Significance
The mammalian cell cytoplasm contains numerous proteins with direct antimicrobial activity. Although these have been extensively studied in the context of viral and bacterial infection, it is unknown whether pathogenic self-propagating proteins, proposed to underlie common neurodegenerative diseases, can be targeted in a similar manner. We studied the ability of tripartite motif protein 21 (TRIM21), a newly identified intracellular antibody receptor, to intercept assemblies of misfolded tau, a cytoplasmic protein that aggregates in patients with Alzheimer’s disease. We developed tau “seeding” assays in human cells and found that TRIM21 could intercept and potently neutralize antibody-labeled tau assemblies. These findings demonstrate that the intracellular immune system can act against self-propagating misfolded proteins, with implications for ongoing attempts to develop antibody-based therapies for neurodegenerative disorders.
Keywords: neurodegeneration, tau, intracellular immunity, antibodies, immunoreceptors
Abstract
Alzheimer’s disease (AD) and other neurodegenerative disorders are associated with the cytoplasmic aggregation of microtubule-associated protein tau. Recent evidence supports transcellular transfer of tau misfolding (seeding) as the mechanism of spread within an affected brain, a process reminiscent of viral infection. However, whereas microbial pathogens can be recognized as nonself by immune receptors, misfolded protein assemblies evade detection, as they are host-derived. Here, we show that when misfolded tau assemblies enter the cell, they can be detected and neutralized via a danger response mediated by tau-associated antibodies and the cytosolic Fc receptor tripartite motif protein 21 (TRIM21). We developed fluorescent, morphology-based seeding assays that allow the formation of pathological tau aggregates to be measured in situ within 24 h in the presence of picomolar concentrations of tau seeds. We found that anti-tau antibodies accompany tau seeds into the cell, where they recruit TRIM21 shortly after entry. After binding, TRIM21 neutralizes tau seeds through the activity of the proteasome and the AAA ATPase p97/VCP in a similar manner to infectious viruses. These results establish that intracellular antiviral immunity can be redirected against host-origin endopathogens involved in neurodegeneration.
The cell’s ability to identify intracellular viruses and bacteria relies on the detection of pathogen-associated molecular patterns (PAMPs) by specialized host receptors. Although highly effective at detecting microbial pathogens, this strategy is poorly equipped to identify host-derived pathogenic species such as aggregated proteins. As an alternative to PAMP detection, recent work has demonstrated that mammalian cells can use host-derived serum proteins, which are normally excluded from the cell interior, to target invading viruses and bacteria in the cytosol. For instance, nonenveloped viruses and bacteria carry antibodies with them into the cytoplasm during infection. These translocated antibodies are then sensed by the cytoplasmically expressed antibody receptor TRIM21 (tripartite motif protein 21), which binds with subnanomolar affinity to the antibody Fc domain (1–4). After binding to antibody, TRIM21 triggers a potent neutralization response that inhibits viral infection. Neutralization of infection is accompanied by degradation of viral components, which requires the activity of the proteasome and the molecular unfoldase, valosin-containing protein (VCP) or p97 (1, 5). Detection of viruses and bacteria by TRIM21 does not rely on microbial PAMPs, as model substrates such as antibody-coated latex beads can be bound and detected by TRIM21 (1, 3). We therefore hypothesized that the intracellular innate immune system could be repurposed to recognize and degrade host-derived pathogenic proteins.
