Abstract
To investigate the role of the translocation-associated gene Pax3:Fkhr in alveolar rhabdomyosarcomas, we generated a Cre-mediated conditional knock-in of Pax3:Fkhr into the mouse Pax3 locus. Exploring embryonic tumor cell origins, we replaced a Pax3 allele with Pax3:Fkhr throughout its expression domain, causing dominant-negative effects on Pax3 and paradoxical activation of the Pax3 target gene, c-Met. Ectopic neuroprogenitor cell proliferation also occurs. In contrast, activation later in embryogenesis in cells that express Pax7 results in viable animals with a postnatal growth defect and a moderately decreased Pax7+ muscle satellite cell pool, phenocopying Pax7 deficiency but remarkably not leading to tumors.
Keywords: Alveolar rhabdomyosarcoma, Pax3:Fkhr, Pax7, FoxO1A, chromosomal translocation, satellite cell
A t(2;13) translocation forms the Pax3:Fkhr fusion gene in 55%-75% of cases for the childhood muscle cancer, alveolar rhabdomyosarcoma (Sorensen et al. 2002). This novel chimeric gene encodes for a transcription factor possessing the paired box and homeodomain DNA-binding domains from the transcription factor Pax3 fused to the potent transactivation domain of the Fkhr transcription factor (Sorensen et al. 2002). Pax3:Fkhr is believed to result in a molecular gain of function that may initiate a deregulated muscle development program in the affected cells.
The roles of Pax3 and Pax7 are best defined in embryogenesis, where they are important transcription regulatory factors in neural tube and myogenic development (Jostes et al. 1990; Goulding et al. 1991). Studies of Pax3-defective Splotch (Auerbach 1954) mice have revealed that Pax3 is an important determinant of p53-dependent dorsal neural tube closure (Pani et al. 2002) and an essential factor for proliferation and migration of muscle precursors from the presomitic mesoderm (Tremblay et al. 1998). Pax7 is most closely related to Pax3, and in general parallels the expression pattern of Pax3 with a slight delay in expression onset (Jostes et al. 1990). Whereas Pax3-defective mice fail to form embryonic hypaxial and epaxial muscles (Tremblay et al. 1998), Pax7-deficient mice have grossly normal embryonic muscle mass but are impaired in the ability form muscle stem cell (satellite cell)-derived postnatal muscle (Oustanina et al. 2004), which normally accounts for a substantial proportion of adult muscle mass.
In an accompanying paper (Keller et al. 2004), we demonstrate that activation of Pax3:Fkhr in a target pool of Myf6+ differentiating muscle produces alveolar rhabdomyosarcoma. In this paper, we determine molecular actions of Pax3:Fkhr by studying its perturbation of well-characterized processes: normal mouse embryogenesis, embryonic myogenesis, and postnatal satellite cell-derived myogenesis. The in vivo functions of Pax3:Fkhr during embryogenesis also provide important insight into the potential timing and cell(s) of origin for this tumor. Most importantly, we demonstrate the surprising result that Pax3:Fkhr expression in Pax7+ satellite cells does not lead to rhabdomyosarcomas.
Results and Discussion
The conditional Pax3:Fkhr (P3Fm) knock-in allele, which allows normal Pax3 expression of that allele until Cre is present, is described in the accompanying paper (Keller et al. 2004) and Supplementary Figure S1A. The complementary Pax7-driven Cre allele was designed to allow Cre expression in the Pax7 expression domain without interfering with the normal Pax7 function (see Materials and Methods; Supplementary Fig. S1B). Pax7ICNm/wt and Pax7ICNm/ICNm mice are phenotypically normal and fertile. To determine whether this Cre was expressed in the expected Pax7 expression domain, lineage analysis was performed by crossing Pax7ICNm/wt mice to ROSA26 LacZ reporter mice (Sorriano 1999). Doubly heterozygous Pax7ICNm/wt Gtrosa26tm1Sor/wt embryos were harvested at embryonic days 10 and 12 (E10 and E12) (Supplementary Fig. S1C,D, respectively). LacZ expression recapitulates the previously described pattern of Pax7 expression (Mansouri et al. 1996) in the somites, midface, and neural tube-derived structures.
