Abstract
A procedure that uses the PCR to make rapid successive steps through a random-primed cDNA library has been developed to provide a method for sequencing very long genes that are difficult to obtain as a single clone. In each successive step, the portions of partial clones that extend out from the region of known DNA sequence are amplified by two stages of PCR with nested, outward-directed primers designed approximately 50 bases in from the end of the known sequence, together with a general primer based on the sequence of the vector. This procedure has been used to determine the coding sequence of the cDNA for the beta heavy chain of axonemal dynein from embryos of the sea urchin Tripneustes gratilla. By starting from a single parent clone, whose translated amino acid sequence overlapped the microsequence of a tryptic peptide of the beta heavy chain, and making 3 such walk steps downstream and 14 walk steps upstream, we obtained a sequence of 13,799 base pairs that had an open reading frame of 13,398 base pairs. This sequence encodes a polypeptide with 4466 residues of Mr 511,804 that is believed to correspond to the complete beta heavy chain of ciliary outer arm dynein.
Full text
PDF

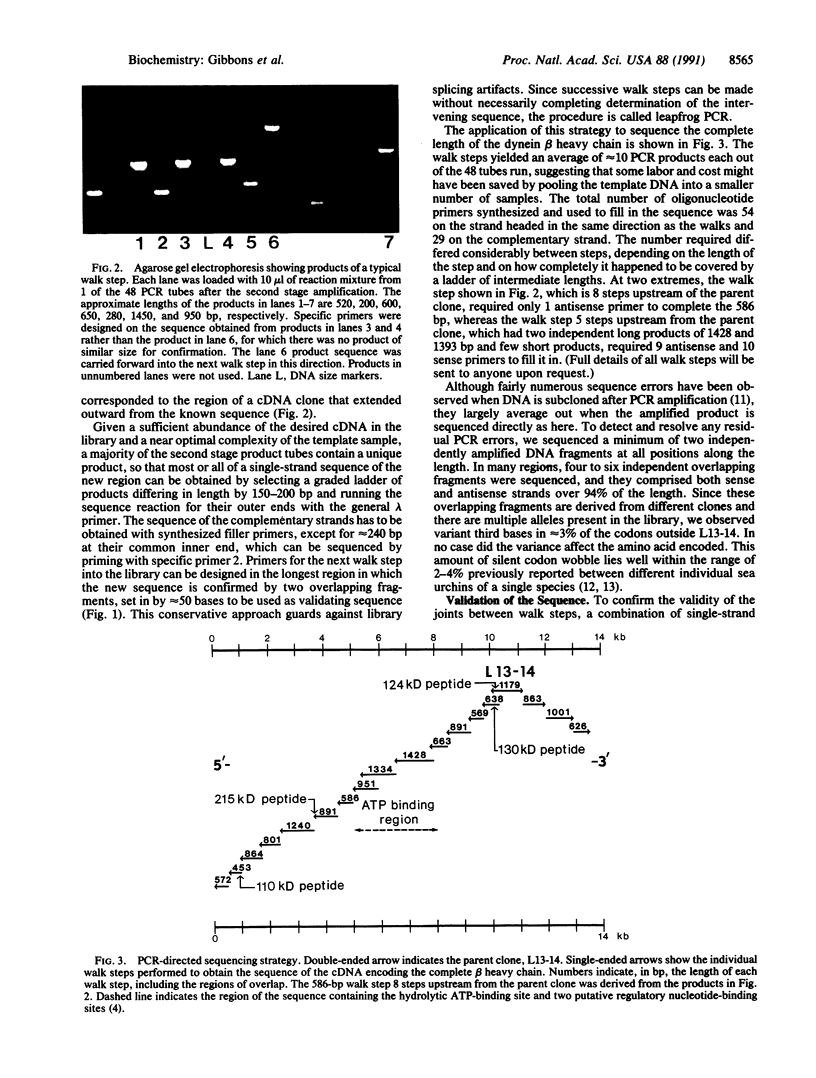


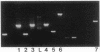
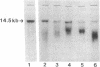
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. Dynein ATPases as microtubule motors. J Biol Chem. 1988 Nov 5;263(31):15837–15840. [PubMed] [Google Scholar]
- Gibbons I. R., Gibbons B. H., Mocz G., Asai D. J. Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature. 1991 Aug 15;352(6336):640–643. doi: 10.1038/352640a0. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mocz G., Tang W. J., Gibbons I. R. A map of photolytic and tryptic cleavage sites on the beta heavy chain of dynein ATPase from sea urchin sperm flagella. J Cell Biol. 1988 May;106(5):1607–1614. doi: 10.1083/jcb.106.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi S. R., Metz E. C. Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra). Mol Biol Evol. 1991 Mar;8(2):227–239. doi: 10.1093/oxfordjournals.molbev.a040642. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Primary structural differences among tubulin subunits from flagella, cilia, and the cytoplasm. Biochemistry. 1978 Jul 11;17(14):2882–2891. doi: 10.1021/bi00607a029. [DOI] [PubMed] [Google Scholar]