Microtubule-associated protein tau occurs in an assembled and hyperphosphorylated state in the cytoplasm of neurons and glial cells in Alzheimer’s disease (AD), progressive supranuclear palsy, chronic traumatic encephalopathy, and other neurodegenerative disorders (6). Tau assemblies applied to the extracellular spaces can seed further aggregation: injection of tau assemblies into the brains of tau transgenic mice promotes the aggregation of intracellular tau (7), and ectopic addition of recombinant tau assemblies promotes the aggregation of intracellular pools in cultured cells (8, 9). Consistent with transcellular propagation of misfolding, tau pathology in AD spreads between connected regions of the brain in a stereotyped manner (10). Seeded propagation of misfolded tau is therefore proposed to underlie many common neurodegenerative disorders. Akin to viral infection, this model of seeded tau propagation relies on the physical transfer of tau assemblies between cells, and is therefore potentially susceptible to interception by antibody. The broad tissue expression of TRIM21 and its ultrahigh affinity for Fc imply that intracellular antibodies will be bound by TRIM21 whenever they occur. Numerous intracellular entities may therefore be targeted by TRIM21, including DNA and RNA viruses (1, 11, 12), cytosolic bacteria (3), and soluble host proteins, including an Fc fragment (5) and a cis-tau conformer (13). However, it is not known whether self-propagating protein assemblies are susceptible to neutralization by TRIM21.
Here we report the development of highly sensitive tau seeding assays, which measure the ability of extracellular tau assemblies to promote the conversion of intracellular, full-length tau from a soluble to aggregated state after their entry to the cell. We demonstrate that tau seeds that enter the cell with attached antibody are rapidly bound and inactivated by TRIM21. Our results demonstrate that given an appropriate danger signal, here antibody, the intracellular immune system is capable of detecting and inactivating self-propagating protein assemblies.
Results
TRIM21 Is Expressed in Neural Cells.
Transcriptomic and immunohistochemical analyses suggest that the cytosolic Fc receptor TRIM21 is expressed at low levels in brain tissue (14, 15). To test whether this basal expression is sufficient for protective intracellular immunity, we challenged primary mouse neural cultures with adenovirus or adenovirus treated with monoclonal antibody 9C12, a model system for intracellular neutralization (2). In neurons derived from wild-type (WT) mice, 9C12 conferred a 40-fold block to infection (Fig. 1A). However, in neurons derived from Trim21−/− mice, a 12-fold block to infection was observed. Basal Trim21 levels can therefore provide a significant block to infection in neurons. This finding is in agreement with previous findings that Trim21 is expressed and active in primary neurons (13). Trim21 is an IFN-stimulated gene whose expression levels determine the potency of intracellular neutralization (2). We tested the effect of type I, II, or III interferons on intracellular neutralization in primary neurons. IFN-α (type I) and IFN-β (type II), but not IFN-λ (type III), up-regulated Trim21 expression (Fig. 1B). IFN-stimulated up-regulation of Trim21 substantially potentiated neutralization, resulting in a >700-fold reduction of infection by antibody in stimulated WT cells (Fig. 1C). In contrast, all IFNs exerted little effect on neutralization in Trim21−/− cells. Levels of neutralization were correlated with, and almost entirely accounted for, by levels of Trim21 mRNA (R2 = 0.94) (Fig. 1D). Importantly, TRIM21 mediated comparable levels of adenovirus neutralization in human H4 neuroglioma and SHSY-5Y neuroblastoma cells, in addition to HeLa and HEK293 lines (Fig. 1E), demonstrating that TRIM21 is widely expressed and active in diverse tissues, including CNS and neural derived cells.
Fig. 1.
Cells derived from neural tissues exert inducible intracellular neutralization via TRIM21. (A) Mouse primary neural cultures derived from WT or Trim21−/− C57BL/6 E18 embryos were challenged with a titration of adenovirus-RFP that was pretreated with PBS or anti-hexon antibody 9C12. (B) Relative levels of Trim21 mRNA in WT neural cultures after stimulation with the indicated IFN compared with untreated (UT) cells, normalized to levels of β-actin mRNA. (C) Levels of neutralization in WT or Trim21−/− neural cultures treated as in B. Fold neutralization was calculated by dividing the level of infection with PBS-treated virus by that of 9C12-treated virus. (D) Fold neutralization observed after IFN stimulation is correlated with Trim21 mRNA levels (Pearson r = 0.99; P < 0.001; R2 = 0.94). (E) Fold neutralization of adenovirus by 9C12 in H4, a model of human microglia; SHSY-5Y, a human cell line with properties of neurons and HeLa and HEK293, commonly used human cell lines. TRIM21 was deleted by CRISPR/Cas9 (T21 KO).
