of buffer containing 10 mM Tris (pH 7.6), 10 mM EDTA, and 0.6% sodium dodecyl sulfate, and incubated at RT for 15 min. NaCl (2 ml at 5 M) was added, and the solution was mixed gently and incubated overnight at 4°C. Chromosomal material was pelleted at 4°C in a Sorval SS-34 rotor at 17,000 × g for 40 min. The supernatant was collected, treated with 50 μg of RNase A/ml at 37°C for 1 h, and then treated with 250 μg of proteinase K/ml for 1 h at 55°C, followed by phenol-chloroform extraction and ethanol precipitation. DNA was collected by centrifugation in a Sorvall HB-6 rotor at 4°C at 13,200 × g for 4 h and resuspended in 10 mM Tris (pH 7.6).
Standard and 2D PFGE.
TRFs were separated by standard pulsed-field gel electrophoresis (PFGE) using a Bio-Rad CHEF DR-III apparatus (Bio-Rad, Hercules, Calif.) as described previously (7) with the run duration shortened to 9 h. For two-dimensional (2D) PFGE, DNA was separated in the first dimension on the same apparatus at RT in a 0.5% pulsed-field agarose gel (Bio-Rad) in 0.5× TBE (44.5 mM Tris base, 44.5 mM boric acid, 1 mM EDTA) with the following settings: 1- to 6-s switch time, 1 V/cm, 120° included angle, 25.5 h duration. The gel was stained with ethidium bromide in 0.5× TBE, destained in 0.5× TBE, and visualized by UV light. The appropriate lanes were then excised. A 1.1% pulsed-field agarose gel in 0.5× TBE containing 300 ng of ethidium bromide/ml was poured around the excised slab, and the second-dimension electrophoresis was carried out in the same apparatus at 4°C in 0.5× TBE containing 300 ng of ethidium bromide/ml with the following conditions: 1- to 6-s switch time, 6 V/cm, 120° included angle for 8.5 h. The gel was visualized using UV light. In-gel hybridization of both standard and 2D PFGE with a [γ-32P](CCCTAA)6 oligonucleotide was done as described previously (7), and the signal was visualized using a Storm 840 PhosphorImager (Molecular Dynamics, Piscataway, N.J.).
DNA preparation for EM analysis.
Nuclei from 2 × 108 cells were collected and psoralen cross-linked as described previously (13), with the following modifications. The nuclei were resuspended in homogenization buffer (10 mM Tris-HCl [pH 7.5], 15 mM NaCl, 60 mM KCl, 1 mM EDTA, 0.1 mM EGTA, 0.15 mM spermine, 0.5 mM spermidine, 0.2% NP-40, 5% sucrose) supplemented with 200 μg of AMT (4′-aminomethyl trioxsalen; Sigma)/ml for cross-linking. Restriction digestion with MboI and AluI (New England Biolabs) was done with enzyme concentrations of 3 U/μg of DNA. The DNA was fractionated using a Pharmacia (Piscataway, N.J.) Gradifrac system with a C16/70 column (Amersham, Piscataway, N.J.) packed with Bio-Gel A15m matrix (Bio-Rad) as described previously (7).
Slot blot analysis of chromatographic fractions for telomeric DNA.
DNA (50 ng) from samples for slot blot analysis was applied to a nylon membrane using a slot blot manifold and was probed using a [γ-32P](CCCTAA)6 oligonucleotide as previously described (7). The membrane was visualized using a Storm 840 PhosphorImager and quantitated using ImageQuant software (Molecular Dynamics).
EM.
Samples were prepared for EM by surface spreading on a denatured protein film as described previously (13) and were analyzed using a Philips CM12 instrument. Images were captured on sheet film and a Gatan multiscan 794 digital camera (Pleasanton, Calif.). Molecule dimensions were determined using Gatan Digital Micrograph software. Images for publication were captured by ACT-1 software (Nikon, Tokyo, Japan) utilizing a Nikon SMZ1000 stereoscope. Image contrast was adjusted by Adobe Photoshop (San Jose, Calif.).
RESULTS
Telomere length and telomerase activity.
