Abstract
A 35-year-old man was admitted to an intensive care unit with unilateral facial swelling and septic shock after multiple presentations to the emergency department with non-specific unilateral pain over the parotid area. A CT scan of his neck showed diffuse right-sided facial soft tissue infection, mastoid effusion and temporal lobe cerebritis. The upper lobes of his lungs had cannonball lesions that were suggestive of septic lung metastases. Blood cultures and ear canal swabs were positive for Burkholderia pseudomallei. The temporal lobe cerebritis eventually developed into an abscess, necessitating a cortical mastoidectomy, craniectomy and temporal lobectomy. After the surgical interventions, antibiotic therapy was continued for a further 6 months. The patient remained well and had no signs of recurrence up to 7 months after the initial presentation.
Background
Melioidosis is an infectious disease of humans and animals that results from the Gram-negative bacterium Burkholderia pseudomallei. The disease occurs endemically in Southeast Asia, Northern Australia, South Asia, China and Taiwan, with the highest incidence in Thailand (2000–3000 cases a year). Likewise, the tropical Northern Territory of Australia has an incidence of 50.2 cases per 100 000 in 2009–2010.1 2 More recently, sporadic cases have been reported in Africa, the Pacific islands and even parts of the Americas.3
Melioidosis has been dubbed ‘the Great Mimicker’, because of its frequent misdiagnosis. There is tremendous variability in the primary mode of presentation, with the severity ranging from chronic localised cutaneous infections to acute overwhelming sepsis and death. The presenting feature is usually pneumonia (>50% patients), but it can also manifest in the genitourinary, skin, bone and neurologic systems.4–6 In a previous study involving 540 cases in tropical Australia over a 20-year period, over half of the patients had bacteraemia and one in five developed septic shock.4 B. pseudomallei is characteristically resistant to most empirical antibiotic regimens used for suspected bacterial sepsis, which means a delay in diagnosis can be fatal. Hence, there is a need for a high index of suspicion in endemic areas.
This case highlights the non-specific manner in which melioidosis presents, where the diagnosis is missed on the initial consultations. It also highlights the diagnostic and management difficulties in managing disseminated abscesses in patients with severe septic shock secondary to melioidosis.
Case presentation
A 35-year-old man presented to the emergency department of the Royal Darwin Hospital, Australia, with a 3-day history of right temporal facial pain that was radiating to the temporomandibular joint, which was exacerbated by chewing. This was associated with night sweats and chills. The patient did not have any significant medical history. On examination, he was afebrile and had tenderness over the right temporal area. The orthopantomogram showed decreased anterior translation of the right mandibular condyle, suggestive of temporomandibular joint dysfunction. The patient was discharged home on non-steroidal anti-inflammatory drugs. He presented again 3 days later with worsening preauricular swelling and temporal pain. This was again diagnosed as a temporomandibular joint issue due to a lack of other significant clinical findings. He was booked for an outpatient Maxillofacial review. No further investigations were ordered at this stage.
Six days after the initial presentation, the patient was brought in by his father to the emergency department with trismus, right parotid and temporal swelling, fevers and acute confusion. It was also noted that the patient had been pig-hunting barefooted and lying on the bare ground for prolonged periods (to fix a car) in the 2 weeks prior to presentation. Examination findings were unremarkable apart from a significant right parotid and right external auditory canal swelling. The right tympanic membrane did not appear inflamed or bulging. A CT scan of the head and neck was ordered for a presumed right parotiditis with abscess formation. The patient developed type 1 respiratory failure and vasodilatory shock soon after admission. He was promptly intubated and admitted to the intensive care unit for supportive care.
Investigations
Blood tests performed on the third presentation showed a raised white cell count (11.8×109) and C reactive protein (283.9 mg/L), and acute kidney injury (creatinine 241 µmol/L). There were also signs of early liver failure on the liver function tests, consistent with the clinical picture of septic shock. His admission HbA1c was 15.8%, which was suggestive of undiagnosed type 2 diabetes.
The CT scan of the head and neck showed inflammation of the right parotid and the soft tissue around it, involving the masseter and pterygoid muscles, external auditory canal and the temporalis muscle (figure 1). There was also complete opacification of the right middle ear and mastoid cavity, without overt bony destruction (figure 2). In the anteroinferior aspect of the right temporal lobe, there was a 2 cm low-density focus with no contrast enhancement that was suggestive of cerebritis. This lesion was not directly anterior to the petrous part of the temporal bone (figure 3). Interestingly, the upper lobes of both lungs had multiple nodular mass lesions suggestive of septic lung metastases (figure 4).