Sensitive in Vitro Tau Seeding Assay.
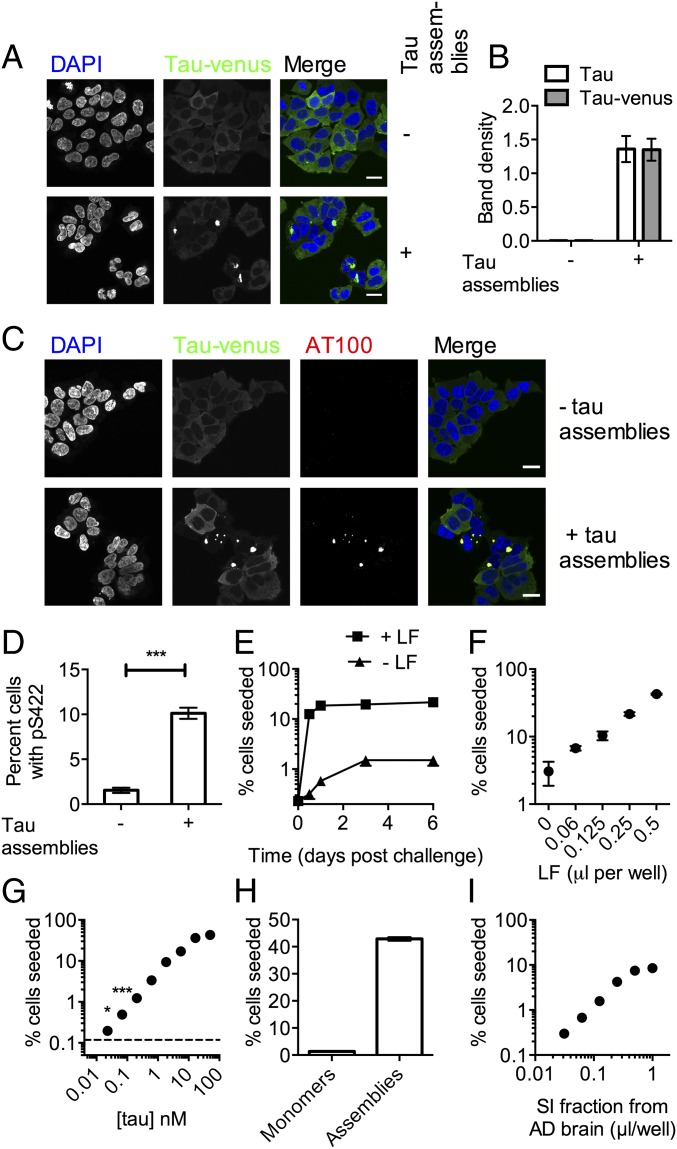
The study of seeded tau propagation relies on transgenic mouse models, in which disease progression is slow and the contribution of seeded vs. spontaneous aggregation is often unclear. Alternatively, in vitro assays allow these contributions to be quantified and dissected, but existing methods rely on aggregation-prone fragments of tau, have suffered from low signal-to-noise ratios, or require laborious biochemical fractionation, which is not amenable to detailed study. We therefore developed a high-content microscopy-based assay using HEK293 cells stably expressing the 0N4R isoform of human tau, bearing frontotemporal dementia-associated mutation P301S with a C-terminal venus tag. Addition of recombinant heparin-assembled P301S tau promoted the conversion of tau-venus from a disperse distribution to bright foci (Fig. 2A). To demonstrate that these foci are associated with tau aggregation, the sarkosyl-insoluble fraction was prepared from cell homogenates. Addition of tau assemblies resulted in a proportion of tau pelleting in the sarkosyl-insoluble fraction, and the venus tag did not substantially alter this process (Fig. 2B and Fig. S1). To investigate whether tau-venus foci recapitulated the pathological characteristics of tauopathies, we used immunostaining for phosphorylated and fibril-specific epitopes. A pan-tau antibody, HT7, colocalized with tau-venus in both untreated cells and seeded cells (Fig. S2). In contrast, tau-venus foci that occurred after seeding, but not soluble tau-venus, were stained by antibodies that depend on phosphorylation of their antigens, anti-pS422 (Fig. S2) (16) and AT100 (pT212, pS214, pT217), which is specific for pathological tau structures (Fig. 2C) (17). To confirm that intracellular expressed tau-venus, rather than seed, was the target of hyperphosphorylation, we challenged cells with tau assemblies bearing point mutation S422A. Phosphorylation at S422 was detected within 12 h of challenge (Fig. 2D), confirming aggregation and hyperphosphorylation of intracellular expressed tau. These observations demonstrate a seeding assay that recapitulates both the aggregation and hyperphosphorylation observed in diseased brains in a widely used human cell line. Furthermore, the results support the close temporal association of intracellular aggregation and hyperphosphorylation.