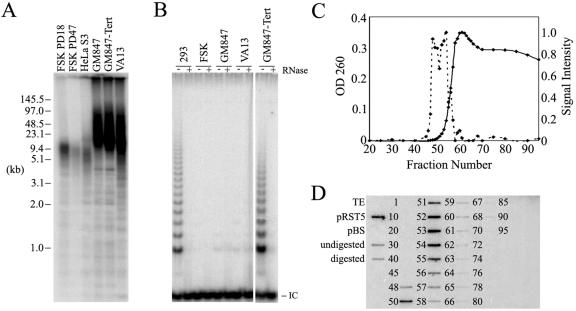
For this investigation we selected the GM847 and VA13 cell lines due to their extensive use in ALT studies. GM847 and VA13 are human skin and lung fibroblast cell lines, respectively, each transformed with the SV40 large T antigen and immortalized by a spontaneous development of the ALT phenotype. A derivative of the GM847 cell line exogenously expressing the protein subunit of telomerase (GM847-Tert) was also used (39). Telomere lengths from the ALT cells were compared to those of primary human foreskin fibroblast (FSK) and HeLa cervical carcinoma S3 subclone cell lines (Fig. 1A). TRFs were generated by digestion of genomic DNA with HinfI and HaeIII, separated by PFGE, and subjected to in-gel hybridization using a telomere-specific probe. Telomeres from the HeLa S3 and FSK cell lines had mean telomere lengths of less than 15 kb, with telomeres shortening concordant with increasing population doublings in the FSK cell line. In the ALT cell lines, telomere lengths were long and heterogeneous, ranging from 6 to 90 kb with the majority between 10 and 50 kb. There was little observable difference in telomere lengths between the GM847 and GM847-Tert cell lines. A sizeable amount of telomeric signal remained in the wells in the GM847, GM847-Tert, and VA13 lanes, likely due to entanglement of some portion of the long telomeric DNA (see EM observations below). Lack of telomerase activity in the ALT cell lines was confirmed by the TRAP assay, which detects the presence of an active telomerase enzyme in cell extracts (Fig. 1B). As expected, the FSK, GM847, and VA13 cell lines lacked telomerase activity, whereas the GM847-Tert cell line exhibited a robust telomerase activity similar to that of the human telomerase-positive 293 kidney cell line.
FIG. 1.
Telomere measurement, telomerase activity, and telomere isolation in GM847, GM847-Tert, and VA13 cells. (A) Total DNA (10 μg) was digested with HinfI/HaeIII and separated by PFGE, and the telomeric material was detected by in-gel hybridization with a [γ-32P](CCCTAA)6 probe. The signal was detected using a PhosphorImager. (B) Telomerase activity as determined by TRAP assay. IC refers to the internal PCR control. (C) Total DNA content and relative telomeric DNA abundance in GM847 psoralen photo-cross-linked fractions following genomic DNA digestion with MboI/AluI and fractionation over an A-15m Bio-Gel column. DNA content (solid line, scale on left) as determined by optical density at 260 nm (OD 260). Telomeric signal intensity (dotted line, scale on right) determined by quantitation of slot blot shown in panel D. (D) Slot blot analysis of selected fractions from the column elution shown in panel C. DNA (50 ng) was applied to a nylon membrane, probed with [γ-32P](CCCTAA)6, and detected by PhosphorImager. Controls included buffer alone (TE), the pRST5 plasmid containing 96 (TTAGGG) repeats, Bluescript (pBS) cloning vector lacking telomeric repeats, undigested GM847 genomic DNA, and MboI/AluI-digested GM847 genomic DNA.
Telomere isolation by gel exclusion chromatography.
To preserve the folded architecture of telomeric DNA for observation by EM, we utilized a method developed previously in which nuclear DNA is psoralen photo-cross-linked in situ (13). After cross-linking, the genomic DNA was digested with MboI and AluI to reduce the nuclear DNA to small fragments while leaving the telomeric DNA undigested. The high-molecular-weight TRFs were separated from the smaller genomic fragments by gel filtration chromatography using Bio-Gel A-15m (Fig. 1C). The earliest eluting fractions containing high-molecular-weight DNA from the cross-linked preparations from GM847 cells were enriched for telomeric content (Fig. 1C and D). The elution profile and slot blot analysis for non-cross-linked preparations from GM847 and both the cross-linked and non-cross-linked preparations from the GM847-tert and VA13 cell lines were similar to the data shown in Fig. 1C and D (data not shown).
Visualization of TRFs.
DNA from the high-molecular-weight, telomere-enriched fractions prepared from ALT cells was examined by surface-spreading EM. Only the initial 4 to 6 fractions containing a strong telomeric signal (fractions 48 to 53 in Fig. 1D) from each preparation were scored. Later fractions contained a majority of molecules of less than 10 kb in length and likely contained a high percentage of nontelomeric DNA. DNA molecules in the initial telomere-enriched fractions commonly ranged from 20 to 100 kb, consistent with telomere lengths measured by PFGE (Fig. 1A). Large tangled masses of DNA were also observed, likely representative of the material that remained in the wells in PFGE (Fig. 1A). In preliminary experiments, EM results were similar in the GM847 and VA13 cell lines and, therefore, experiments were not repeated for quantitation using the VA13 cell line.
Extrachromosomal telomeric repeat circles.