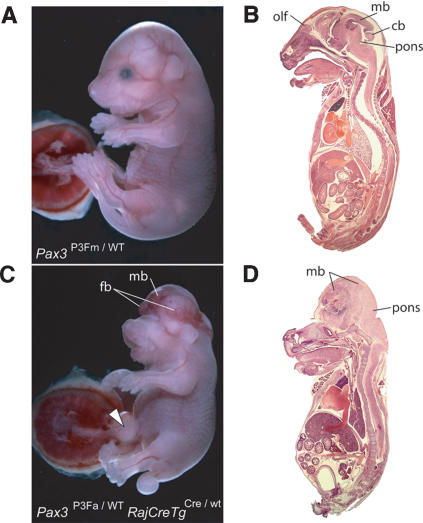
Pediatric cancers are frequently associated with chromosomal translocations, and these translocations are often asymptomatically present at birth (Greaves and Wiemels 2003). To replicate a prenatal Pax3:Fkhr translocation for alveolar rhabdomyosarcomas, we triggered Pax3:Fkhr in the Pax3 expression domain from the earliest time point of Pax3 promoter activation by breeding conditional Pax3P3Fm/wt mice to transgenic mice expressing Cre ubiquitously from early gestation (Schwenk et al. 1995) (we denote this transgene locus as RajCreTg). The Pax3:Fkhr transcript was confirmed by sequencing the RT-PCR product of Pax3P3Fa/wt RajCreTgCre/wt E10.5 embryos (see Supplementary Fig. S2). Pax3P3Fa/wt RajCreTgCre/wt mice were born in normal Mendelian ratios but displayed severe birth defects (see Fig. 1A-D and its legend). Elements of this phenotype are reminiscent of the Splotch (Auerbach 1954) and Splotch-delayed (Tremblay et al. 1998) homozygous mutant phenotypes. This partial phenocopy of Pax3 deficiency includes cranioschisis, exencephaly, and midface abnormalities (Tremblay et al. 1998) and characteristic skeletal dysplasia (see Supplemental Material; Supplementary Fig. S3). The hypoplastic dorsal root ganglia, normally organized but hypoplastic limb muscle, and thinned diaphragm of Pax3P3Fa/wt RajCreTgCre/wt mice are similar but do not fully phenocopy the absent dorsal root ganglia, absent hypaxial (limb) muscle, and absent diaphragm reported for Splotch mice (see Supplementary Fig. S4). Pax3P3Fa/wt RajCreTgCre/wt mice had normal respiratory competency for several hours under isothermal conditions. Phenotypes of Splotch mice not present in Pax3P3Fa/wt RajCreTgCre/wt mice include spina bifida of incomplete penetrance and cardiac conotruncal defects (truncus arteriosus, ventricular septal defects), whereas Pax3P3Fa/wt RajCreTgCre/wt mice have distinctive phenotypes such as forebrain hypoplasia, anophthalmia, disorganized midbrain overgrowth, and omphalocoele.
Figure 1.
Early embryonic activation of Pax3:Fkhr. (A,B) Whole-mount and sagittal section of an unaffected E18.5 embryo harboring a single Pax3:Fkhr allele (Pax3P3Fm/wt) that had not been activated by Cre. (C,D) Whole-mount and sagittal section of a mutant E18.5 embryo harboring a single Pax3:Fkhr allele that has been activated by the Rajewsky Cre driver (Pax3P3Fa/wt RajCreTgCre/wt). Note cranioschisis, exencephaly, anophthalmia, omphalocoele (open arrowhead), foreshortened snout, and slightly foreshortened limbs. In the mutant, the forebrain (fb) is hypoplastic and laterally displaced by the midbrain. Note absence of the cerebellum (cb) and olfactory lobe (olf), as well as overproliferation and anteriorization of the midbrain (mb).
We concluded that Pax3P3Fa/wt RajCreTgCre/wt mice partially phenocopy the Splotch and Splotch-delayed phenotypes for Pax3 deficiency, suggesting the activity of the single wild-type allele of Pax3 in Pax3P3Fa/wt RajCreTgCre/wt mice is at least partially suppressed by the dominant-negative action of Pax3:Fkhr; however, novel features of our mice suggested that Pax3:Fkhr has additional embryonic targets as well.
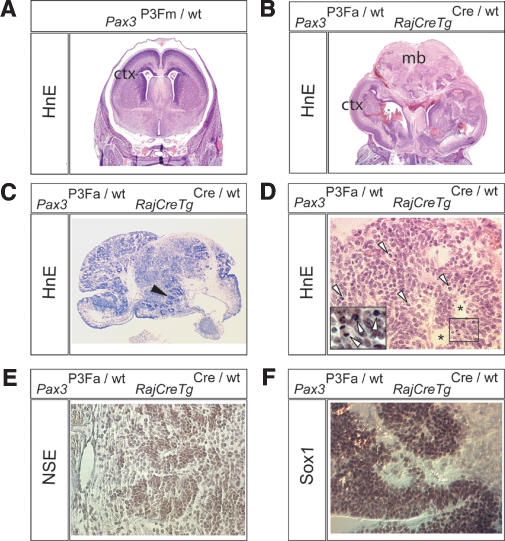
We explored the midbrain overgrowth of newborn Pax3P3Fa/wt RajCreTgCre/wt mice to determine whether these cells had undergone hamartomatous or neoplastic transformation. By histology (Fig. 2A,B), the midbrain and cortex was highly disorganized. Pseudorosettes with a high mitotic index were present in an increasing caudal-to-rostral gradient within the midbrain (Fig. 2C-F). Immunohistochemistry to determine the lineage of the pseudorosetting cells revealed that they express neuron-specific enolase (Fig. 2E) but not the markers of mature neuronal (synaptophysin, PGP9.5, S100, 68-kDa neurofilament, β-3-tubulin), glial (GFAP), ependymal (AE1.3), or mesenchymal (myogenin, desmin, vimentin) differentiation (data not shown). Electron microscopy revealed cells with high nuclear-to-cytoplasmic ratios without neurofilaments or other distinguishing features (data not shown). Because of the apparent undifferentiated state of these cells, we tested them for the expression of Sox1 (Fig. 2F), a marker of primitive embryonic neural stem cells (Aubert et al. 2003), which was positive. We therefore suggest that these ectopic, mitotically active cells represent retained primitive neuroectodermal precursors. The mitogenic effect Pax3:Fkhr has on Sox-1-expressing neural stem cells in the midbrain is especially interesting from the point of view that there may be tissue-specific cofactors that allow Pax3:Fkhr to induce proliferation in these cells but not others. This result also raises the interesting possibility that mechanisms underlying this Pax3:Fkhr-mediated neural stem cell proliferation could be elucidated and used for expansion of primitive embryonic neural stem cells in other contexts.
