Abstract
Objective
Mutations in the LMNA gene encoding lamins A and C of the nuclear lamina are a frequent cause of cardiomyopathy accounting for 5–8% of familial dilated cardiomyopathy (DCM). Our aim was to study disease onset, presentation and progression among LMNA mutation carriers.
Methods
Clinical follow-up data from 27 LMNA mutation carriers and 78 patients with idiopathic DCM without an LMNA mutation were collected. In addition, ECG data were collected and analysed systematically from 20 healthy controls.
Results
Kaplan-Meier analysis revealed no difference in event-free survival (death, heart transplant, resuscitation and appropriate implantable cardioverter-defibrillator therapy included as events) between LMNA mutation carriers and DCM controls (p=0.5). LMNA mutation carriers presented with atrial fibrillation at a younger age than the DCM controls (47 vs 57 years, p=0.003). Male LMNA mutation carriers presented with clinical manifestations roughly a decade earlier than females. In close follow-up non-sustained ventricular tachycardia was detected in 78% of LMNA mutation carriers. ECG signs of septal remodelling were present in 81% of the LMNA mutation carriers, 21% of the DCM controls and none of the healthy controls giving a high sensitivity and specificity for the standard ECG in distinguishing LMNA mutation carriers from patients with DCM and healthy controls.
Conclusions
Male LMNA mutation carriers present clinical manifestations at a younger age than females. ECG septal remodelling appears to distinguish LMNA mutation carriers from healthy controls and patients with DCM without LMNA mutations.
Keywords: LMNA mutations, Lamin A/C, septal remodelling
Key questions.
What is already known about this subject?
Although ventricular dilation and dysfunction may remain less severe than in other forms of dilated cardiomyopathy, the penetrance of cardiolaminopathy mutations is almost complete often resulting in serious arrhythmias or heart failure. Cardiomyopathy-causing LMNA mutations often present with typical ECG abnormalities, such as atrioventricular block and atrial or ventricular arrhythmias.
What does this study add?
We report that most LMNA mutation carriers present with non-sustained ventricular tachycardia (NSVT) in close follow-up. In addition, we reaffirm that male LMNA mutation carriers have an earlier disease onset than females. We suggest that LMNA mutation carriers likely benefit from close follow-up. We also present a new ECG entity, septal remodelling, present in most LMNA mutation carriers and suggesting that the pathological process leading to cardiolaminopathy typically affects the myocardial septum.
How might this impact on clinical practice?
The detection of septal remodelling in standard ECG should lead to an echocardiogram and thorough enquiry of cardiac family history.
Even asymptomatic LMNA mutation carriers need follow-up to detect atrial fibrillation and NSVTs.
Introduction
LMNA mutations are prevalent in familial dilated cardiomyopathy (DCM), accounting for about 5–8% of the cases.1 The typical early manifestations of LMNA mutations are ECG abnormalities including flat P wave, atrioventricular block, supraventricular and ventricular arrhythmias.2 3 LMNA mutations pose a risk for sudden cardiac death, and consequently implantable cardioverter-defibrillator (ICD) implantation has been suggested as primary prophylaxis for all LMNA mutation carriers, or after further risk assessment.4 5 Cardiolaminopathy often follows an age-dependent disease progression in which the ECG and rhythm abnormalities tend to precede structural heart disease and systolic impairment, which in turn often does not fulfil the echocardiography criteria for DCM due to milder dilation of the left ventricle or dilation with preserved ejection fraction.3 6 Lately LMNA mutations have also been linked to familial forms of mainly right ventricular disease manifestations resembling arrhythmogenic right ventricular cardiomyopathy.7 8
Structural heart disease can result in general, non-localising ECG changes, such as left ventricular hypertrophy (LVH), ST depression, widening of the QRS complex, and P terminal force.9 10 On the other hand, ECG changes in leads overlying affected regions can reflect regional disease processes. For instance, narrow and deep q waves are typical for localised wall thickening in hypertrophic cardiomyopathy.11
Late gadolinium enhancement (LGE) in cardiac MRI (CMR) is considered an effective tool in showing myocardial scarring.12 In LMNA mutation carriers LGE has been mainly seen in the basal or mid-ventricular septum suggesting a possible localised disease process.13 14
The aim of this study was to assess disease presentation, progression and clinical outcome in symptomatic and asymptomatic LMNA mutation carriers.