Fig. 2.
Exogenously added tau assemblies induce aggregation and phosphorylation of intracellular tau. (A) P301S tau-venus expressed in HEK293 cells with or without addition of heparin-assembled recombinant P301S tau to culture supernatant. (B) Sarkosyl insoluble tau 3 d after challenge with P301S tau assemblies or mock treatment. Levels determined by Western blot and normalized to total tau. (C) Cells treated as in A stained with phosphorylated tau fibril-specific antibody AT100. (D) Levels of staining with anti-pS422 12 h postchallenge with P301S/S422A tau assemblies. (E) Time course of seeding by 100 nM P301S tau assemblies in the presence or absence of 0.5 µL LF. (F) Percentage HEK293 cells that exhibit aggregated tau-venus 3 d postchallenge with 200 nM P301S tau assemblies with indicated dose of LF. (G–I) Percentage of cells seeded after challenge in the presence of LF with (G) a titration of P301S tau assemblies, (H) 50 nM monomeric or aggregated P301S tau, or (I) AD brain sarkosyl insoluble (SI) fraction. *Unpaired t test P < 0.05; ***P < 0.0001. Error denotes SEM. In B, D, and F, n = 3 replicate experiments; in E and G–I, n = 9–24 fields per condition. (Scale bars, 20 µm.)
Fig. S1.
Biochemical fractionation of cells after seeding. HEK293 expressing P301S 0N4R tau either with or without a C-terminal venus tag were exposed to recombinant P301S tau assemblies. After 3 d, cells were harvested and the sarkosyl insoluble (SI) fraction was collected. Input and SI fractions were probed with anti-tau antibody HT7 or anti-GAPDH.
Fig. S2.
Characterization and quantification of tau-venus seeding assay. HEK293 cells expressing P301S tau-venus were challenged with tau assemblies before fixing and staining with (A) pan-tau antibody HT7 or (B) phosphorylation-specific antibody anti-pS422. (C) HEK293 tau-venus cells were challenged with 100 nM tau assemblies in the presence of LF and fixed in 4% paraformaldehyde 2 d postchallenge. Nikon NIS Elements program was used to detect seeded vs. unseeded cells. Nuclei were identified by Hoechst staining of DNA, and cells were scored for the presence of tau puncta within a region of interest around the nuclei. Cells scored as seeded are labeled with red overlay, whereas cells scored as not seeded are labeled with blue overlay. The calculated percentage of cells seeded for the field is displayed in white. (Scale bar, 0.1 mm.)
Cytoplasmic Delivery of Tau Is Rate-Limiting to Seeding.
