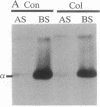
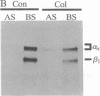
Abstract
Polarized epithelial cells target distinct sets of membrane and secretory proteins to their apical and basolateral domains. Here we examine whether constitutively secreted and membrane proteins that are bound for the same domain share the same carrier vesicles. To address the issue, differential effects of microtubule depolymerization on basolateral protein targeting in the polarized Madin-Darby canine kidney II cell line were studied. We find that the basolateral insertion of the active, ouabain-binding Na+,K(+)-ATPase and of a set of very late antigen integrins is little affected by microtubule disruption. Under equivalent conditions, the basolateral secretion of the basement membrane protein laminin is strongly suppressed. More specifically, it is demonstrated that microtubules are involved in targeting laminin, but not integrins, from the compartment related to the accumulation of newly synthesized proteins at 20 degrees C (trans-Golgi network) to the basolateral domain. Our study also reveals that laminin associated with basolateral binding sites interacts with those sites only secondarily to secretion. The data provide evidence for a branch in the basolateral targeting pathway, with secreted and membrane proteins loaded into distinct carrier vesicles.
Full text
PDF

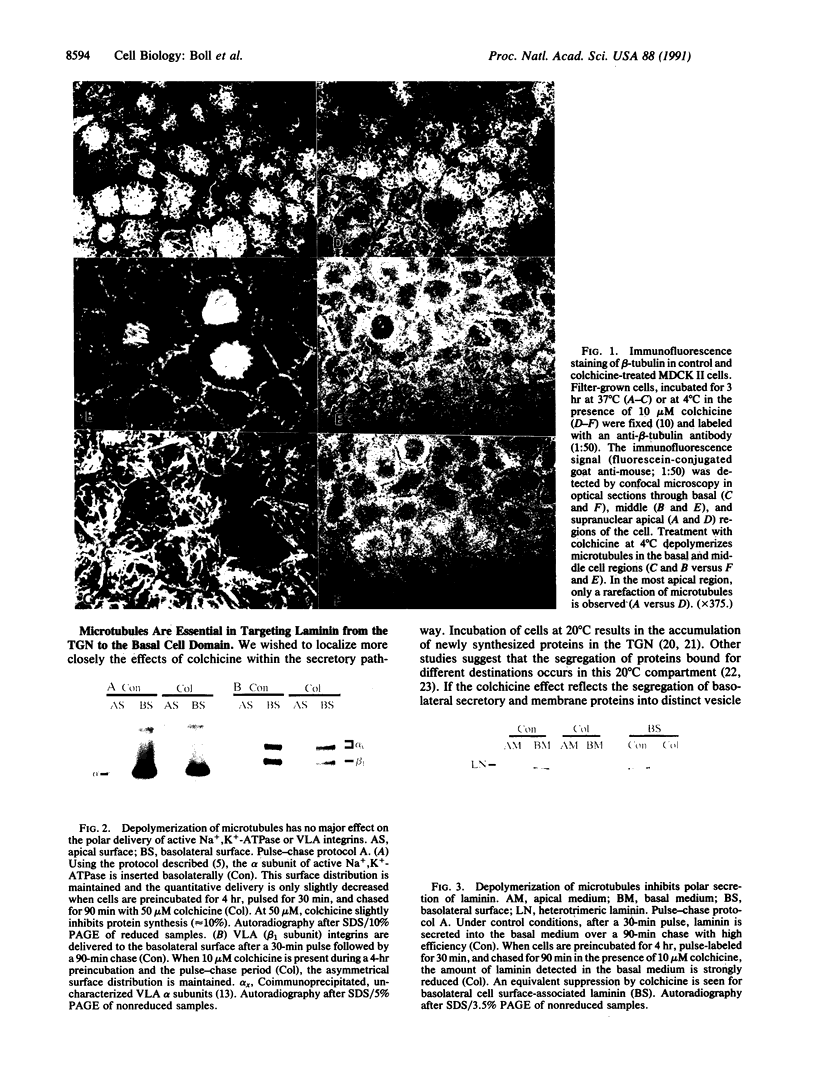

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achler C., Filmer D., Merte C., Drenckhahn D. Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J Cell Biol. 1989 Jul;109(1):179–189. doi: 10.1083/jcb.109.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Parton R., Kuznetsov S. A., Schroer T. A., Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990 Aug 24;62(4):719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., McKinnon W. C., Mostov K. E. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 1):2365–2373. doi: 10.1083/jcb.111.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- De Almeida J. B., Stow J. L. Disruption of microtubules alters polarity of basement membrane proteoglycan secretion in epithelial cells. Am J Physiol. 1991 Jul;261(1 Pt 1):C691–C700. doi: 10.1152/ajpcell.1991.261.1.C691. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Eilers U., Klumperman J., Hauri H. P. Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2). J Cell Biol. 1989 Jan;108(1):13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hubbard A. L., Stieger B., Bartles J. R. Biogenesis of endogenous plasma membrane proteins in epithelial cells. Annu Rev Physiol. 1989;51:755–770. doi: 10.1146/annurev.ph.51.030189.003543. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Mâle P., Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990 Nov;9(11):3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Microtubules, membrane traffic, and cell organization. Cell. 1990 Apr 6;61(1):5–7. doi: 10.1016/0092-8674(90)90206-t. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Parczyk K., Haase W., Kondor-Koch C. Microtubules are involved in the secretion of proteins at the apical cell surface of the polarized epithelial cell, Madin-Darby canine kidney. J Biol Chem. 1989 Oct 5;264(28):16837–16846. [PubMed] [Google Scholar]
- Pfeffer S. R. Mannose 6-phosphate receptors and their role in targeting proteins to lysosomes. J Membr Biol. 1988 Jul;103(1):7–16. doi: 10.1007/BF01871928. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Sabatini D. D. Microtubule-acting drugs lead to the nonpolarized delivery of the influenza hemagglutinin to the cell surface of polarized Madin-Darby canine kidney cells. J Cell Biol. 1987 Feb;104(2):231–241. doi: 10.1083/jcb.104.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas P. J., Misek D. E., Vega-Salas D. E., Gundersen D., Cereijido M., Rodriguez-Boulan E. Microtubules and actin filaments are not critically involved in the biogenesis of epithelial cell surface polarity. J Cell Biol. 1986 May;102(5):1853–1867. doi: 10.1083/jcb.102.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Willemsen R., van Kerkhof P., Slot J. W., Geuze H. J., Lodish H. F. Vesicular stomatitis virus glycoprotein, albumin, and transferrin are transported to the cell surface via the same Golgi vesicles. J Cell Biol. 1983 Dec;97(6):1815–1822. doi: 10.1083/jcb.97.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A., Bennett M. K., Antony C., Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990 Sep;111(3):987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M. J., Matlin K. S. Microtubule perturbation inhibits intracellular transport of an apical membrane glycoprotein in a substrate-dependent manner in polarized Madin-Darby canine kidney epithelial cells. Cell Regul. 1990 Nov;1(12):921–936. doi: 10.1091/mbc.1.12.921. [DOI] [PMC free article] [PubMed] [Google Scholar]