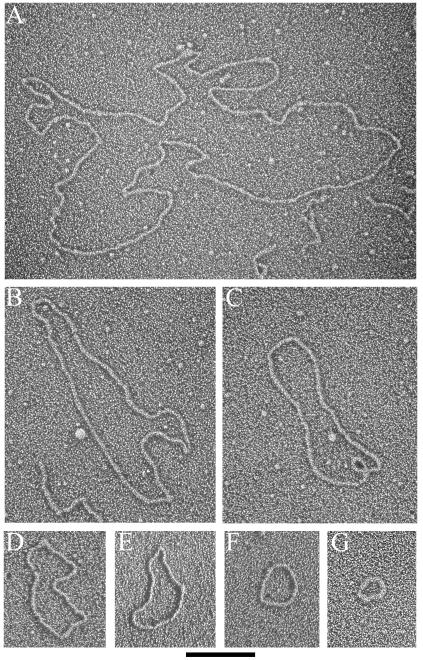
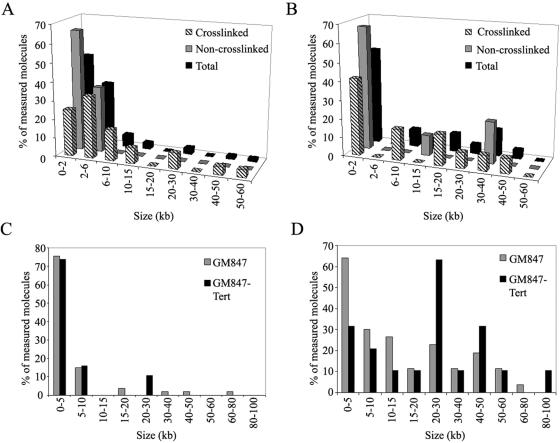
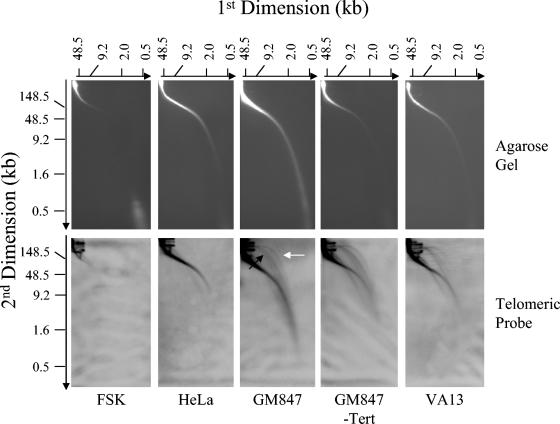
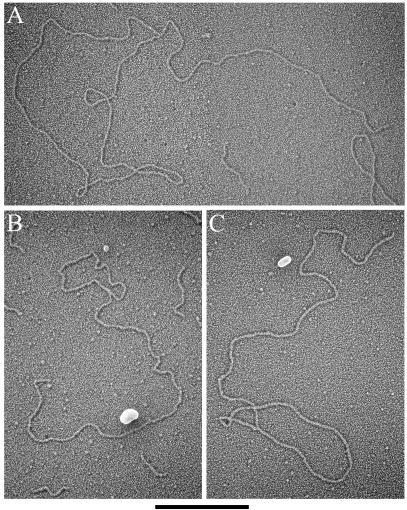
Previous examination of telomere-enriched, high-molecular-weight fragments isolated from human, mouse, and garden pea cells yielded two major structural species, long linear duplex DNA and t-loops, defined as a duplex DNA loop and connected duplex tail (7, 13). The most striking observation in the telomere-enriched fractions from ALT cells is that they also contain a major species of extrachromosomal circular DNA molecules that are not present in non-ALT cells (Fig. 2). In the high-molecular-weight fractions of cross-linked DNA preparations from GM847 cells, 4.5% of the total molecules scored were circles (Table 1) with a size range of 0.8 to 56.8 kb. The size distribution was skewed towards smaller circles, with 25% of circles being ≤2 kb and 58% of circles being ≤6 kb in total length (Fig. 3A). In non-cross-linked preparations from GM847, extrachromosomal circles represented 4.8% of the molecules scored, indicating that the circular form was not an artifact of psoralen cross-linking (Table 1). The extrachromosomal circles from the non-cross-linked preparations ranged from 0.4 to 6.1 kb. Circular molecules were also present in GM847-Tert cells with properties similar to those of the GM847 cell line (Table 1). In the cross-linked preparations from GM847-Tert, the circles ranged from 0.7 to 47.8 kb, whereas in the non-cross-linked preparations circles ranged from 0.7 to 32.7 kb. However, there was a difference in the abundance of circular molecules observed in the cross-linked (4.0%) and non-cross-linked (1.7%) preparations from GM847-Tert cells (Table 1). Extrachromosomal circles have not been observed previously above a background level of 0.2 to 0.5% in any of our preparations from primary human foreskin fibroblasts (N. Quinney and J. Griffith, unpublished data), primary human blood lymphocytes (13), or telomerase-positive HeLa cervical carcinoma subclones 1.2.11 and S3 (13). Extrachromosomal circles were also not present in preparations from mouse liver or Pisum sativum (common garden pea) root and shoot cells (7, 13).
FIG. 2.
Extrachromosomal DNA circles from GM847 and GM847-Tert cells. EMs of circular DNA molecules observed in the telomere-enriched fractions from GM847 and GM847-Tert cells. DNA was prepared for EM by surface spreading with cytochrome c and rotary shadowcasting. Images are shown in negative contrast. Circle lengths are 22.1, 8.9, 6.2, 3.9, 3.0, 1.6, and 0.8 kb for A through G, respectively. Bar is equivalent to 1 kb.
TABLE 1.
Summary of EM observations of the telomere-enriched fractions from GM847 and GM847-Tert cells
| Cell type | n | t-loops (%) | Circles (%) | Y molecules (%) |
|---|---|---|---|---|
| GM847 | ||||
| Cross-linked | 423 | 39 (9.2) | 19 (4.5) | 15 (3.5) |
| Non-cross-linked | 820 | 19 (2.3) | 39 (4.8) | 5 (0.6) |
| GM847-Tert | ||||
| Cross-linked | 303 | 21 (6.9) | 12 (4.0) | 8 (2.6) |
| Non-cross-linked | 974 | 13 (1.3) | 17 (1.7) | 5 (0.5) |
FIG. 3.