Figure 1.
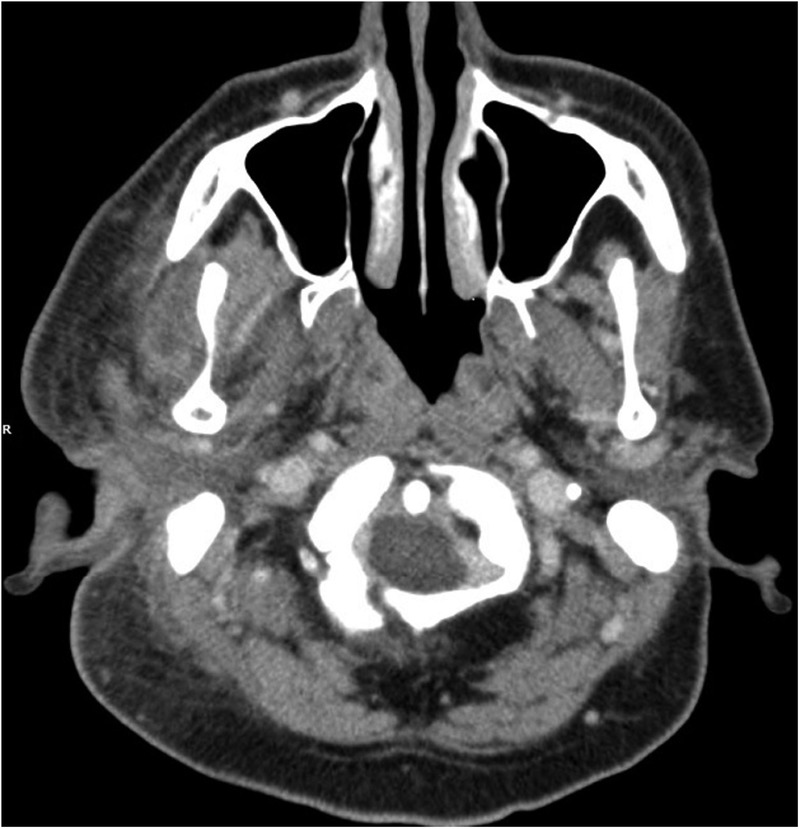
CT scan on admission showing soft tissue inflammation over the right periparotid/periauricular region.
Figure 2.
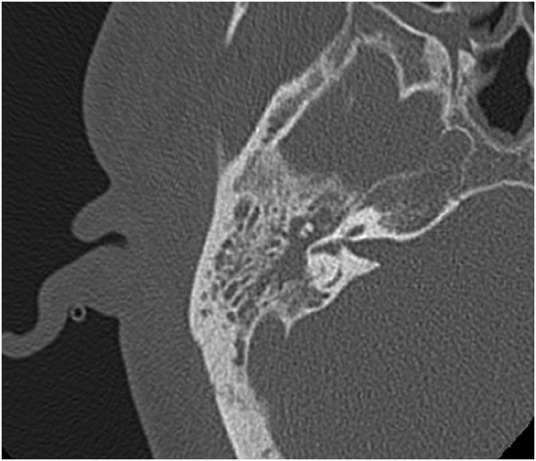
Bony window of admission CT scan demonstrating complete opacification of the right mastoid cavity without any signs of bony erosion.
Figure 3.
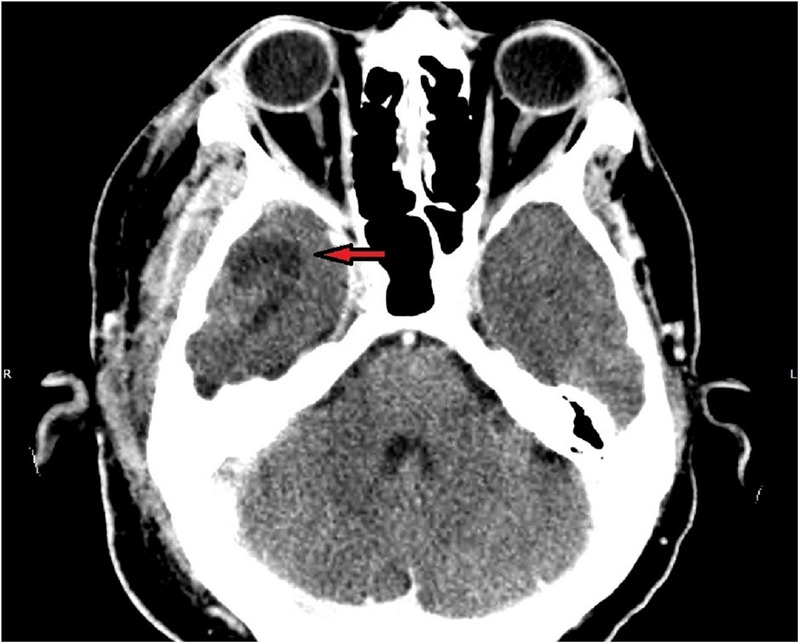
Sigmoid shaped hypodensity in the right temporal lobe (red arrow) with no ring enhancement, suggestive of cerebritis. Note the position of this lesion—it is not directly related to the right temporal bone.