Methods
Patients and controls
This longitudinal retrospective study included all identified adult LMNA mutation carriers from Helsinki and Kuopio University Hospitals willing to participate in a follow-up study. Twenty-seven LMNA mutation carriers were recruited to the study between 1999 and 2010. The mutation carriers, each harbouring one of five LMNA mutations ((NM_170707.3 (LMNA), c427T>C, p.(Ser143Pro) in exon 2; c.394G>C, p.(Ala132Pro) in exon 2; c.568C>T, p.(Arg190Trp) in exon 3; c. 1493delG, p.(Ala499Leufs*49) in exon 9; c. 1085delT, p.(Leu363Trpfs*117) in exon 6) were either probands or their family members from nine families identified in two previous studies.15 16 Clinical follow-up data from the LMNA mutation carriers and DCM controls were collected up to 31 December 2014. Control patients (n=78) with idiopathic DCM were collected from a patient database retrospectively. Probands with DCM, diagnosed and recruited before 2010 were included as DCM controls; patients recruited after possible heart transplantation, were excluded to minimise possible collection bias. Only probands who have been tested for cardiomyopathy-causing mutations using OsSeq, a next-generation-sequencing method, as described before were included to ensure that there were no LMNA mutation carriers among the DCM controls.17
Concerning the ECG abnormalities, the study patients were also compared with an available cohort of 20 (7 men, 13 women) healthy controls.
General
The criteria used for the diagnosis of DCM were Left ventricular end-diastolic diameter (LVEDD)>27 mm/m2 and Left ventricular ejection fraction (LVEF)<45%.15 Clinical end point data were collected from all available hospital records. First incidences of atrial fibrillation, non-sustained ventricular tachycardia (NSVT), resuscitation or appropriate ICD therapy, cardiogenic embolism and pacemaker/ICD implantations were recorded. NSVT was defined as more than three consecutive ventricular beats in 24-hour Holter or clinical exercise test. To avoid bias, NSVTs were not recorded in the DCM controls due to the less vigorous follow-up of the DCM control group compared with the LMNA mutation carrier group. Owing to the small number of major clinical events in the LMNA mutation carrier group, a composite end point of resuscitation, appropriate ICD therapy, death and heart transplant was used. In LMNA mutation carriers with available CMR data, the presence of the ECG parameter septal remodelling was compared with CMR findings from a previous article;13 the CMR methods were presented earlier.
The study patients gave written informed consent. The study was approved by the Ethics Committee of the Helsinki University Central Hospital (Decision number: Dnro 322/E5/03, Research permit number: T1010K0019).
ECG analyses
One standard 12-lead ECG recorded by 50 mm/s speed of each LMNA mutation carrier, DCM control and healthy control was analysed in a systematic manner manually by one investigator (KN) blinded to the clinical data. The ECG recording was from the time of study recruitment. The age at the time of ECG recording was 42 years for all LMNA mutation carriers; 49 years for those with DCM (LMNA-DCM subgroup), and 37 years for those without. The following definitions were used in the ECG analyses: first-degree atrioventricular block was defined as PR interval >200 ms,18 P terminal force as negative portion of the P wave in lead V1≥0.4 mm/s,19 flat P wave as P-wave amplitude <1 mm in lead II, and broad P wave as ≥120 ms in lead II20 LVH was defined according to the Sokolow-Lyon criteria,21 or Cornell voltage duration product (QRS-duration (ms)×(RaVL in mm+SV3 in mm with 6 mm added for women) ≥2440),22 23 for ST segment depression we used ≥0.5 mm if the pattern was horizontal or descending, and ≥1 mm if ascending in ≥2 adjacent leads measured at the J point+60 ms,24 for T-wave inversion ≥1 mm in ≥2 adjacent leads, except for leads aVR and V1 was used.25 For fragmented QRS in ≥2 adjacent leads we used the definitions by Das et al.26 Septal fragmentation was considered present if there was QRS fragmentation in ≥2 septal leads (V1–V3). Non-specific intraventricular conduction defect was defined as QRS duration ≥120 ms not fulfilling criteria for right or left bundle branch block. Owing to the high proportion of implanted pacemakers, we used anamnestic data of AV block in addition to the findings in the ECG used for analysis.
In addition to the established ECG changes, we introduced a novel ECG parameter, ‘septal remodelling’, which we define as one of the following present in V1–V3: (1) pathological Q waves in ≥2 parallel leads, or (2) septal fragmentation as defined above, (3) poor R-wave progression (R wave <3 mm) in leads V1–V3 accompanied by QRS fragmentation, or disorderly distributed R-wave amplitudes, either RV2>RV3 or RV1>RV2. The possibility of lead switch was considered by assessing the morphology of the P and S waves in the precordial leads, and no suspicious cases were observed.27 Any Q wave ≥40 ms in duration, or ≥3 mm deep, or qR-ratio ≥0.25, in ≥2 parallel leads except lead aVR was considered pathological.28
Statistical methods
Continuous variables were analysed using Student's t-test. The normality of continuous variables was assessed using the Shapiro-Wilk test for normality. In the few instances, where the variables were not normally distributed Mann-Whitney U test was used. For categorical variables, the χ2 test was used when the expected count of 80% or more of the cells was ≥5. Otherwise the Fisher's exact test was used. All statistical tests were two-sided with a 5% level of significance, and no adjustments were made for multiplicity. However, concerning the ECG analyses the number of paired-wise comparisons (LMNA vs DCM and LMNA vs healthy control) was accounted for by multiplying the p values obtained by 2. The Kaplan-Meier analysis was used for survival analysis. SPSS V.22 was used for statistical analyses.