Summary of extrachromosomal circle (t-circles) and t-loop observations by EM. (A and B) Distribution of extrachromosomal DNA circle size as a percentage of scored molecules observed in the telomere-enriched fractions from GM847 (A) and GM847-Tert (B) cells. (C and D) Distribution of the loop portion (C) and total t-loop length (D) as a percentage of scored molecules from the psoralen cross-linked, telomere-enriched fractions from GM847 and GM847-Tert cells.
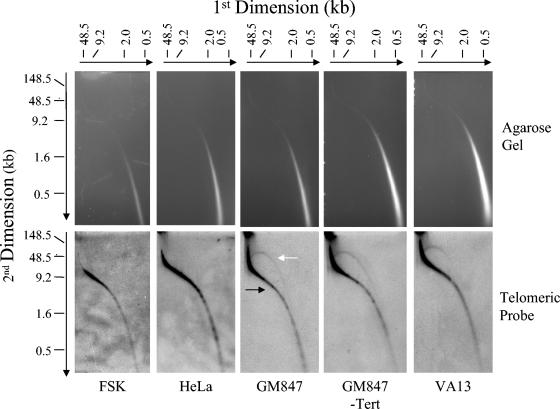
To determine if the extrachromosomal circles observed here by EM contained telomeric repeats, we employed 2D PFGE to resolve the very large circular molecules. For these experiments, TRFs of non-cross-linked DNA prepared from ALT, FSK, and HeLa cervical carcinoma subclone 1.2.11 (telomerase-positive cell line with telomeres up to 40 kb) were separated by neutral-neutral 2D PFGE and the telomeric content was detected by in-gel hybridization with a telomere-specific probe (Fig. 4). In the ALT and non-ALT cells, a smear of telomeric signal with a migration pattern typical of linear DNA was observed, consistent with telomere lengths measured by one-dimensional PFGE (compare Fig. 4 and 1). However, in the ALT cell lines (GM847, GM847-Tert, and VA13) a second prominent arc of telomeric material with a pattern characteristic of circular DNA was present. The circular arc was heterogeneous in size, consistent with the size of the extrachromosomal circles observed by EM (Fig. 3A and B). To demonstrate that the circular telomeric DNA observed was extrachromosomal in origin, we utilized the Hirt protocol to precipitate the large chromosomal material in lysates from ALT and non-ALT cells (18). Material from the supernatant was then electrophoretically separated utilizing the same 2D PFGE protocol (Fig. 5). In both ALT and non-ALT cells, some chromosomal DNA remains unprecipitated, as seen by the smear of telomeric signal running consistent with linear DNA. In addition, arcs of telomeric DNA with a mobility consistent with supercoiled and open circular forms were present. However, as described above, they were only observed in the ALT cells. Similar to the TRF 2D PFGE experiment, the ECTR circle lengths were heterogeneous, with sizes spanning the same broad size range as seen by EM (Fig. 3A and B). To establish consistency in nomenclature (42), we will refer to the ECTR circles as t-circles.
FIG. 4.
2D PFGE of TRFs from ALT and non-ALT cells. Total DNA (20 μg) was digested with HinfI/HaeIII and then separated by 2D PFGE. Telomeric material was detected by in-gel hybridization with a [γ-32P](CCCTAA)6 probe and was visualized using a PhosphorImager. The black and white arrows indicate linear- and circular-form DNA, respectively.
FIG. 5.
2D PFGE of Hirt supernatant from ALT and non-ALT cells. An equal number of cells (107) were Hirt precipitated, and the supernatant was separated by 2D PFGE. Telomeric material was detected by in-gel hybridization with a [γ-32P](CCCTAA)6 probe and was visualized by PhosphorImager. The black and white arrows indicate supercoiled- and open-circular-form DNA, respectively.
Telomere loops.
One possible explanation for the increased intertelomeric recombination observed in ALT cells is an improper maintenance of telomere capping, increasing the likelihood of recombination between telomeres. We therefore examined the high-molecular-weight DNA fractions from ALT cells for the presence of t-loops and recombination intermediates. t-loops were observed in the GM847 and GM847-Tert cells with similar characteristics (Fig. 6 and Table 1). In the GM847 cross-linked preparations, 9.2% of the total molecules scored were arranged into a t-loop structure (Table 1), with the earliest fractions (1 to 2 after the initial telomeric signal) containing a higher percentage of looped molecules (16%) than the later fractions (4 to 6 after the initial telomeric signal, 8.1%). Fifty-three t-loops were measured from GM847 cross-linked preparations, with the loop portion of the t-loop ranging from 0.5 to 70.2 kb. The loop portion of t-loops from GM847 cells was generally small, with 76% of the molecules possessing a loop portion of ≤5 kb, with a median loop size of 2.4 kb (Fig. 3C). Total length (loop plus tail) of t-loops observed in GM847 cells ranged from 0.9 to 78.6 kb (Fig. 3D), consistent with telomere lengths measured by PFGE (Fig. 1). In non-photo-cross-linked preparations from GM847 cells, 2.3% of the molecules were arranged into t-loops (Table 1), suggesting that psoralen cross-linking is required to efficiently maintain loop structure, as observed previously (7, 13).