Figure 2.
Overproliferation of primitive neuroectodermal cells for early embryonic activation of Pax3:Fkhr. Histological (HnE) brain sections of a Pax3P3Fm/wt E18.5 embryo (A) and a mutant Pax3P3Fa/wt RajCreTgCre/wt E18.5 embryo (B-F). (A,B) Coronal section showing a global disorganization of the cortex (ctx) and overgrowth of the midbrain (mb) for mutant embryos. (C) More anterior coronal section of the mutant showing multiple zones of proliferation characterized by pseudorosettes (black arrowheads). (D) High-power view of pseudorosettes (asterisks) demonstrating multiple mitotic figures (open arrowheads and inset). (E) Positive immunohistochemical staining of pseudorosetting cells for neuron-specific enolase (NSE). (F) Positive Sox1 immunohistochemical staining of pseudorosetting cells from the anterior-most region of the mutant midbrain.
It is especially interesting that Pax3:Fkhr appears to have these previously unsuspected gain-of-function properties in tissues of the optic placode and midbrain whose patterning and development are known to be affected by other Pax family members. Although Pax2 and Pax6 expression was preserved (see Supplemental Material; Supplementary Fig. S6), it remains possible that Pax3:Fkhr alters expression of downstream Eya, Six, Dach, and Groucho genes that normally interact with these Pax genes during development. It will be useful to take into account the recent report of transfection experiments (Xia and Barr 2004) for which oncogenic or growth suppressive effects of Pax3:Fkhr were correlated with low versus high expression levels, respectively.
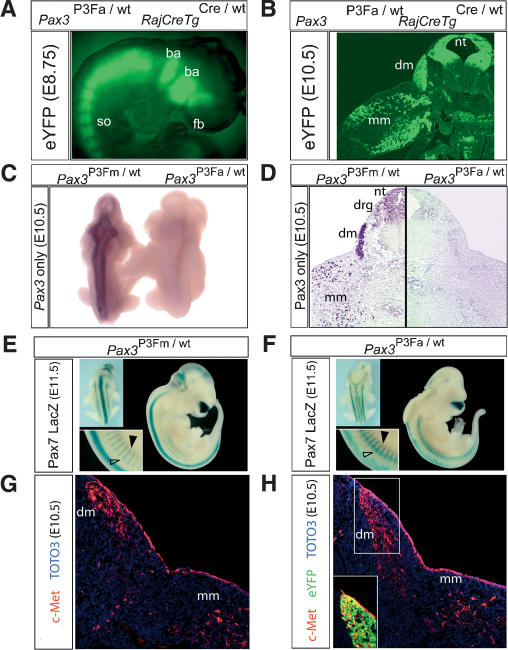
Because of the similarity of our Pax3P3Fa/wt RajCreTgCre/wt phenotype to the Splotch phenotype of homozygous Pax3 deficiency, we tested whether Pax3:Fkhr was expressed in the expected Pax3 domain or ectopically in a pattern influenced by 3′ Fkhr genomic regulatory elements, and whether expression of the normal allele of Pax3 was affected. The eYFP marker for Pax3:Fkhr was expressed strongly in a pattern consistent with normal Pax3 promoter activity (see Fig. 3A,B and its legend). RNA in situ hybridization using a riboprobe specific for the wild-type allele of Pax3 revealed complete absence of wild-type Pax3 expression (Fig. 3C) in the Pax3P3Fa/wt RajCreTgCre/wt E10.5 mutant embryo, establishing that Pax3:Fkhr suppresses Pax3 transcript levels. Immunohistochemistry using an antibody specific to the C terminus of wild-type Pax3 protein confirmed the near-complete absence of Pax3 in the Pax3P3Fa/wt RajCreTgCre/wt mutant (Fig. 3D). This absence of Pax3 transcripts and protein accounts for the similarities to the Splotch phenotype.
Figure 3.