Results
General
The frequencies and incidence ages of clinical manifestations are reported in table 1. The mean age at previous follow-up or major end point was 48 years for LMNA mutation carriers and 59 years for the DCM controls (p<0.001). Twelve LMNA mutation carriers fulfilled the criteria for DCM. Pacemakers were more common in the LMNA-DCM subgroup than in the DCM control group (83% vs 47%, p=0.047). NSVT was very common among the LMNA mutation carriers (78%). Heart transplants were more frequent in the LMNA-DCM subgroup than in the DCM control group (25% vs 11%), but the difference was not statistically significant. Atrial fibrillation was also more common among LMNA mutation carriers than in the DCM controls; p=0.007 when comparing the LMNA-DCM subgroup to the DCM control group. LMNA mutation carriers had their first episode of atrial fibrillation at a younger age than the DCM controls (47 vs 57 years, p=0.003). All the cases of likely cardiogenic thromboembolic events among the LMNA mutation carriers occurred among the LMNA-DCM subgroup. The frequency of thromboembolic events in the LMNA-DCM subgroup was roughly twice as high as in the DCM control group, but the difference was not statistically significant.
Table 1.
The frequencies and incidence ages of clinical LMNA mutation or cardiomyopathy manifestations
| All LMNA mutation carriers, n=27 |
LMNA mutation carriers with DCM, that is, LMNA-DCM subgroup, n=12 |
DCM controls, n=78 | |||
|---|---|---|---|---|---|
| N (%) | p Value* | N (%) | p Value* | N (%) | |
| AF | 15 (55.6) | NS | 11 (91.7) | 0.007 | 39 (50.0) |
| NSVT | 21 (77.8) | 11 (91.7) | |||
| Pacemaker (any, including ICDs) | 16 (59.3) | NS | 10 (83.3) | 0.020 | 37 (47.4) |
| ICD | 9 (33.3) | NS | 6 (50.0) | NS | 26 (33.3) |
| Thrombosis | 4 (14.8) | NS | 4 (33.3) | NS | 12 (15.4) |
| Major end point | 7 (25.9) | 0.039 | 7 (58.3) | NS | 38 (48.7) |
| Males | 13 (48.1) | 0.026 | 6 (50.0) | NS | 56 (71.8) |
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age at DCM diagnosis | 48.7 (10.7) | NS | 48.2 (10.4) | NS | 47.4 (12.2) |
| Age at AF | 46.9 (10.9) | 0.003 | 47.9 (10.7) | 0.014 | 56.9 (10.1) |
| Age at NSVT | 45.6 (11.8) | 49.1 (10.1) | |||
| Age at PM | 49.1 (10.6) | NS | 49.6 (11.1) | NS | 55.4 (13.0) |
| Age at ICD | 48.5 (11.4) | NS | 52.0 (12.7) | NS | 53.0 (12.9) |
| Age at thrombosis | 52.6 (10.0) | NS | 52.6 (10.0) | NS | 54.8 (12.5) |
| Age at major end point | 51.0 (8.7) | NS | 51.0 (8.7) | NS | 59.0 (14.2) |
Comparisons of all the LMNA mutation carriers and those LMNA mutation carriers fulfilling the criteria for DCM to the DCM controls.
*Comparisons to all DCM controls.
AF, atrial fibrillation; DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator; NS, not significant; NSVT, non-sustained ventricular tachycardia; PM, pacemaker.
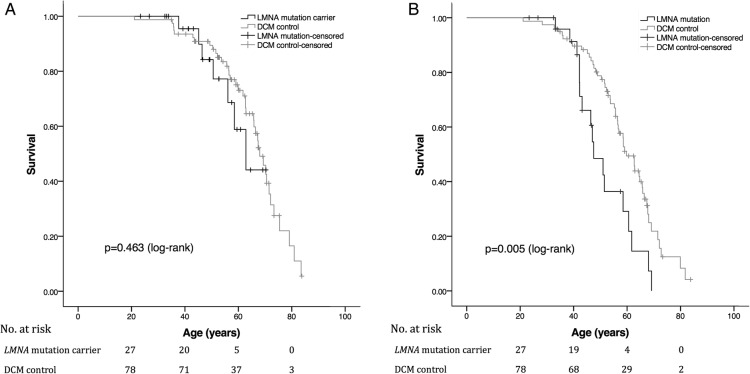
There were less major end points in the entire LMNA carrier group, and more in the LMNA-DCM subgroup than in the DCM control group. However, looking at major end points including deaths, heart transplantations and resuscitations, Kaplan-Meier analysis (figure 1A) revealed no difference in event-free survival between LMNA mutation carriers and DCM controls. When pacemakers were included in survival analysis, a statistically significant difference was seen; median age estimate for LMNA mutation carriers to first event was 48 years (CI 42 to 53) compared with 60 years (CI 55 to 64, p=0.005) for DCM controls (figure 1B).