FIG. 6.
t-loops from GM847 and GM847-Tert cells. EMs of t-loop molecules observed in the telomere-enriched fractions of GM847 and GM847-Tert DNA. DNA was prepared for EM as described for Fig. 2 and is shown in negative contrast. Loop and tail sizes are 19.0 and 8.4, 4.8 and 9.6, and 7.2 and 9.5 kb for panels A through C, respectively. Bar, 2 kb.
Similar results were obtained in experiments using GM847-Tert cells. In the cross-linked preparations, 6.9% of the molecules were observed arranged into a t-loop structure, with the loop portion ranging from 0.9 to 29.8 kb and a median size of 2.6 kb (Table 1). The loop portion of the t-loops observed in GM847-Tert cells was also generally small, with 74% of measured loops being ≤5 kb in length. The distribution of total t-loop lengths (loop plus tail) in the GM847-Tert cross-linked preparations ranged from 2.0 to 90.3 kb (Fig. 3D), consistent with the telomere measurement by PFGE (Fig. 1). In non-cross-linked preparations from GM847-Tert cells, only 1.3% of the molecules were arranged into t-loops, reflecting the dependence on psoralen cross-linking for structural preservation (Table 1).
Other DNA forms were also present in the ALT telomeric preparations. Y-shaped molecules were observed at frequencies of 3.5 and 2.6% of the total molecules scored in cross-linked preparations from GM847 and GM847-Tert, respectively. In the absence of cross-linking, the corresponding values fell to 0.6 and 0.4%, respectively (Table 1). In both cell lines these DNA structures were large, with the total amount of DNA composing the three arms in Y molecules from GM847 cells ranging from 16 to 99 kb, suggesting that they are derived from the long telomeric DNA in ALT cells. While these molecules could be recombination or break-induced replication intermediates, we suspect they are most likely t-loops that have broken within the loop portion of the molecule. This conclusion is supported by the requirement of psoralen cross-linking to maintain both t-loop and Y-molecule structure. We also observed a small percentage (<1%) of molecules resembling Holliday Junctions (data not shown).
DISCUSSION
We report here that nuclei of human ALT cells frequently contain free DNA circles comprised of telomeric repeats similar to what has been seen in a K. lactis telomerase mutant and C. parapsilosis mitochondria. Further, we observed that telomeres in ALT cells are commonly arranged into t-loops, though on average the loop portion is small, similar to measurements observed in non-ALT cell lines. Together, these observations support the possibility of a roll-and-spread mechanism of telomere maintenance in human ALT cells.
Several lines of evidence suggest that the t-loops and t-circles described in this study are composed of telomeric DNA. First, there is a close correlation between the size distribution of t-loops observed by EM and telomere measurement by PFGE. Second, the high-molecular-weight DNA observed by EM in the excluded fractions that hybridized with a telomeric probe reached sizes of over 100 kb, and the majority of molecules were greater than 10 kb in length. In previous telomere preparations from HeLa S3 and FSK cells, very few molecules of lengths greater than 20 kb were observed. Thus, it is unlikely that very long satellite repeat DNA is present as a major contaminant in the excluded fractions following the extensive digestion of the genomic DNA. Third, the t-loops observed are consistent with previous descriptions of the telomere structure by EM (7, 13, 27, 28, 31). Finally, 2D PFGE confirmed the presence of telomeric repeats in the extrachromosomal circular material.
In our examination of TRFs from ALT cells, linear DNA molecules were the most commonly observed DNA form. We believe that the percentage of telomeres maintained in a t-loop structure through extensive preparation is a function of cross-linking efficiency, which is low, and therefore expect the bulk of telomeres to lose their looped form during enrichment (7, 13). The percentage of looped molecules observed in this study was lower than what was reported previously for HeLa cells (13). Several reasons may contribute to this. The long telomeric DNA in ALT cells is likely more prone to shearing, reducing the number of observed t-loops. This is supported by the relatively high percentage of Y molecules observed in these experiments. Portions of very long molecules were also commonly hidden from view by extension into the bar portion of the EM mounting grid and were not counted. Therefore, the number of large looped molecules is likely misrepresented. In previous experiments using HeLa cells (13), the telomeres were more homogenous in length and were present in a smaller number of elution fractions. Therefore, only the fraction(s) containing the highest percentage of t-loops were reported. In this study, the heterogeneous telomere sizes in ALT cells required that we score a greater number of fractions and present an average of these data. Although some early fractions did contain almost 20% t-loops, averaging several fractions lowered the percentage of total observed looped molecules.