Pax3:Fkhr, Pax3/7, and target gene expression with early embryonic activation of Pax3:Fkhr. (A) Expression of the Pax3:Fkhr marker, eYFP, in somites (so), branchial arches (ba), and anterior forebrain (fb) of an E8.75 embryo. (B) eYFP marker of Pax3:Fkhr expression in a transverse section of the forelimb of an E10.5 Pax3P3Fa/wt RajCreTgCre/wt embryo. (nt) Neural tube; (dm) dermomyotome; (mm) migrating myoblasts. (C) Whole-mount RNA in situ hybridization using a probe specific for only wild-type Pax3. (Right) Neural tube signal is absent for the mutant Pax3P3Fa/wt RajCreTgCre/wt embryo. (D) Immunohistochemistry demonstrates near complete absence of wild-type Pax3 protein in the mutant Pax3P3Fa/wt RajCreTgCre/wt embryo in an axial section of the forelimb. (E,F) Expression of Pax7 using a LacZ reporter reveals preserved Pax7 expression in the unaffected Pax3P3Fm/wt (E) and the mutant Pax3P3Fa/wt RajCreTgCre/wt (F) embryo. However, the neural tube (F, upper left inset), dorsal root ganglia, and dermomyotome (F, lower left inset, open and black arrowheads, respectively) were severely perturbed. (G) Immunohistochemistry for c-Met in an axial cross-section of the unaffected Pax3P3Fm/wt embryo at the level of the forelimb. (H) c-Met immunohistochemistry in an axial section of the mutant Pax3P3Fa/wt RajCreTgCre/wt embryo, demonstrating maintenance of c-Met expression despite Pax3 absence. (Inset) The eYFP marker of Pax3:Fkhr expression is coexpressed in c-Met-expressing cells of the dermomytome (yellow).
The mechanism for the decreased Pax3 transcript level remains to be determined; however, in Pax3:Fkhr-expressing human tumor cell lines, the level of Pax3 mRNA is fivefold lower than the level of Pax3:Fkhr mRNA (Davis and Barr 1997) despite similar RNA stabilities, suggesting a transcriptional level of action. Because Pax3 transcription is positively autoregulated by its own protein product (Ridgeway and Skerjanc 2001), a reasonable model for suppression of Pax3 transcription by Pax3:Fkhr is that the fusion protein negatively regulates Pax3 transcription through the Pax3 cis-autoregulatory element.
To account for the differences between the Pax3P3Fa/wt RajCreTgCre/wt phenotype and Splotch, we examined expression of the Pax3-related gene, Pax7, and downstream targets of Pax3. For Pax7 expression, we generated mutant and normal E11.5 embryos carrying a targeted replacement of Pax7 with LacZ (Mansouri et al. 1996). Pax7-LacZ expression was present in the P3Fa/wtRajCreTgCre/wt mutant, but Pax3-Pax7-derived structures such as the neural tube, dorsal root ganglia, and dermomyotome were perturbed (Fig. 3E,F). To determine whether Pax3:Fkhr may partially compensate for Pax3 loss in hypaxial muscle, we examined expression of a Pax3 downstream target gene, c-Met, in cells of the dermomyotome that migrate into the forelimb at E10.5 (Fig. 3G,H). Despite the absence of Pax3 in the Pax3P3Fa/wt RajCreTgCre/wt mutant embryo, c-Met expression was increased and found to be coexpressed with the Pax3:Fkhr marker, eYFP. This result demonstrates that Pax3:Fkhr may partially compensate for Pax3 loss by transcriptional activation of some Pax3 target genes in developing hypaxial muscle. This result is also consistent with in vitro data demonstrating that c-Met is a target of Pax3:Fkhr (Ginsberg et al. 1998). Interestingly, broad activation of the c-Met signaling pathway in conjunction with inactivation of the Ink4a/ARF locus results in non-alveolar rhabdomyosarcomas in mice at high frequency (Sharp et al. 2002).