Figure 1.
(A). Kaplan-Meier plot of event-free survival in 27 LMNA mutation carriers and 78 DCM control patients. Death, heart transplantation, resuscitation or appropriate ICD therapy included as events. Median age estimate for LMNA mutation carriers to first event was 63 years (CI 53 to 72) compared with 68 years (CI 64 to 72) for DCM controls. No statistically significant difference in event-free survival between the two groups was observed (p=0.463, log-rank test). (B). Kaplan-Meier plot of event-free survival in 27 LMNA mutation carriers and 78 DCM control patients. Deaths, heart transplants, resuscitations, appropriate ICD therapy or pacemaker implantations included as events. Median age estimate for LMNA mutation carriers was 48 years (CI 42 to 53) compared with 60 years (CI 55 to 64) for DCM controls (p=0.005, log-rank test). DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator.
The Finnish founder mutation Ser143Pro was the most common mutation among the LMNA mutation carriers, with 15 of the 27 mutation carriers harbouring it.15 There were no statistically significant differences between the Ser143Pro mutation carriers compared with the other LMNA mutation carriers (Ala132Pro, Arg190Trp, c. 1493delG, c. 1085delT) concerning the frequencies or incidence ages of the clinical manifestations. Of the DCM controls 28% had a genetic diagnosis. The most common cardiomyopathy-causing mutations among the DCM controls were truncating titin gene mutations, which explained 18% of cases.
Differences between men and women
The frequencies and incidence ages of clinical manifestations in male and female LMNA mutation carrier and DCM control subgroups are presented in table 2. Male LMNA mutation carriers presented with clinical manifestations at an earlier age than female LMNA mutation carriers. This difference is illustrated in figure 2. There was a trend for a higher incidence of atrial fibrillation among the male LMNA mutation carriers compared with the women. Men presented with atrial fibrillation at an earlier age than female LMNA mutation carriers (41 vs 54 years, p=0.016). They also received pacemakers and ICDs at a younger age than females (42 vs 59 years for any pacemakers, p=0.001, 42 vs 63 for ICDs, p<0.001). In addition, they developed DCM at a younger age than women (42 vs 54 years, p=0.042). The vast majority of male and female LMNA mutation carriers had recorded NSVT episodes. Although there was no gender difference in the frequencies of NSVTs, men appeared to have the first recorded episode roughly 10 years earlier than women (41 vs 50 years, NS).
Table 2.
The frequencies and incidence ages of clinical LMNA mutation or cardiomyopathy manifestations
|
LMNA-males, N=13 N (%) |
p Value LMNA vs DCM | Male DCM controls, N=56 N (%) |
LMNA-females, N=14 N (%) |
p Value LMNA vs DCM | Female DCM controls,N=22 N (%) |
p Value LMNA-males vs LMNA-females |
|
|---|---|---|---|---|---|---|---|
| AF | 8 (61.5) | NS | 29 (51.8) | 7 (50.0) | NS | 10 (45.5) | NS |
| NSVT | 10 (76.9) | 11 (78.6) | NS | ||||
| Pacemaker (any, including ICDs) | 9 (69.2) | NS | 26 (46.4) | 7 (50.0) | NS | 11 (50.0) | NS |
| ICD | 6 (46.2) | NS | 16 (28.6) | 3 (21.4) | NS | 10 (45.5) | NS |
| Thrombosis | 2 (15.4) | NS | 8 (14.3) | 2 (14.3) | NS | 4 (18.2) | NS |
| Major end point | 5 (38.5) | NS | 27 (48.2) | 2 (14.3) | 0.03 | 11 (50.0) | NS |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age at DCM diagnosis | 42.2 (7.0) | NS | 47.8 (11.4) | 54.1 (10.3) | NS | 46.3 (14.2) | 0.042 |
| Age at AF | 40.9 (9.3) | <0.001 | 55.4 (8.7) | 53.7 (8.6) | NS | 61.0 (13.1) | 0.016 |
| Age at NSVT | 40.5 (9.7) | 50.3 (11.9) | NS | ||||
| Age at PM | 41.8 (4.1) | <0.001 | 55.8 (12.7) | 58.5 (8.6) | NS | 54.6 (14.5) | 0.001 |
| Age at ICD | 41.5 (3.6) | 0.007 | 51.2 (11.6) | 62.7 (6.1) | NS | 55.8 (14.8) | <0.001 |
| Age at thrombosis | 48.5 (12.1) | NS | 56.7 (10.2) | 56.8 (9.3) | NS | 51.1 (17.4) | NS |
| Age at major end point | 47.7 (7.6) | NS | 60.1 (13.9) | 59.5 (4.8) | NS | 56.3 (15.1) | NS |
Comparisons of male LMNA mutation carriers to male DCM controls, female LMNA mutation carriers to female DCM controls and male LMNA mutation carriers to female LMNA mutation carriers.