The observation of t-loops in this study indicates that ALT cells are able to form and maintain t-loops on at least some portion of their chromosome ends. Although we initially expected to see large loop portions in the t-loops from ALT cells due to the long telomeres, the majority of the loop portions measured in both GM847 and GM847-Tert cells were small, similar to previous observations from non-ALT cells (13). In non-ALT human cells, telomere length is commonly less than 10 kb, with the majority of the telomeric DNA present in the loop portion of the t-loop (13). Although the telomeres are elongated in ALT cells, the machinery that generates the loop portion of the t-loop likely remains unchanged and therefore loop size would presumably remain at wild-type length. Within the cell, the t-loop may serve to anchor a higher order chromatin structure at the telomere. When the majority of the telomere is encompassed in the loop portion of the t-loop, the telomere would be highly protected within the higher ordered structure. However, in ALT cells where a large majority of the telomere is commonly not encompassed within the loop portion of the t-loop, a greater length of telomeric DNA would be excluded from a higher order structure and would remain relatively exposed, increasing the likelihood of recombination. Therefore, the small loop size observed relative to the total telomere length may predispose ALT telomeres to homologous recombination.
The observation of t-circles in this study is consistent with the presence of ECTR within the nuclei of ALT cells (32, 41, 47) and indicates that at least some fraction of these molecules is in a circular structure. This is consistent with the roll-and-spread mechanism of telomere expansion in K. lactis survivors and C. parapsilosis mitochondria. Although it is known that some ECTR DNA molecules reside in the nuclear APBs of ALT cells, we cannot determine if the t-circles we observed by EM and 2D PFGE originate from within the APBs. However, given the large number of circles observed and the small fraction of ALT cells containing APBs, it is likely that the circles are present regardless of APB presence within the cell.
Results from these experiments indicated that there was little difference in the abundance of t-loops and t-circles between GM847 and GM847-Tert cells (Table 1). The distribution of circle sizes observed by EM in both GM847 and GM847-Tert cells are skewed towards smaller circles, with a limited number of large circles observed in both cell types (Fig. 3A and B). In GM847-Tert, the distribution of circle size measured by EM differs slightly from the same measurements in GM847 (Fig. 3A and B). However, data from the 2D PFGE experiments suggests that there is little difference in the size distribution and abundance of t-circles from the two cell lines (Fig. 4 and 5). Therefore, differences observed by EM are likely due to the limitations of sample size in the microscopy experiments. Very little difference in the loop portion of t-loops was observed between GM847 and GM847-Tert cells. In GM847-Tert preparations there were fewer small t-loops (loop plus tail size of <5 kb) than in the GM847 preparations (Fig. 3D). This likely reflects the ability of telomerase to elongate the shortest telomeres in the GM847-Tert cell line, therefore reducing the number of short telomeres (35). Together these results suggest that the presence of the catalytic subunit of telomerase affects neither the generation of t-circles nor the ability to maintain t-loops in the GM847 cell line. This is consistent with previous results indicating that expression of Tert in the GM847 cell lines does not affect the ALT phenotype (35).
How the t-circles are generated in ALT cells was not determined in this study, though possibilities include an intratelomeric recombination event or the improper resolution of the t-loop at the loop junction (22, 42). Expected products of both events include the release of a free DNA circle and are consistent with data from ALT somatic cell hybrids that display rapid telomere shortening after inhibition of the ALT phenotype (34, 35). This would be expected if mutations that facilitate the development of the ALT phenotype alter the t-loop from being resistant to recombination to being permissive of recombination. Extrachromosomal circles containing telomeric repeats have also been observed previously in human cells exhibiting genomic instability and may suggest that some level of instability is required for t-circle generation (36). Another possibility is that linear ECTR molecules are ligated in the nucleus to form circles, as repetitive linear DNA would be expected to easily circularize due to the complementary ends. t-circles may also be present at the early stages of development, as seen in the amphibian Xenopus laevis (8), and their presence in ALT cells could reflect the improper upregulation of a developmental pathway. t-circles may also function to elongate telomeres directly via homologous recombination. For example, incorporation of a 50-kb circle would result in the immediate elongation of a telomere to the very large size observed in ALT cells.
Initiation of rcr on t-circles would be greatly facilitated by the presence of a nick or small gap exposing a 3′ hydroxyl group. Inefficient ligation following any of the above mechanisms of circle formation will allow a nick or small gap to occur. The observation by 2D PFGE (Fig. 5) of a substantial portion of the t-circles in an open circular form indicates that they are capable of serving as an rcr template. However, in the absence of a nick or gap, strand invasion, possibly from a telomeric 3′ overhang, could also facilitate the initiation of rcr on a t-circle template. Although rcr intermediates have been observed by EM in DNA preparations from a K. lactis telomerase mutant and C. parapsilosis mitochondria, in this study we were unable to distinguish t-loops from rcr intermediates due to their similar structure.
While the data presented here do not refute other postulated ALT mechanisms, the presence of t-circles in human ALT cells provides a possible explanation for how nascent telomeric DNA is synthesized in ALT cells. Following an initial rcr event, the newly synthesized telomeric DNA could insert into the chromosomal telomeres by homologous recombination. Once a small number of telomeres are elongated, intertelomeric recombination or break-induced replication would facilitate elongation of the short telomeres within the ALT cell. The newly synthesized telomeric DNA following rcr could also be processed into smaller linear telomeric DNA molecules, each capable of circularizing for subsequent use as an rcr template. Thus, the roll-and-spread mechanism may also function to constantly propagate t-circle formation in ALT cells.