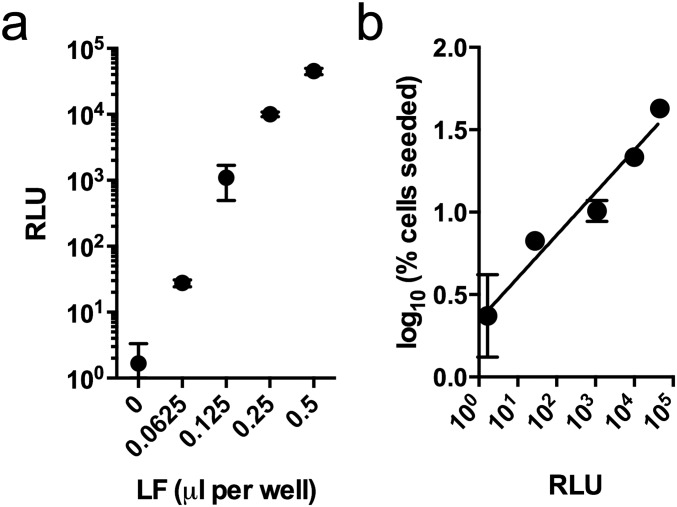
Previous observations in cell-based models of tau seeding support the hypothesis that entry to the cell, rather than aggregation itself, is rate-limiting (9). We used cationic lipids (Lipofectamine 2000; LF) to promote uptake and disrupt endosomal membranes. LF substantially increased both the rate and overall level of aggregation in our assay (Fig. 2E), using automated image analysis (Fig. S2). The data suggest that seeded aggregation is a rapid process that occurs largely within 12 h, whereas uptake and endosomal rupture are inefficient and, in the absence of LF, rate-limiting. LF increased the percentage of seeded cells in a manner proportional to its ability to deliver a luciferase-encoding plasmid (Fig. 2F and Fig. S3), consistent with intracellular delivery of tau assemblies being the cause of increased seeding by LF. In the presence of LF, seeding increased in a dose-dependent manner with tau concentration over more than three orders of magnitude (Fig. 2G). Statistically significant seeding, relative to a LF-treated control, was detected using 23-pM tau assemblies. In contrast, the addition of monomeric tau did not induce seeding (Fig. 2H). The sarkosyl-insoluble fraction of Alzheimer’s diseased brain tissue was also competent at inducing seeding (Fig. 2I). These data demonstrate a sensitive and quantitative assay using full-length tau that responds to the presence of tau assemblies, both recombinant and clinically derived, by propagating intracellular, phosphorylated tau aggregates. Moreover, they demonstrate that cytosolic entry is rate-limiting and that once inside the cell, trace amounts of seed, left unrestricted, are capable of rapidly initiating tau aggregation.
Fig. S3.
LF increases tau seeding proportionally to its capacity to deliver DNA. (A) Transfection efficiency as measured by relative luminescence units (RLUs) of cells 24 h after treatment with a luciferase-encoding plasmid and indicated doses of LF. (B) The level of seeding after challenge with P301S tau assemblies was found to correlate with the ability of LF to transfect plasmid DNA, as measured by plasmid-expressed luciferase readings. Pearson r, 0.96; P < 0.01. Error denotes SEM.
Antibodies Prevent Seeding but Poorly Block Entry.
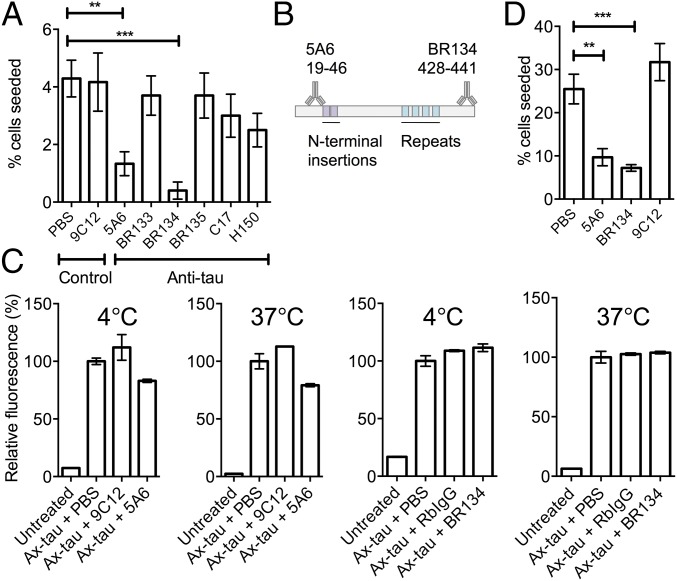
Next, we used our assay to identify antibodies that reduce seeding. A panel of phosphorylation-independent anti-tau and control antibodies (Table S1) was screened, of which mouse monoclonal IgG1, 5A6 (18), and rabbit polyclonal antibody, BR134 (19), were found to prevent seeding by recombinant P301S tau assemblies in the absence of LF (Fig. 3 A and B). The ability of 5A6 and BR134 to prevent binding and uptake of AlexaFluor488-labeled tau assemblies to cells was tested. Binding (at 4 °C) and uptake (at 37 °C) were marginally reduced (∼20%) by 5A6, but not by BR134 (Fig. 3C). Consistent with previous observations (20), these results suggest that entry-blocking contributes to neutralization. However, for both 5A6 and BR134, the majority of neutralization was not accounted for by this mechanism. Moreover, neutralization was observed when assembled P301S tau-antibody complexes were introduced by LF (Fig. 3D). Together, these results suggest that neutralization by 5A6 and BR134 occurs largely postentry.