AF, atrial fibrillation; DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator; NS, not significant; NSVT, non-sustained ventricular tachycardia; PM, pacemaker.
Figure 2.
The incidence ages of clinical LMNA mutation manifestations. Squares represent males, circles females. DCM, dilated cardiomyopathy; ICD, implantable cardioverter-defibrillator.
ECG
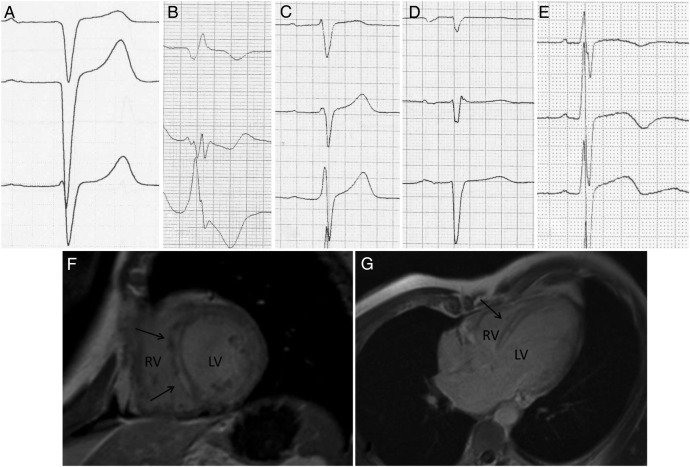
The ECG characteristics of the LMNA mutation carriers, DCM controls and healthy controls are presented in table 3. The novel ECG pattern, septal remodelling proved to be very frequent (81%) in LMNA mutation carriers, while this ECG pattern was present in only 21% of DCM controls and in none of the healthy controls. The differences were even more striking when combining the ECG parameters flat P wave and AV block to septal remodelling in the analyses. The sensitivities, specificities, and positive and negative predictive values for septal remodelling in classifying LMNA mutation carriers from the two control groups are given in table 4. Figure 3 shows ECGs with varying forms of septal remodelling together with CMR images of an LMNA mutation carrier presenting LGE in the septum. We had available CMR data from 17 of the 27 LMNA mutation carriers. In 76% of the cases (n=13), septal remodelling in the ECG and septal LGE in CMR were concordantly present. In the remaining four cases, either septal remodelling in ECG (n=2) or LGE in CMR (n=2) was present.
Table 3.
The ECG characteristics of the LMNA mutation carriers, DCM controls and healthy controls
| LMNA mutation carriers, N=27% | DCM controls, N=78% | p Value LMNA vs DCM |
Healthy controls, N=20% | p Value LMNA vs healthy control |
|
|---|---|---|---|---|---|
| Rhythm | |||||
| Sinus rhythm | 70.4 | 75.6 | NS | 100 | NS |
| AF | 11.1 | 16.7 | 0 | ||
| Other* | 18.5 | 7.7 | 0 | ||
| First AV block | 37.0 (55.6)† | 15.4 (20.7) | 0.034 | 0 | 0.006 |
| Current or previous AV block | 59.3 | 24.4 | 0.002 | 0 | <0.001 |
| PTF | 22.2 (30.0) | 30.8 (40.0) | NS | 10.0 | NS |
| Flat P wave | 33.3 (45.0) | 6.4 (8.3) | 0.002 | 0 | 0.012 |
| Broad P wave | 7.4 (10.0) | 5.1 (6.7) | NS | 0 | NS |
| LVH | 7.4 (11.8) | 20.5 (34.0) | NS | 0 | NS |
| ST depression | 18.5 (29.4) | 32.1 (53.2) | NS | 5.0 | NS |
| T inversion | 7.4 (11.8) | 32.1 (53.2) | 0.012 | 5.0 | NS |
| QRS fragmentation | 37.0 | 33.3 | NS | 5.0 | 0.028 |
| Septal fragmentation | 22.2 | 6.4 | NS | 0 | 0.062 |
| Septal remodelling | 81.5 | 20.5 | <0.001 | 0 | <0.001 |
| Septal remodelling, flat P wave, or current or previous AV block | 96.3 | 41.0 | <0.001 | 0 | <0.001 |
| LBBB | 7.4 | 20.5 | NS | 0 | NS |
| RBBB | 0 | 2.6 | NS | 0 | NS |
| NSIVCD | 3.7 | 7.7 | NS | 0 | NS |
*Physiological pacemaker or third AV block.
