Abstract
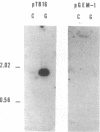
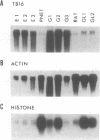
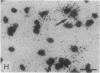
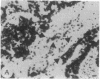
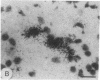
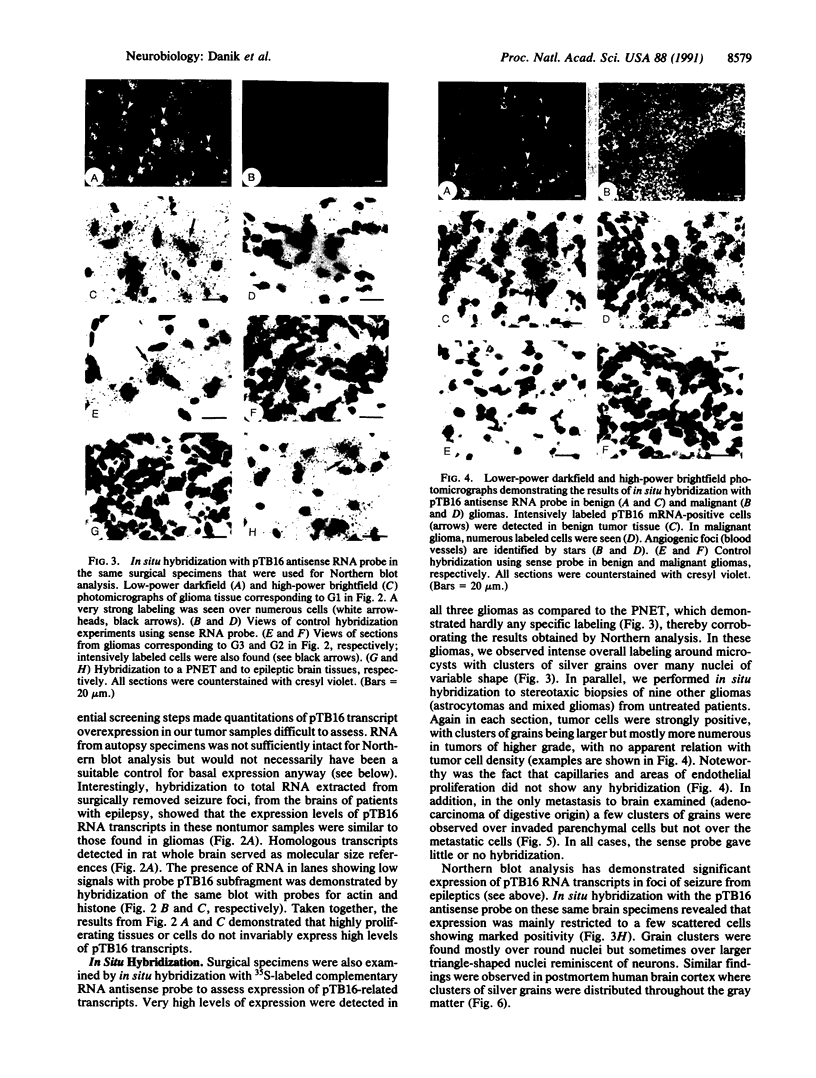
Clone pTB16 has been isolated by differential screening of a human glioma cDNA library. Northern blot analysis has shown that pTB16 expression is several times (greater than 11-fold) higher in gliomas than in a primitive neuroectodermal tumor. This observation was supported by in situ hybridization and extended to nine other gliomas. Expression was virtually absent in adenocarcinoma cells metastasized to brain. Malignant gliomas showed stronger hybridization than benign gliomas, while blood capillaries did not show hybridization. pTB16 mRNA was also shown to be expressed in established glioma cell lines and at high levels in epileptic foci, indicating that expression of the gene may be limited to certain cell types and that its upregulation is not merely a consequence of cellular proliferation. Nucleotide sequence analysis identified pTB16 as the human counterpart for rat testicular sulfated glycoprotein 2 (SGP-2), whose function in the reproductive system remains unknown. Although SGP-2 transcripts, and hence pTB16, were recently shown to be increased in neurodegenerative diseases such as scrapie in hamsters and Alzheimer disease in humans, our observations with brain tumors and epilepsy are suggestive of a role for pTB16 in neuropathologies in general and support the hypothesis of its involvement in tissue remodeling and cell death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Cox K. H., Angerer R. C. Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol. 1987;152:649–661. doi: 10.1016/0076-6879(87)52071-7. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Chen C. L., Feng Z. M., Marshall A., Bardin C. W. Rat clusterin isolated from primary Sertoli cell-enriched culture medium is sulfated glycoprotein-2 (SGP-2). Biochem Biophys Res Commun. 1988 Aug 30;155(1):398–404. [PubMed] [Google Scholar]
- Cheng C. Y., Mathur P. P., Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry. 1988 May 31;27(11):4079–4088. doi: 10.1021/bi00411a026. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Full-length cDNA clones: vector-primed cDNA synthesis. Methods Enzymol. 1987;152:371–389. doi: 10.1016/0076-6879(87)52044-4. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Bohmont C. W., Liu N. G., Tourtellotte W. W. Changes in brain gene expression shared by scrapie and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7260–7264. doi: 10.1073/pnas.86.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz I. B., Burdzy K., Sétchell B., Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983 Jun;28(5):1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- Griswold M. D., Morales C., Sylvester S. R. Molecular biology of the Sertoli cell. Oxf Rev Reprod Biol. 1988;10:124–161. [PubMed] [Google Scholar]