Table S1.
List of antibodies used in this study
| Antibody | Epitope/immunogen | Type | Source |
| 9C12 | Adenovirus hexon | Mouse IgG1 | (43) |
| 5A6 | Tau amino acids 19–46 | Mouse IgG1 | (18) |
| BR133 | Tau amino acids 1–16 | Rabbit polyclonal | (19) |
| BR134 | Tau amino acids 428–441 | Rabbit polyclonal | (19) |
| BR135 | Tau amino acids 323–355 | Rabbit polyclonal | (19) |
| C-17 | Tau C terminus | Goat polyclonal | Santa Cruz Biotechnology sc-1995 |
| H150 | Tau N terminus amino acids 1-150 | Rabbit polyclonal | Santa Cruz Biotechnology sc-5587 |
| AT100 | Tau pT212, pS214, pT217 | Mouse IgG1 | (44) |
| pS422 | Tau pS422 | Rabbit monoclonal | (16) |
| HT7 | Tau amino acids 159–163 | Mouse IgG1 | Thermo Fisher Scientific MN1000 |
Fig. 3.
Neutralization of tau seeding can occur independent of entry blocking. (A) Percentage HEK293 tau-venus cells seeded 5 d after challenge with P301S tau assemblies that were mixed with indicated antibodies for 1 h before addition to culture supernatant. (B) Tau epitopes identified by 5A6 and BR134. (C) Relative fluorescence of HEK293 cells treated with Alexa488-labeled tau assemblies at indicated temperature. (D) Percentage HEK293 tau-venus cells seeded after challenge with 100 nM P301S tau assemblies incubated with indicated antibody in the presence of LF. **Unpaired t test P < 0.01; ***P < 0.0001. Error denotes SEM. In A and G, n = 10–20 fields per condition; C–F, duplicate experiments. (Scale bars, 20 µm.)
TRIM21 Neutralizes Tau Seeding.
Cytosolic Fc receptor TRIM21 mediates the highly efficient postentry neutralization of antibody-bound nonenveloped viruses and soluble toxic tau precursors (1, 2, 13, 21). To investigate whether tau seeding can be neutralized by TRIM21, we used CRISPR/Cas9 to disrupt TRIM21 in HEK293 cells expressing P301S tau-venus and confirmed protein knockout by immunoblot (Fig. 4A). HEK293 cells take up tau aggregates by macropinocytosis (22), but lack expression of FcγRs, permitting analysis of neutralization in the absence of cell surface antibody receptors. Disruption of TRIM21 by CRISPR/Cas9 reduced the ability of mouse monoclonal antibody 9C12 to neutralize adenovirus infection (Fig. 1E), as has previously been demonstrated by small hairpin RNA and genetic ablation (2). Neutralization of tau seeding by 5A6 and BR134 was significantly reduced in cells that lacked TRIM21 (Fig. 4B). TRIM21-mediated neutralization of adenovirus proceeds without a lag phase and is dose-dependent until reaching a plateau termed the “persistent fraction.” This represents a proportion of infection that cannot be prevented, but whose level is dependent on TRIM21 expression levels (2, 23). TRIM21-mediated neutralization of tau seeding occurred with log-linear dynamics and without a detectable lag-phase (Fig. 4C). This suggests that, as in the case of adenovirus neutralization, a threshold antibody stoichiometry is not required, but that neutralization increases incrementally with antibody occupancy. A persistent fraction was observed for tau neutralization, whose level was increased in cells lacking TRIM21 (Fig. 4D). Thus, neutralization of tau seeding occurs with similar dynamics as neutralization of adenovirus infection. To determine whether the nature of the tau seed affects neutralization, we tested whether TRIM21 can neutralize the sarkosyl-insoluble fraction from AD brain, which contains tau fibrils. We found that BR134 reduced seeding in unmodified HEK293 cells, but provided no protection when TRIM21 was deleted (Fig. 4E). Thus, tau assemblies from recombinant and clinical samples can be neutralized in the intracellular domain in a similar manner to viral infection.