†The prevalence in brackets of only those applicable.
AF, atrial fibrillation; AV block, atrioventricular block; DCM, dilated cardiomyopathy; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; NS, not significant; NSIVCD, non-specific intraventricular conduction defect; PTF, P terminal force; RBBB, right bundle branch block.
Table 4.
The sensitivities, specificities, and PPV and NPV for (ECG) septal remodelling in classifying LMNA mutation carriers from the DCM controls, healthy controls or the combined control group of DCM controls and healthy controls
| Septal remodelling, N (%) | Current or previous AV block, flat P wave or septal remodelling, N (%) | |
|---|---|---|
| LMNA mutation carriers | 22 (81.5) | 26 (96.3) |
| Healthy controls | 0 | 0 |
| DCM controls | 16 (20.5) | 32 (41.0) |
| LMNA vs DCM controls | Per cent | Per cent |
| Sensitivity | 81.5 | 96.3 |
| Specificity | 79.5 | 59.0 |
| PPV | 57.9 | 44.8 |
| NPV | 92.5 | 97.9 |
| LMNA vs healthy controls | ||
| Sensitivity | 81.5 | 96.3 |
| Specificity | 100 | 100 |
| PPV | 100 | 100 |
| NPV | 80 | 95.2 |
| LMNA vs DCM or healthy controls | ||
| Sensitivity | 81.5 | 96.3 |
| Specificity | 83.7 | 67.3 |
| PPV | 57.9 | 44.8 |
| NPV | 94.3 | 98.5 |
DCM, dilated cardiomyopathy; NPV, negative predictive value; PPV, positive predictive value.
Figure 3.
(A–E) Example ECGs presenting septal remodelling. (A) Q waves in V1–V2; (B) broad QRS with fragmentation in V2–V3, and a Q wave in V1; (C) RV1>RV2 with fragmented QRS in V2; (D) poor R-wave progression with QRS fragmentation in V2; (E) RV2>RV3 with fragmented QRS in V1. 3. (F and G) CMR image of an LMNA mutation carrier. Arrows point to the areas showing late gadolinium enhancement. CMR, cardiac MRI; LV, left ventricle; RV, right ventricle.
There were no mutation-specific ECG findings in this study when comparing all the five mutations, or the Finnish founder mutation Ser143Pro (n=15) to the other four mutations as one group (n=12).
Discussion
Our data suggest a difference in the age of disease onset in cardiolaminopathy between men and women; men presented roughly a decade earlier than women regarding all studied clinical manifestations. Concerning the age of first incidence of atrial fibrillation, age of pacemaker or ICD implantation, and age at DCM diagnosis, the difference was statistically significant. Also in a previous study of LMNA mutation carriers, the frequencies of malignant ventricular arrhythmias, end-stage heart failure and mortality were higher in men than in women, but the age of the first cardiac involvement was similar in both genders.29 We suggest that the disease onset in LMNA mutation carriers in general takes place earlier in men than in women.
The incidence of ventricular arrhythmias is known to be high in LMNA mutation carriers with prevalence numbers over 50% cited in several studies.14 30 The prevalence of 78% (for NSVT) in this study is even higher, which may be explained by repeated documentations of Holter ECGs and clinical exercise testing of the same individuals over several years. This finding suggests that even if ventricular arrhythmias have not been detected in an individual LMNA mutation carrier, they likely will be in regular follow-up. Some years ago, it was even suggested that ICDs should be implanted prophylactically to all LMNA mutation carriers.4 Recently, a risk assessment scheme was suggested to identify those LMNA mutation carriers at greater risk for malignant ventricular arrhythmias.5 We agree with the proposed strategy for individual risk stratification among LMNA mutation carriers when considering the need for an ICD. However, the high prevalence of NSVT highlights the need for close follow-up of these patients, as the clinical presentation is likely to progress. It can also be speculated that close follow-up and timely interventions could reduce the risk of events, or slow down disease progression. Our results suggest a similar or lower incidence of major clinical end points, including heart transplantations, resuscitations or deaths, in LMNA mutation carriers than in DCM controls. The incidence of major end points was higher in the older DCM control group, but Kaplan-Meier analysis showed a similar event-free survival rate in the LMNA mutation carrier and the DCM control groups.