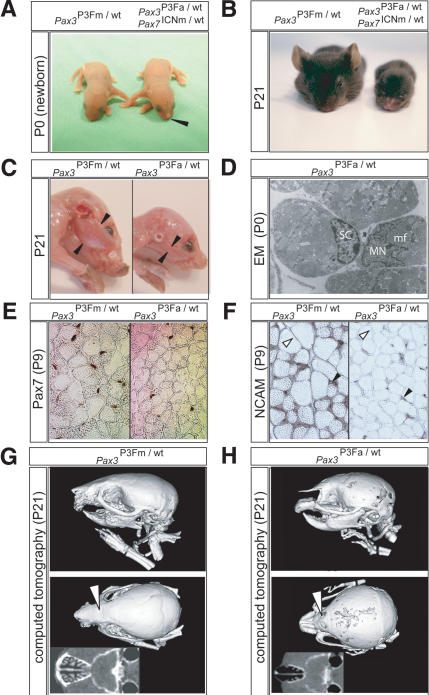
To study the consequences of later embryonic activation of Pax3:Fkhr in the overlap of Pax3 and Pax7 expression domains, we bred conditional Pax3:Fkhr mice to Pax7-Cre mice. Pax3P3Fa/wt Pax7ICNm/wt pups were represented at normal Mendelian ratios and displayed the mild birth defects of a narrowed nose (Fig. 4A). Although initially equal in size to Pax3P3Fm/wt Pax7wt/wt littermates, the Pax3P3Fa/wt Pax7ICNm/wt pups showed evidence of severe postnatal growth retardation by age 3 wk, at which time they weighed less than one-third of their unaffected littermates (Fig. 4B). The reduced weight of the postnatal day 21 (P21) neonates is primarily the result of extreme reduction of muscle mass (Fig. 4C), with an accompanying mild reduction in myofiber diameter by histology (data not shown). To determine whether this reduction in muscle mass was a consequence of reduced satellite cells (Oustanina et al. 2004), electron microscopy was performed, revealing that satellite cells were present in normal weight Pax3P3Fa/wt Pax7ICNm/wt mutant P0 pups (Fig. 4D), but in fewer numbers than control littermates. To determine more precisely whether the number of satellite cells was reduced in Pax3P3Fa/wt Pax7ICNm/wt mice postnatally, we harvested the vastus lateralis muscle of P9 mouse pups and performed Pax7 immunohistochemistry to detect satellite cells, counterstaining myofiber nuclei with DAPI (Fig. 4E). We found a significant reduction in the percentage of satellite cells for mutant muscle compared with normal (10% vs. 15.2%, p < 0.001, n = 11,805 non-vessel nuclei counted). Myofiber density was significantly higher in mutants as compared with normals (515 vs. 349 cells per field, p < 0.001) as a result of reduced cross-sectional area of individual myofibers (affected myofiber cross-sectional area was 68% of control), which is similar to findings for Pax7-/- mice (Oustanina et al. 2004). The proportion of activated myoblasts coexpressing Pax7 and Myogenin in mutant and control mice appear comparable (data not shown); however, immunohistochemical staining for N-CAM, a cell surface marker of myofibers that have undergone fusion with a satellite-cell-derived myoblast (Covault et al. 1986), showed severely reduced N-CAM expression at the surface of mature myofibers from Pax3P3Fa/wt Pax7ICNm/wt mutant mice (Fig. 4F). Taken together, these results suggest that the small myofiber diameter at P21 is a result of the smaller satellite cell pool that is impaired in self-renewal capacity as are Pax7-/- mice (Oustanina et al. 2004), resulting in fewer satellite-cell-derived activated myoblasts being available to fuse with existing mature myofibers. This functional defect appears to have been present at birth but was significantly exacerbated postnatally.
Figure 4.
Phenotype caused by later embryonic activation of Pax3:Fkhr in the Pax7 expression domain. (A) P0 mouse pups harboring a single Pax3:Fkhr allele not activated by Cre (Pax3P3Fm/wt Pax7wt/wt, left) or a single Pax3:Fkhr allele that had been activated by Pax7-Cre (Pax3P3Fa/wt Pax7ICNm/wt, right). Note pointed nose of the mutant (black arrowhead). (B) Severe runting at P21 of the mutant (right, weight 3 g) compared with the unaffected pup (left, 10.5 g). (C) Reduced masseter muscle mass (flanked by black arrowheads) of the pointed-nose mutant (right) in comparison to the unaffected control (left). (D) Electron microscopy (12,838×) demonstrating the presence of satellite cells, SC, in Pax3P3Fa/wt Pax7ICNm/wt mice. (MN) Myofiber nucleus; (mf) adjacent myofibrils. (E) Pax7 immunohistochemistry demonstrating lower satellite cell frequency and one-third smaller myofiber cross-sectional diameter in vastus lateralis skeletal muscle of Pax3P3Fa/wt Pax7ICNm/wt mutant mice (right) compared with Pax3P3Fm/wt Pax7wt/wt control mice (left). (F) Immunohistochemistry for N-CAM, demonstrating cell surface expression in myofibers of Pax3P3Fm/wt Pax7wt/wt control mice (left) but not mutant Pax3P3Fa/wt Pax7ICNm/wt mice (right). N-CAM expression is present in satellite cells (black arrowheads) from both animals. (G,H) Computed tomography 46-μm3 resolution scans of the unaffected (G) and mutant (H) P21 pup skulls. Note absence of the rostral portion of the premaxilla (pm), hypoplasia of the lacrimal bones (open arrows), and hypoplasia of the nasal turbinates (insets).
For the midface defects, computed tomography scan at P21 demonstrated maxillary and lacrimal bone hypoplasia, agenesis of the rostral premaxilla, and severely underdeveloped nasal turbinates relative to control littermates (Fig. 4G,H). Because of the increased work of breathing associated with a collapsible nasal airway, only a limited number of Pax3P3Fa/wt Pax7ICNm/wt mice have survived to 3.5 mo of age (lethality was in part ameliorated by serial fostering followed by weaning to a gel-based source of hydration). None of these animals having ubiquitous Pax3:Fkhr rearrangements in their Pax7+ satellite cells developed alveolar rhabdomyosarcoma; this is a remarkable observation, because it is widely thought that alveolar rhabdomyosarcomas arise from satellite cells.