The results presented here suggest that the mechanism of telomere maintenance in human ALT cells may parallel observations of K. lactis survivors and C. parapsilosis mitochondria (30, 42, 43). In the future, it will be important to determine what mutations allow t-circles to be generated and maintained in ALT cells as well as the exact mechanistic roles t-circles serve in the ALT phenotype. It will also be important to compare laboratory- versus cancer-derived ALT cell lines to determine if the naturally occurring ALT phenotype mimics the laboratory-derived state.
ADDENDUM IN PROOF
Following completion of this work, similar results on the appearance of t-circles were communicated to us by Titia de Lange (Rockefeller University, New York, N.Y.) (R. Wang, A. Smogorzewska, and T. de Lange, Cell, in press).
Acknowledgments
We thank Shelia Stewart (Washington University, St. Louis, Mo.) for her kind gift of the ALT cell lines used in this study. Deepa Subramanian (University of North Carolina at Chapel Hill) and Lubomir Tomaska (Comenius University, Bratislava, Slovakia) are thanked for critical review of the manuscript. Scott DeWire (UNC-CH) and members of the Griffith laboratory are thanked for their experimental suggestions.
This work was supported in part by grants from the National Institutes of Health (GM31819) and The Ellison Foundation. J.D.G. is an Ellison Senior Scholar.
REFERENCES
- 1.Bechter, O. E., J. W. Shay, and W. E. Wright. 2004. The frequency of homologous recombination in human ALT cells. Cell Cycle 3:547-549. [PubMed] [Google Scholar]
- 2.Bechter, O. E., Y. Zou, J. W. Shay, and W. E. Wright. 2003. Homologous recombination in human telomerase-positive and ALT cells occurs with the same frequency. EMBO Rep. 4:1138-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechter, O. E., Y. Zou, W. Walker, W. E. Wright, and J. W. Shay. 2004. Telomeric recombination in mismatch repair deficient human colon cancer cells after telomerase inhibition. Cancer Res. 64:3444-3451. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 5.Bryan, T. M., A. Englezou, L. Dalla-Pozza, M. A. Dunham, and R. R. Reddel. 1997. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 3:1271-1274. [DOI] [PubMed] [Google Scholar]
- 6.Bryan, T. M., A. Englezou, J. Gupta, S. Bacchetti, and R. R. Reddel. 1995. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14:4240-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesare, A. J., N. Quinney, S. Willcox, D. Subramanian, and J. D. Griffith. 2003. Telomere looping in P. sativum (common garden pea). Plant J. 36:271-279. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, S., and M. Mechali. 2002. Formation of extrachromosomal circles from telomeric DNA in Xenopus laevis. EMBO Rep. 3:1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Counter, C. M., A. A. Avilion, C. E. LeFeuvre, N. G. Stewart, C. W. Greider, C. B. Harley, and S. Bacchetti. 1992. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11:1921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange, T., L. Shiue, R. M. Myers, D. R. Cox, S. L. Naylor, A. M. Killery, and H. E. Varmus. 1990. Structure and variability of human chromosome ends. Mol. Cell. Biol. 10:518-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunham, M. A., A. A. Neumann, C. L. Fasching, and R. R. Reddel. 2000. Telomere maintenance by recombination in human cells. Nat. Genet. 26:447-450. [DOI] [PubMed] [Google Scholar]
- 12.Girardi, A. J., F. C. Jensen, and H. Koprowski. 1965. SV40-induced transformation of human diploid cells: crisis and recovery. J. Cell Physiol. 65:69-83. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 14.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 15.Harley, C. B., N. W. Kim, K. R. Prowse, S. L. Weinrich, K. S. Hirsch, M. D. West, S. Bacchetti, H. W. Hirte, C. M. Counter, C. W. Greider, et al. 1994. Telomerase, cell immortality, and cancer. Cold Spring Harbor Symp. Quant. Biol. 59:307-315. [DOI] [PubMed] [Google Scholar]
- 16.Henson, J. D., A. A. Neumann, T. R. Yeager, and R. R. Reddel. 2002. Alternative lengthening of telomeres in mammalian cells. Oncogene 21:598-610. [DOI] [PubMed] [Google Scholar]
- 17.Herbig, U., W. A. Jobling, B. P. Chen, D. J. Chen, and J. M. Sedivy. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell. 14:501-513. [DOI] [PubMed] [Google Scholar]
- 18.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 19.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 20.Londono-Vallejo, J. A., H. Der-Sarkissian, L. Cazes, S. Bacchetti, and R. R. Reddel. 2004. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 64:2324-2327. [DOI] [PubMed] [Google Scholar]
- 21.Lundblad, V., and E. H. Blackburn. 1993. An alternative pathway for yeast telomere maintenance rescues est1-senescence. Cell 73:347-360. [DOI] [PubMed] [Google Scholar]
- 22.Lustig, A. J. 2003. Clues to catastrophic telomere loss in mammals from yeast telomere rapid deletion. Nat. Rev. Genet. 4:916-923. [DOI] [PubMed] [Google Scholar]