We introduce a novel ECG concept, septal remodelling, as a readily available clinical tool to assess cardiac involvement in LMNA mutation carriers. In our study of 27 LMNA mutation carriers of varying ages, 81% presented with this ECG finding compared with 21% of the DCM controls and none of the healthy controls. When combining this abnormality with the previously described ECG signs associated with laminopathy, flat P waves and AV block, all but one of the LMNA mutation carriers had at least one of these abnormalities.
ST segment depression, markers of increased left ventricular mass (LVH and broad QRS), and elevated filling pressure (P terminal force) are established ECG signs of structural heart disease.9 10 ECG changes associated with regional disease processes, mainly fibrosis or necrosis, are less well established. We suggest that septal remodelling in the ECG leads V1–V3 represents a localised disease process typical to cardiolaminopathy. Our hypothesis is compatible with the fact that CMR studies using LGE as a marker for cardiac fibrosis suggest disease localisation in the basal and mid-ventricular septum and the basal left ventricular wall in LMNA mutations.13 14 In this study in 76% (13/17) of the LMNA mutation carriers with available CMR data, septal remodelling in the ECG and LGE in the septum were concordant. ECG septal remodelling was very common in LMNA mutation carriers, quite rare in DCM controls and absent in healthy controls. However, it seems logical that any regional disease process, such as myocardial infarction or myocarditis, resulting in necrosis or fibrosis, would be reflected in a similar way on the 12-lead ECG. Therefore, we propose further clinical assessment, including family history and cardiac imaging to rule out structural heart disease, in individuals without a history of cardiac disease, who present with septal remodelling in their 12-lead ECG. Genetic testing may also be appropriate, taking into account the entire clinical presentation.
Owing to the explorative nature of this study, multiplicity correction was not performed.
In conclusion, our results suggest that male LMNA mutation carriers appear to present with cardiolaminopathy manifestations roughly a decade earlier than women. The high incidence of atrial fibrillation and ventricular arrhythmias in LMNA mutation carriers supports close follow-up to enable timely initiation of medical and device-based antiarrhythmic therapy.
Finally, we introduce a novel ECG parameter, septal remodelling, which was frequently observed in LMNA mutation carriers and seemed to differentiate these individuals from patients with other forms of DCM.
Limitations
The number of LMNA mutation carriers in this study was rather small. It is possible that some non-significant statistical comparisons could have reached statistical significance in a larger patient material. Our definition of ECG septal remodelling is not based on exact experimental or CMR-based correlations between regional disease processes and electrophysiological changes. However, it represents a collection of previously published ECG parameters of localised heart disease.
Acknowledgments
The authors thank Sini Weckström (RN) for invaluable assistance in data collection, Tero Vahlberg for excellent statistical consultation and Santtu Ollila for expert image editing.
Footnotes
Contributors: LO, KN, MH, JuK, PP and TH participated in data collection, data analysis, drafting and revision of the manuscript. MJ, RJ, MK, JoK, SK, EP and ER participated in data collection, drafting and revision of the manuscript.
Funding: Aarne Koskelo Foundation, Finnish Foundation for Cardiovascular Research, Finnish Medical Foundation, Governmental subsidy (EVO-grants: TYH2014208, TYH2012120, TYH7106, TYH200921, TYH4241, TYH2205), and Ida Montin Foundation.
Competing interests: None declared.
Ethics approval: Ethics Committee of the Helsinki University Central Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Jacoby D, McKenna WJ. Genetics of inherited cardiomyopathy. Eur Heart J 2012;33:296–304. 10.1093/eurheartj/ehr260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodt C, Siegfried JD, Hofmeyer M et al. Temporal relationship of conduction system disease and ventricular dysfunction in LMNA cardiomyopathy. J Card Fail 2013;19:233–9. 10.1016/j.cardfail.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Berlo JH, de Voogt WG, van der Kooi AJ et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med (Berl) 2005;83:79–83. 10.1007/s00109-004-0589-1 [DOI] [PubMed] [Google Scholar]
- 4.Meune C, Van Berlo JH, Anselme F et al. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med 2006;354:209–10. 10.1056/NEJMc052632 [DOI] [PubMed] [Google Scholar]
- 5.van Rijsingen IA, Arbustini E, Elliott PM et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol 2012;59:493–500. 10.1016/j.jacc.2011.08.078 [DOI] [PubMed] [Google Scholar]