Figure 4.
CT scan of the chest demonstrating multiple cannonball lesions (red arrows).
After the first 3 days of admission, blood cultures that were sent off on an Ashdown broth grew Gram-negative bacilli that were identified as B. pseudomallei. The diagnosis of melioidosis was also confirmed by growth of the bacteria in sputum samples and a right ear canal swab.
Differential diagnosis
The microbiological confirmation of melioidosis was unavailable in the first few days of admission, although there was a high index of suspicion among the treating clinicians due to the behaviour of the disease and the local endemicity of B. pseudomallei. Provisional treatment with broad-spectrum antibiotics was used, given the overall clinical presentation of septic shock. The nature of the right mastoid effusion on CT was uncertain.
This could have been the primary source of infection (mastoiditis or mastoid abscess) or a sympathetic effusion secondary to the adjacent soft tissue inflammation. The other matter for consideration was whether the cerebritis was secondary to the mastoid effusion or due to a haematogenous spread of the infection. The temporal lobe lesion was not directly adjacent to the mastoid/middle ear cavity, making the likelihood of direct spread less likely. Hence, it was unclear whether a cortical mastoidectomy would be beneficial, especially in the context of a critically ill patient with an infection of the soft tissue overlying the postauricular surgical site.
Owing to the severe septic shock and high risk for surgery, a right myringocentesis/tympanostomy was performed by the bedside instead of a cortical mastoidectomy to (1) drain any potential middle ear collection and (2) obtain microbiological specimens. Surprisingly, no middle ear fluid/pus was drained. However, the external auditory canal was sufficiently oedematous that a transudate/exudate sample from the canal was retrieved and sent for culture, which grew B. pseudomallei.
Treatment
The patient was treated with intravenous meropenem and Bactrim. He had profound vasodilatory shock, which required high dose norepinephrine, vasopressin and epinephrine. He was also treated with granulocyte colony-stimulating factor, based on previous evidence of its efficacy in severe melioidosis. His acute kidney injury required continuous veno-venous haemofiltration. He also developed hyperglycaemia while critically ill, requiring an insulin infusion to main euglycemia.
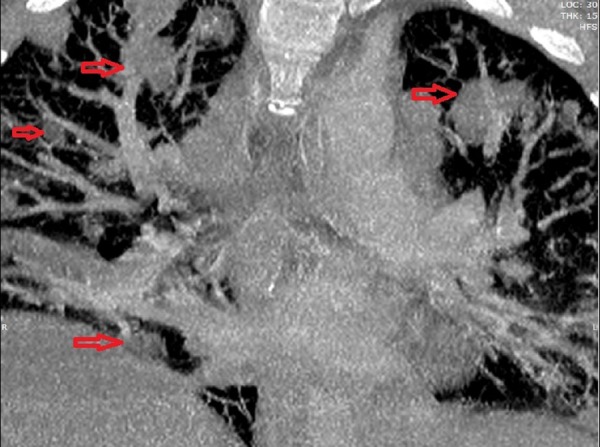
An MRI was performed on day 7 after admission, when the patient stabilised on the initial antibiotic and supportive management. This showed ongoing diffuse myositis and oedema in the soft tissues around the parotid gland (figure 5). Intracranially, the meninges along the floor and lateral right middle cranial fossa showed signal enhancement, with a 4×2.5×0.6 mm epidural collection overlying the right tentorium. The temporal lobe lesion had begun to show early signs of abscess formation with peripheral enhancement (figure 6). Also, there was an absence of flow void in the right transverse and sigmoid sinuses suggestive of thrombosis.
Figure 5.