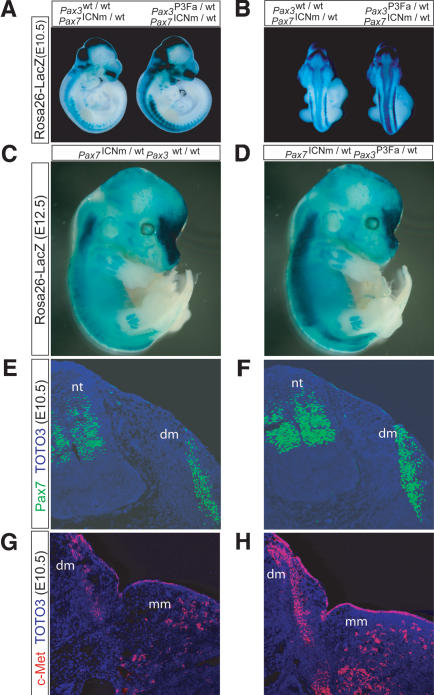
Collectively, the reduced muscle mass, maxillary and lacrimal bone hypoplasia, and nasal turbinate abnormalities of Pax3P3Fa/wt Pax7ICNm/wt mice strongly suggest a phenocopy of the Pax7 deficiency (Mansouri et al. 1996; Oustanina et al. 2004). To determine whether the Pax3:Fkhr allele was directly suppressing Pax7 transcription, we bred a ROSA26 LacZ reporter gene (Sorriano 1999) into Pax3P3Fa/wt Pax7ICNm/wt mutant mice and controls to act as a readout of Pax7 promoter activity and cell survival in E10.5 and E11.5 embryos (Fig. 5A-D). We encountered a range of LacZ reporter activity for which the Pax7 promoter activity of Pax3P3Fa/wt Pax7ICNm/wt mice was either equivalent or increased as compared with Pax3wt/wt Pax7ICNm/wt control mice. An example of increased activity is shown in Figure 5A-D. The increased expression of Pax7 in Pax3P3Fa/wt Pax7ICNm/wt mice may be understood in terms of Pax3:Fkhr-mediated suppression of Pax3 expression, because Pax3 is normally a repressor of Pax7 expression (Borycki et al. 1999) (in other words, Pax7 expression increases because Pax3 no longer suppresses Pax7 transcription). At the protein level, Pax7 was also found to be increased in the neural tube and dermomyotome of Pax3P3Fa/wt Pax7ICNm/wt E10.5 embryos (Fig. 5E,F). As before for severely affected Pax3P3Fa/wt RajCreTgCre/wt mutant embryos, the structurally less perturbed Pax3P3Fa/wt Pax7ICNm/wt E10.5 embryos demonstrated increased expression of the Pax3 downstream target, c-Met (Fig. 5G,H). This Pax7 knockout phenocopy, despite increased Pax7 expression, could be explained by dominant-negative effects of Pax3:Fkhr on Pax7 targets, rather than effects on expression of Pax7 itself. Unfortunately, at present downstream targets unique to Pax7 but not shared by Pax3 remain to be identified. A working model for how Pax3:Fkhr modulates Pax3, Pax7, and other downstream targets is shown in Supplementary Figure S7.
Figure 5.
Pax7 and c-Met expression with embryonic activation of Pax3:Fkhr in the Pax7 expression domain. (A,B) Rosa26 LacZ reporter activity in E10.5 embryos harboring a targeted Pax7-Cre allele alone (Pax7ICNm/wt, left) or a targeted Pax7-Cre allele and a single, activated Pax3:Fkhr allele (Pax3P3Fa/wt Pax7ICNm/wt, right). Reporter activity suggests maintenance or increased level of Pax7 promoter activity in the neural tube and somites, as well as new Pax7 promoter activity in the maxillary and mandibular branches of branchial arch 1. (C,D) Unaffected (C) and affected (D) E12.5 embryos demonstrating sustained Pax7 promoter activity. (E,F) Pax7 immunohistochemistry in an axial cross-section at the level of the forelimb of the unaffected and affected embryos, demonstrating maintenance of Pax7 protein expression. (G,H) c-Met immunohistochemistry in an axial cross-section at the level of the forelimb of the unaffected and affected embryos, demonstrating increased expression of c-Met in the affected embryo (H).
Recent transfection studies have shown that in embryonal rhabdomyosarcoma cells forced to express the alveolar rhabdomyosarcoma oncogene Pax3:Fkhr, Pax3 levels are increased and Pax7 levels are decreased (Tomescu et al. 2004). We have no clear explanation for the differences between this study and ours, except to suggest that embryonal rhabdomyosarcomas may not be the starting point or an equivalent cellular context for alveolar rhabdomyosarcomas.