- 6.Hershberger RE, Morales A, Siegfried JD. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet Med 2010;12:655–67. 10.1097/GIM.0b013e3181f2481f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollila L, Kuusisto J, Peuhkurinen K et al. Lamin A/C mutation affecting primarily the right side of the heart. Cardiogenetics 2013;3:e1. [Google Scholar]
- 8.Quarta G, Syrris P, Ashworth M et al. Mutations in the lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2012;33:1128–36. 10.1093/eurheartj/ehr451 [DOI] [PubMed] [Google Scholar]
- 9.Momiyama Y, Mitamura H, Kimura M. ECG characteristics of dilated cardiomyopathy. J Electrocardiol 1994;27:323–8. 10.1016/S0022-0736(05)80270-5 [DOI] [PubMed] [Google Scholar]
- 10.Kasser I, Kennedy JW. The relationship of increased left atrial volume and pressure to abnormal P waves on the electrocardiogram. Circulation 1969;39:339–43. [DOI] [PubMed] [Google Scholar]
- 11.Charron P, Dubourg O, Desnos M et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation 1997;96:214–19. 10.1161/01.CIR.96.1.214 [DOI] [PubMed] [Google Scholar]
- 12.McCrohon JA, Moon JC, Prasad SK et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003;108:54–9. 10.1161/01.CIR.0000078641.19365.4C [DOI] [PubMed] [Google Scholar]
- 13.Holmström M, Kivistö S, Heliö T et al. Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson 2011;13:30 10.1186/1532-429X-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forleo C, Carmosino M, Resta N et al. Clinical and functional characterization of a novel mutation in lamin a/c gene in a multigenerational family with arrhythmogenic cardiac laminopathy. PLoS ONE 2015;10:e0121723 10.1371/journal.pone.0121723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kärkkäinen S, Heliö T, Miettinen R et al. A novel mutation, Ser143Pro, in the lamin A/C gene is common in Finnish patients with familial dilated cardiomyopathy. Eur Heart J 2004;25:885–93. 10.1016/j.ehj.2004.01.020 [DOI] [PubMed] [Google Scholar]
- 16.Kärkkäinen S, Reissell E, Heliö T et al. Novel mutations in the lamin A/C gene in heart transplant recipients with end stage dilated cardiomyopathy. Heart 2006;92:524–6. 10.1136/hrt.2004.056721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinrinade O, Ollila L, Vattulainen S et al. Genetics and genotype-phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur Heart J 2015;36:2327–37. 10.1093/eurheartj/ehv253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng S, Keyes MJ, Larson MG et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 2009;301:2571–7. 10.1001/jama.2009.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arevalo AC, Spagnuolo M, Feinstein AR. A simple electrocardiographic indication of left atrial enlargement. A study of young patients with rheumatic heart disease. JAMA 1963;185:358–62. [DOI] [PubMed] [Google Scholar]
- 20.Macfarlane P. Comprehensive electrocardiology. Karger Publisher, 2011. [Google Scholar]
- 21.Sokolow M, Lyon T. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–86. 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- 22.Okin PM, Roman MJ, Devereux RB et al. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol 1995;25:417–23. 10.1016/0735-1097(94)00371-V [DOI] [PubMed] [Google Scholar]
- 23.Okin PM, Devereux RB, Nieminen MS et al. Relationship of the electrocardiographic strain pattern to left ventricular structure and function in hypertensive patients: the LIFE study. Losartan Intervention For End point. J Am Coll Cardiol 2001;38:514–20. [DOI] [PubMed] [Google Scholar]
- 24.Lakdawala NK, Thune JJ, Maron BJ et al. Electrocardiographic features of sarcomere mutation carriers with and without clinically overt hypertrophic cardiomyopathy. Am J Cardiol 2011;108:1606–13. 10.1016/j.amjcard.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konno T, Fujino N, Hayashi K et al. Differences in the diagnostic value of various criteria of negative T waves for hypertrophic cardiomyopathy based on a molecular genetic diagnosis. Clin Sci (Lond) 2007;112:577–82. 10.1042/CS20060293 [DOI] [PubMed] [Google Scholar]
- 26.Das MK, Khan B, Jacob S et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–501. 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- 27.Zema MJ, Kligfield P. Electrocardiographic poor R wave progression. I: correlation with the Frank vectorcardiogram. J Electrocardiol 1979;12:3–10. [DOI] [PubMed] [Google Scholar]
- 28.Dumont CA, Monserrat L, Soler R et al. Interpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance. Eur Heart J 2006;27:1725–31. 10.1093/eurheartj/ehl101 [DOI] [PubMed] [Google Scholar]
- 29.van Rijsingen IA, Nannenberg EA, Arbustini E et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail 2013;15:376–84. 10.1093/eurjhf/hfs191 [DOI] [PubMed] [Google Scholar]
- 30.Hasselberg NE, Edvardsen T, Petri H et al. Risk prediction of ventricular arrhythmias and myocardial function in lamin A/C mutation positive subjects. Europace 2014;16:563–71. 10.1093/europace/eut291 [DOI] [PubMed] [Google Scholar]