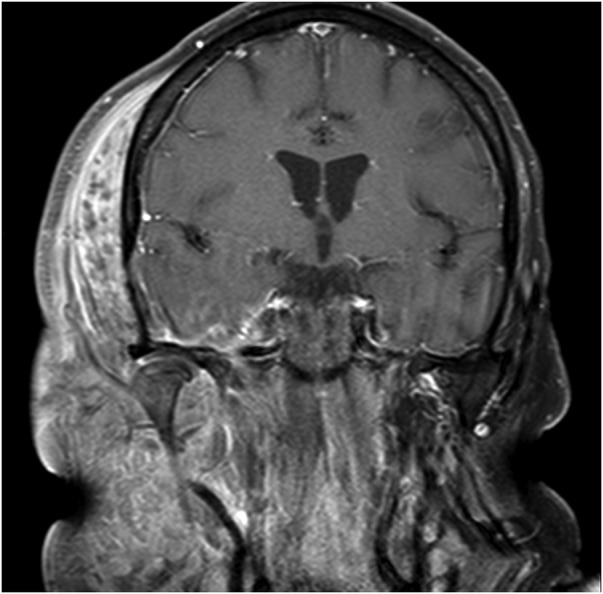
MRI 7 days after admission. The extent of the soft tissue infection and swelling became much more diffuse.
Figure 6.
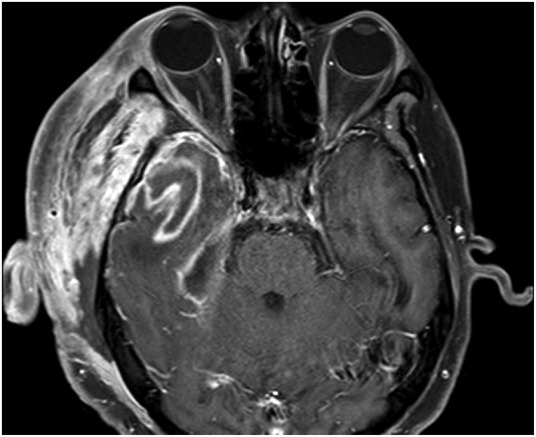
MRI 7 days after admission. The previous temporal lobe lesion now shows ring enhancement, suggestive of abscess formation.
The patient underwent a right cortical mastoidectomy after the MRI findings were noted. Intraoperatively, there was diffuse oedema with large amounts of exudate in the soft tissue overlying the right mastoid process. Apart from granulation tissue in the mastoid cavity, no purulent material was seen in the middle ear. A needle aspiration of the sigmoid sinus did not reveal any clot in it. The patient remained stable postoperatively.
A progress MRI was performed 5 days after the surgery, which showed no signs of sigmoid sinus thrombosis. However, the right temporal lobe lesion had developed into a 25 mm abscess. The patient subsequently underwent a right craniectomy and temporal lobectomy.
Postoperatively, he was further treated with 8 weeks of intravenous antibiotics (meropenem for 4 weeks, then ceftazidime for 4 weeks). This was followed by oral Bactrim therapy for 6 months. He continued to have persistent right ear discharge, which was treated with regular ear toileting.
Outcome and follow-up
The patient was discharged home 2 months after his initial presentation. He remains under close surveillance by the infectious diseases physicians, neurosurgeons and otolaryngologists. The patient has been well with no signs of deterioration or recurrence 7 months after the initial presentation. He was diagnosed and treated for type 2 diabetes due to the persisting hyperglycaemia—this may have been his risk factor for developing melioidosis.
Discussion
The clinical presentation, severity of disease and outcomes in melioidosis are influenced by the inoculating dose, route of infection and, most importantly, the human host risk factors. The key risk factors include diabetes, hazardous alcohol use, chronic lung disease, chronic renal disease, thalassaemia, immunosuppressive therapy and cancer.7 In a previous study, it was shown that the vast majority of fatalities occurred in patients with one or more of the above risk factors.2 4 7 A history of either percutaneous inoculation or inhalation/ingestion of contaminated soil and/or water is important to elicit. It has been noted that inhalation of the bacteria becomes the predominant route of transmission in events of tropical monsoonal storms, cyclones, hurricanes and typhoons, most likely secondary to aerosolisation of the bacteria from the soil.8–11 In retrospect, the barefooted pig-hunting and close proximity to soil in the monsoon season in Northern Australia probably caused the inoculation in our patient, who had undiagnosed diabetes.
In Thailand and Cambodia, the most common presentation for children with melioidosis is suppurative parotitis, which is rare in Australia.12 This is most likely thought to reflect ingestion of contaminated water, as described above. This case also demonstrates the need to be circumspect when considering surgical intervention in patients with melioidosis that can have multifocal abscess that with direct or haematogenous spread. It is difficult to pinpoint whether the right facial inflammation seen in our patient was primarily due to parotiditis or a generalised myositis/fasciitis of the temporal region. It is difficult to comment, even in retrospect, whether the cortical mastoidectomy was therapeutically useful in this case, given the atypical location of the temporal lobe abscess.