- Griswold M. D., Roberts K., Bishop P. Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry. 1986 Nov 18;25(23):7265–7270. doi: 10.1021/bi00371a003. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirszbaum L., Sharpe J. A., Murphy B., d'Apice A. J., Classon B., Hudson P., Walker I. D. Molecular cloning and characterization of the novel, human complement-associated protein, SP-40,40: a link between the complement and reproductive systems. EMBO J. 1989 Mar;8(3):711–718. doi: 10.1002/j.1460-2075.1989.tb03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- May P. C., Johnson S. A., Poirier J., Lampert-Etchells M., Finch C. E. Altered gene expression in Alzheimer's disease brain tissue. Can J Neurol Sci. 1989 Nov;16(4 Suppl):473–476. doi: 10.1017/s0317167100029796. [DOI] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. M. Nick translation. Methods Enzymol. 1987;152:91–94. doi: 10.1016/0076-6879(87)52012-2. [DOI] [PubMed] [Google Scholar]
- Michel D., Gillet G., Volovitch M., Pessac B., Calothy G., Brun G. Expression of a novel gene encoding a 51.5 kD precursor protein is induced by different retroviral oncogenes in quail neuroretinal cells. Oncogene Res. 1989;4(2):127–136. [PubMed] [Google Scholar]
- Munoz D. G., McNab B., George D. H., Johnson D. Absence of gliosis in the brains of epileptic fowl. Can J Neurol Sci. 1988 Nov;15(4):409–412. [PubMed] [Google Scholar]
- O'Bryan M. K., Baker H. W., Saunders J. R., Kirszbaum L., Walker I. D., Hudson P., Liu D. Y., Glew M. D., d'Apice A. J., Murphy B. F. Human seminal clusterin (SP-40,40). Isolation and characterization. J Clin Invest. 1990 May;85(5):1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. J., Christie D. L. The primary structure of glycoprotein III from bovine adrenal medullary chromaffin granules. Sequence similarity with human serum protein-40,40 and rat Sertoli cell glycoprotein. J Biol Chem. 1990 Apr 25;265(12):6617–6623. [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Buttyan R., Benson M., Cheng H. Gene expression during the early phases of regression of the androgen-dependent Shionogi mouse mammary carcinoma. Cancer Res. 1988 Nov 15;48(22):6309–6312. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester S. R., Skinner M. K., Griswold M. D. A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod. 1984 Dec;31(5):1087–1101. doi: 10.1095/biolreprod31.5.1087. [DOI] [PubMed] [Google Scholar]