In important previous studies, germ-line expression of the Pax3:Fkhr fusion gene under the control of the Pax3 promoter has resulted in embryonic lethality without midbrain overproliferation, anophthalmia, or forebrain abnormalities (for references and comparisons, see Supplementary Table 1). In several instances, the other models represent hypomorphs of the early embryonic activation of our conditional model. However, each of these Pax3:Fkhr alleles had design differences such as being a transgene or being constructed from an incomplete cDNA instead of genomic DNA that may have affected the spectrum of Pax3:Fkhr activities in separate tissues. In our conditional knock-in model, we closely recapitulate the cis-regulatory changes caused by the real translocation in human tumors. At the time of Cre expression, we remove the 3 kb of Pax3 containing exons 8-10 and juxtapose a previously silent 9.3-kb genomic fragment of Fkhr containing exons 2 and 3 and the full 3′-untranslated region. By using murine genomic DNA from both Pax3 and Fkhr, we avoided potential complications of species-specific regulatory element sequence. The value of this approach is demonstrated by the markedly different phenotypes we see in our mice with early embryonic activation of Pax3:Fkhr. Furthermore, with this conditional model we have the added advantage of studying the effects of Pax3:Fkhr at midgestation, thereby bypassing the earlier Pax3:Fkhr expression that we show results in massive perturbation of the central nervous system. The later, less severe (yet intriguing) phenocopy of Pax7 deficiency we report here allows for closer comparisons of gene expression levels in tissues whose structures are not so severely perturbed.
With regard to the timing and cell of origin for human tumors possessing Pax3:Fkhr translocations, the severity of birth defects in Pax3P3Fa/wt RajCreTgCre/wt or Pax3P3Fa/wt Pax7ICNm/wt mice and associated lethality suggests that the translocation event in humans is unlikely to occur either as a germ-line mutation or as a mosaic in a broad range of embryonic myogenic precursors. One could speculate that a small subset of embryonically or postnatally derived Pax3/Pax7-coexpressing satellite cells could give rise to an alveolar rhabdomyosarcoma; however, our results demonstrate that Pax3P3Fa/wt Pax7ICNm/wt mice carrying the activated Pax3:Fkhr allele in the entire Pax7+ subcompartment of the satellite cell pool do not develop tumors even up to 3.5 mo of age, well into the young adulthood of these mice. This unexpected result that Pax7+ satellite cells do not give rise to alveolar rhabdomyosarcomas is made more remarkable by the observation in the accompanying paper (Keller et al. 2004) that Pax3:Fkhr activation in a target pool of Myf6+ differentiating myofibers leads to alveolar rhabdomyosarcoma tumors. These provocative findings, that mature differentiating skeletal muscle and not satellite cells gives rise to alveolar rhabdomyosarcomas, asks us to reconsider the widely held assumption that only muscle stem cells give rise to rhabdomyosarcomas.
Materials and methods
Isolation of genomic clones
A 21-kb genomic clone spanning exon 8 to the 3′-untranslated region of Pax7 was isolated from a λ bacteriophage library of mouse strain SvJ-129 (Stratagene).
Production of gene-targeted mouse lines
Generation of the Pax3:Fkhr conditional allele is described in the accompanying paper (Keller et al. 2004). For the Pax7-Cre mouse line, a targeting vector was constructed that placed an ires-Cre-FRT-Neo-FRT cassette in the ClaI site within the 3′-untranslated region of the Pax7 gene following the stop codon in exon 10. This targeting vector was electroporated into R1 embryonic stem cells that were then subjected to positive and negative selection. Three of 144 clones were identified as positive by screening with EcoRI genomic DNA digests with Southern hybridization using a 3′ external probe. Germ-line mice were generated as described in our accompanying paper (Keller et al. 2004). For genotyping by PCR, see Supplemental Material.
Pax7-Cre and Pax7-LacZ expression analysis
Whole-mount detection of the tissue-specific CRE expression for the Pax7-Cre mouse line was performed by crossing that line to ROSA26 LacZ reporter mice (Sorriano 1999). Embryos were harvested at E10, E10.5, E12, or E12.5 and as described in our accompanying paper (Keller et al. 2004). Whole-mount LacZ expression in Pax7-LacZ E11.5 embryos was detected similarly (Mansouri et al. 1996).
Immunohistochemistry and fluorescence
Immunohistochemistry of sections was performed as described in our accompanying paper (Keller et al. 2004), and as detailed in Supplemental Material. For whole-mount eYfp fluorescence detection, E8.75 embryos placed in LabTek Chamber Slides (Nunc) and visualized by epifluorescence microscopy on a Zeiss Axiovert 200M microscope (Intelligent Imaging Innovations) using a YFP filter and Slidebook integration and de-convolution software (Intelligent Imaging Innovations).
RNA in situ hybridization
RNA in situ hybridization was performed as previously described (Lufkin 2003) using an 843-bp probe unique to native Pax3 only (GenBank accession no. 6679210: nucleotides 1464-2214) that was derived from a reverse-transcriptase-amplified cDNA from wild-type E11 mouse embryo total RNA, which had been subcloned into the pCRII-TOPO plasmid (Invitrogen Life Technologies).