Fig. 4.
Tau seeding is neutralized via TRIM21. (A) Immunoblot for TRIM21 in HEK293 tau-venus cells treated with control vector expressing Cas9 only or with additional TRIM21-directed guide RNA. (B) Relative levels of seeding by recombinant P301S tau assemblies after treatment with indicated antibody in the presence of LF. (C) Titration of 5A6 against tau seeding in HEK293. (D) Titration of 5A6 or isotype control antibody 9C12 against tau seeding. (E) Relative level of seeding by AD brain sarkosyl insoluble fraction alone or in complex with BR134 in HEK293 cells in the presence of LF. (F) Percentage of tau aggregates within cell boundaries that colocalize with myc-TRIM21. (G) Confocal microscope images of cells expressing mCherry-TRIM21 fixed 1 h after application of tau-venus assemblies purified from human cells in the presence of LF. (Scale bar, 5 µm.) **Unpaired t test P < 0.01; ***P < 0.0001. Error denotes SEM. In B–E, n = 8–12 fields per condition; in G, n = 13 fields per condition. n.d., none detected.
TRIM21 Is Rapidly Recruited to Antibody-Bound Tau Assemblies.
The fast kinetics of seeded tau aggregation imply that for TRIM21 to be effective, it must be rapidly recruited to incoming antibody-coated seeds. To test this, we challenged HEK293 cells expressing myc-tagged TRIM21 with tau assemblies and examined the localization of TRIM21 after 4 h (Fig. S4 and Fig. 4F). Alternatively, we challenged HEK293 cells expressing mCherry-TRIM21 with the sarkosyl-insoluble fraction prepared from seeded tau-venus-expressing cells and fixed cells at 1 h (Fig. 4G). In both cases, TRIM21 adopted a disperse cytoplasmic distribution in cells that were treated with unlabeled tau assemblies. However, TRIM21 reorganized to bright foci surrounding antibody-coated tau assemblies during challenge. As has been observed for antibody-bound viruses and bacteria (1, 3), a subset of antibody-bound P301S tau assemblies did not colocalize with TRIM21 and is likely endosomal. We conclude that TRIM21 is recruited to tau assemblies shortly after their entry to the cytoplasm.
Fig. S4.
Tau assemblies colocalize with TRIM21 when pretreated with antibody. Confocal microscope images of immunofluorescence staining for myc-TRIM21 and tau in HEK293 cells that were untreated or 4 h after challenge with tau assemblies treated with 5A6 or PBS in the presence of LF. (Scale bar, 10 µm.)
TRIM21 Is the Sole Protective Fc Receptor in a Human Neuronal Cell Line.