Isolation of B. pseudomallei by culture, preferably using Ashdown broth, remains the diagnostic gold standard but can take many days. Rapid diagnostic systems, such as direct real-time PCR assays, are in use but are less sensitive.13 14 In this case, the diagnosis was delayed partly because melioidosis was not initially considered to be a differential diagnosis in a young man who was thought to be ‘healthy’ and ‘not at risk’. This demonstrates the need for a high index of suspicion, so that diagnostic investigations and empirical treatment can be initiated promptly. This is especially so in susceptible patients who have travelled to or live in endemic areas.
Current guidelines for the treatment of melioidosis recommend a 2-week intensive therapy with either ceftazidime or meropenem, followed by oral eradication therapy using trimethoprim and sulfamethoxazole for 3–6 months.2 There is some evidence that suggests better outcomes with meropenem in severe melioidosis with septic shock.15 Hence, our patient was treated with meropenem initially while he was critically ill, before stepping down to ceftazidime. Recurrence occurs in ∼1 in 16 patients, often within the first year. Hence, these patients will need monitoring over the longer term after completion of the eradication therapy.16 17
Learning points.
Burkholderia pseudomallei is a Gram-negative bacterium that can cause severe sepsis, especially in immunocompromised patients.
A high index of suspicion, especially in travellers or residents in endemic regions, is required to recognise and treat this condition early, because the clinical presentation can be very variable.
Prolonged antibiotic therapy with regular surveillance is important for disease eradication and prevention of recurrence.
Footnotes
Contributors: TLL was the primary author and was responsible for drafting and finalising the manuscript. SL, GC and HP contributed to the review and revision of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Currie BJ, Fisher DA, Howard DM et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis 2000;31:981–6. 10.1086/318116 [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 2015;36:111–25. 10.1055/s-0034-1398389 [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Dance DAB, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 2008;102(Suppl 1):S1–4. 10.1016/S0035-9203(08)70002-6 [DOI] [PubMed] [Google Scholar]
- 4.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010;4:e900 10.1371/journal.pntd.0000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev 1991;4:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012;367:1035–44. 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 7.Suputtamongkol Y, Chaowagul W, Chetchotisakd P et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 1999;29:408–13. 10.1086/520223 [DOI] [PubMed] [Google Scholar]
- 8.Chen YL, Yen YC, Yang CY et al. The concentrations of ambient Burkholderia pseudomallei during typhoon season in endemic area of melioidosis in Taiwan. PLoS Negl Trop Dis 2014;8:e2877 10.1371/journal.pntd.0002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng AC, Jacups SP, Gal D et al. Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int J Epidemiol 2006;35:323–9. 10.1093/ije/dyi271 [DOI] [PubMed] [Google Scholar]
- 10.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 2003;9:1538–42. 10.3201/eid0912.020750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo TJ, Ang LW, James L et al. Melioidosis in a tropical city state, Singapore. Emerg Infect Dis 2009;15:1645–7. 10.3201/eid1510.090246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leelarasamee A. Melioidosis in Southeast Asia. Acta Trop 2000;74:129–32. [DOI] [PubMed] [Google Scholar]
- 13.Kaestli M, Richardson LJ, Colman RE et al. Comparison of TaqMan PCR assays for detection of the melioidosis agent Burkholderia pseudomallei in clinical specimens. J Clin Microbiol 2012;50:2059–62. 10.1128/JCM.06737-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peacock SJ, Chieng G, Cheng AC et al. Comparison of Ashdown's medium, Burkholderia cepacia medium, and Burkholderia pseudomallei selective agar for clinical isolation of Burkholderia pseudomallei. J Clin Microbiol 2005;43:5359–61. 10.1128/JCM.43.10.5359-5361.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AC, Fisher DA, Anstey NM et al. Outcomes of patients with melioidosis treated with meropenem. Antimicrob Agents Chemother 2004;48:1763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarovich DS, Ward L, Price EP et al. Recurrent melioidosis in the Darwin Prospective Melioidosis Study: improving therapies mean that relapse cases are now rare. J Clin Microbiol 2014;52:650–3. 10.1128/JCM.02239-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limmathurotsakul D, Chaowagul W, Chierakul W et al. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis 2006;43:979–86. 10.1086/507632 [DOI] [PubMed] [Google Scholar]