Acknowledgments
The monoclonal antibodies developed by Atsushi Kawakami and Miyuki Yamamoto were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). We appreciate a C-terminal-specific Pax3 antibody from Jonathan Epstein. We thank Mary Blandford, Kristin Deatherage, Jennifer Edwards, Misha S. Capecchi, Nichole Sanderson, Nathan Marsala, Fred Clayton, Lyn Pedone, and Daniel Fults for technical assistance and advice. This work was supported by a Scott Carter Foundation Fellowship to C.K. and by a K08 award from the National Cancer Institute (1K08 CA90438-01) to C.K.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1243904.
References
- Aubert J., Stavridis, M.P., Tweedie, S., O'Reilly, M., Vierlinger, K., Li, M., Ghazal, P., Pratt, T., Mason, J.O., Roy, D., et al. 2003. Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. 100 Suppl 1: 11836-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R. 1954. Analysis of the developmental defects of a lethal mutation in the house mouse. J. Exper. Zool. 127: 305-329. [Google Scholar]
- Borycki A.G., Li, J., Jin, F., Emerson, C.P., and Epstein, J.A. 1999. Pax3 functions in cell survival and in pax7 regulation. Development 126: 1665-1674. [DOI] [PubMed] [Google Scholar]
- Covault J., Merlie, J.P., Goridis, C., and Sanes, J.R. 1986. Molecular forms of N-CAM and its RNA in developing and denervated skeletal muscle. J. Cell Biol. 102: 731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. and Barr, F.G. 1997. Fusion genes resulting from alternative chromosomal translocations are overexpressed by gene-specific mechanisms in alveolar rhabdomyosarcoma. Proc. Natl. Acad. Sci. 94: 8047-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg J.P., Davis, R.J., Bennicelli, J.L., Nauta, L.E., and Barr, F.G. 1998. Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 58: 3542-3546. [PubMed] [Google Scholar]
- Goulding M.D., Chalepakis, G., Deutsch, U., Erselius, J.R., and Gruss, P. 1991. Pax3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 10: 1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M.F. and Wiemels, J. 2003. Origins of chromosome translocations in childhood leukaemia. Nat. Rev. Cancer 3: 639-649. [DOI] [PubMed] [Google Scholar]
- Jostes B., Walther, C., and Gruss, P. 1990. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 33: 27-37. [DOI] [PubMed] [Google Scholar]
- Keller C., Arenkiel, B.R., Coffin, C.M., El-Bardeesy, N., DePinho, R.A., and Capecchi, M.R. 2004. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: Cooperativity of Ink4a/ARF and Trp53 loss of function. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Lufkin T. 2003. In situ hybridization of whole mount embryos with RNA probes. In Manipulating the mouse embryo (eds. M.G. Andras Nagy et al.), pp. 670-682. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mansouri A., Stoykova, A., Torres, M., and Gruss, P. 1996. Dysgenesis of cephalic neural crest derivatives in Pax7-/- mutant mice. Development 122: 831-838. [DOI] [PubMed] [Google Scholar]
- Oustanina S., Hause, G., and Braun, T. 2004. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 23: 3430-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L., Horal, M., and Loeken, M.R. 2002. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: Implications for Pax-3-dependent development and tumorigenesis. Genes & Dev. 16: 676-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway A.G. and Skerjanc, I.A. 2001. Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J. Biol. Chem. 276: 19033-19039. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron, U., and Rajewsky, K. 1995. A Cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23: 5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R., Recio, J.A., Jhappan, C., Otsuka, T., Liu, S., Yu, Y., Liu, W., Anver, M., Navid, F., Helman, L.J., et al. 2002. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat. Med. 8: 1276-1280. [DOI] [PubMed] [Google Scholar]
- Sorensen P.H., Lynch, J.C., Qualman, S.J., Tirabosco, R., Lim, J.F., Maurer, H.M., Bridge, J.A., Crist, W.M., Triche, T.J., and Barr, F.G. 2002. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the children's oncology group. J. Clin. Oncol. 20: 2672-2679. [DOI] [PubMed] [Google Scholar]
- Sorriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21: 70-71. [DOI] [PubMed] [Google Scholar]
- Tomescu O., Xia, S.J., Strezlecki, D., Bennicelli, J.L., Ginsberg, J., Pawel, B., and Barr, F.G. 2004. Inducible short-term and stable long-term cell culture systems reveal that the PAX3-FKHR fusion oncoprotein regulates CXCR4, PAX3, and PAX7 expression. Lab. Invest. 84: 1060-1070. [DOI] [PubMed] [Google Scholar]
- Tremblay P., Dietrich, S., Mericskay, M., Schubert, F.R., Li, Z., and Paulin, D. 1998. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. (Orlando) 203: 49-61. [DOI] [PubMed] [Google Scholar]
- Xia S.J. and Barr, F.G. 2004. Analysis of the transforming and growth suppressive activities of the PAX3-FKHR oncoprotein. Oncogene 2004 Aug 2 [Epub ahead of print]. [DOI] [PubMed]