- 23.Makarov, V. L., Y. Hirose, and J. P. Langmore. 1997. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88:657-666. [DOI] [PubMed] [Google Scholar]
- 24.Masutomi, K., E. Y. Yu, S. Khurts, I. Ben-Porath, J. L. Currier, G. B. Metz, M. W. Brooks, S. Kaneko, S. Murakami, J. A. DeCaprio, R. A. Weinberg, S. A. Stewart, and W. C. Hahn. 2003. Telomerase maintains telomere structure in normal human cells. Cell 114:241-253. [DOI] [PubMed] [Google Scholar]
- 25.McEachern, M. J., and E. H. Blackburn. 1996. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 10:1822-1834. [DOI] [PubMed] [Google Scholar]
- 26.Moyzis, R. K., J. M. Buckingham, L. S. Cram, M. Dani, L. L. Deaven, M. D. Jones, J. Meyne, R. L. Ratliff, and J. R. Wu. 1988. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 85:6622-6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Jordan, J. L., G. A. Cross, T. de Lange, and J. D. Griffith. 2001. T-loops at trypanosome telomeres. EMBO J. 20:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murti, K. G., and D. M. Prescott. 1999. Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops. Proc. Natl. Acad. Sci. USA 96:14436-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan, S., C. Groff-Vindman, and M. J. McEachern. 2003. Factors influencing the recombinational expansion and spread of telomeric tandem arrays in Kluyveromyces lactis. Eukaryot. Cell 2:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan, S., and M. J. McEachern. 2002. Recombinational telomere elongation promoted by DNA circles. Mol. Cell. Biol. 22:4512-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikitina, T., and C. L. Woodcock. 2004. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 166:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogino, H., K. Nakabayashi, M. Suzuki, E. Takahashi, M. Fujii, T. Suzuki, and D. Ayusawa. 1998. Release of telomeric DNA from chromosomes in immortal human cells lacking telomerase activity. Biochem. Biophys. Res. Commun. 248:223-227. [DOI] [PubMed] [Google Scholar]
- 33.Olovnikov, A. M. 1973. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41:181-190. [DOI] [PubMed] [Google Scholar]
- 34.Perrem, K., T. M. Bryan, A. Englezou, T. Hackl, E. L. Moy, and R. R. Reddel. 1999. Repression of an alternative mechanism for lengthening of telomeres in somatic cell hybrids. Oncogene 18:3383-3390. [DOI] [PubMed] [Google Scholar]
- 35.Perrem, K., L. M. Colgin, A. A. Neumann, T. R. Yeager, and R. R. Reddel. 2001. Coexistence of alternative lengthening of telomeres and telomerase in hTERT-transfected GM847 cells. Mol. Cell. Biol. 21:3862-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regev, A., S. Cohen, E. Cohen, I. Bar-Am, and S. Lavi. 1998. Telomeric repeats on small polydisperse circular DNA (spcDNA) and genomic instability. Oncogene 17:3455-3461. [DOI] [PubMed] [Google Scholar]
- 37.Shay, J. W., and S. Bacchetti. 1997. A survey of telomerase activity in human cancer. Eur. J. Cancer 33:787-791. [DOI] [PubMed] [Google Scholar]
- 38.Smogorzewska, A., and T. de Lange. 2002. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 21:4338-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart, S. A., W. C. Hahn, B. F. O'Connor, E. N. Banner, A. S. Lundberg, P. Modha, H. Mizuno, M. W. Brooks, M. Fleming, D. B. Zimonjic, N. C. Popescu, and R. A. Weinberg. 2002. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA 99:12606-12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng, S. C., and V. A. Zakian. 1999. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:8083-8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokutake, Y., T. Matsumoto, T. Watanabe, S. Maeda, H. Tahara, S. Sakamoto, H. Niida, M. Sugimoto, T. Ide, and Y. Furuichi. 1998. Extra-chromosomal telomere repeat DNA in telomerase-negative immortalized cell lines. Biochem. Biophys. Res. Commun. 247:765-772. [DOI] [PubMed] [Google Scholar]
- 42.Tomaska, L., M. J. McEachern, and J. Nosek. 2004. Alternatives to telomerase: keeping linear chromosomes via telomeric circles. FEBS Lett. 567:142-146. [DOI] [PubMed] [Google Scholar]
- 43.Tomaska, L., J. Nosek, A. M. Makhov, A. Pastorakova, and J. D. Griffith. 2000. Extragenomic double-stranded DNA circles in yeast with linear mitochondrial genomes: potential involvement in telomere maintenance. Nucleic Acids Res. 28:4479-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Zglinicki, T., G. Saretzki, W. Docke, and C. Lotze. 1995. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 220:186-193. [DOI] [PubMed] [Google Scholar]
- 45.Watson, J. D. 1972. Origin of concatemeric T7 DNA. Nat. New Biol. 239:197-201. [DOI] [PubMed] [Google Scholar]
- 46.Wright, W. E., V. M. Tesmer, K. E. Huffman, S. D. Levene, and J. W. Shay. 1997. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 11:2801-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeager, T. R., A. A. Neumann, A. Englezou, L. I. Huschtscha, J. R. Noble, and R. R. Reddel. 1999. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59:4175-4179. [PubMed] [Google Scholar]