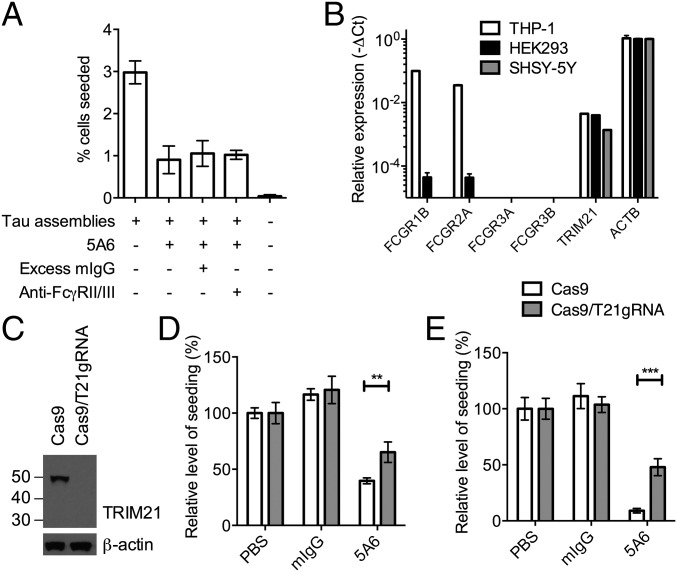
It has previously been demonstrated that antibody receptors FcγRII/III expressed on the surface of neurons in brain slices mediates uptake of uncomplexed anti-tau antibodies (24, 25). The internalized antibodies are proposed to interact with cellular tau pools in a compartment that stains positive for markers of the endolysosome. Such a mechanism could potentially affect the seeded aggregation of cytosolic tau, either by increasing the uptake of tau–antibody immune complexes to neurons or by diverting immune complexes to endolysosomal degradation pathways. We therefore addressed whether cell surface Fc receptors enhance or interfere with seeding/neutralization in a human neuronal cell line. We established a seeding assay in the human neuroblastoma cell line SHSY-5Y by expressing P301S tau-venus in a similar manner as in HEK293s (Fig. S5). Seeding was observed in both the presence and absence of lipofectamine and cells bearing seeded tau aggregates stained positive with AT100. The presence of excess IgG or an antibody that blocks FcγRII/III did not alter observed levels of seeding by tau–antibody complexes (Fig. 5A). These data are suggestive of an absence of FcγR activity in these cells. Directly testing for the expression FcγRs in SHSY-5Y cells revealed minimal or no expression for both high-affinity (CD64, FcγRIB) and low-affinity (CD32 FcγRIIA; CD16a, FcγRIIIA; CD16b, FcγRIIIB) Fc receptors, similar to HEK293s (Fig. 5B). In contrast, we observed strong FcγRI and FcγRII expression in the monocyte-derived cell line THP-1, consistent with its role as an antigen-presenting cell. Conversely, TRIM21 mRNA was detected in all cell lines at comparable levels. These findings are in agreement with data from cultured mouse neurons and transcriptomic databases, where cell surface FcγR expression in neurons is found to be very low or absent (14, 26). Tau neutralization in SHSY-5Y cells occurred similarly to HEK293s. In both the presence and absence of LF, seeding was neutralized by 5A6, but significantly reduced on TRIM21 depletion (Fig. 5 C–E). Thus, TRIM21 appears to be the only FcR expressed and functional in neurons, and therefore the sole receptor capable of effecting the neutralization of prion-like proteins that replicate in the cytoplasm of neurons.
Fig. S5.
Seeding in SHSY-5Y cells. (A) P301S tau-venus was expressed in SHSY-5Y cells and challenged with tau assemblies. Immunostaining reveals only cells bearing tau aggregates (indicated with arrows) stain positive with AT100. (B and C) Levels of seeding in SHSY-5Y cells after challenge with (B) 3.3 µM recombinant tau assemblies for 3 d or (C) 250 nM recombinant tau assemblies and LF for 24 h.
Fig. 5.
TRIM21 is the only protective Fc receptor in SHSY-5Y neuroblastoma cells. (A) Levels of seeding in SHSY-5Y cells treated as indicated in the absence of LF. Excess mouse IgG (mIgG) or an antibody that blocks the low-affinity FcγRII and FcγRIII did not alter levels of seeding after challenge with tau-5A6 complexes. (B) Levels of indicated mRNA transcripts in HEK293, SHSY-5Y, and the monocyte cell line THP-1. Relative levels were determined by RT-qPCR, using the −ΔCt method relative to β-actin (ACTB). (C) Immunoblot for TRIM21 in SHSY-5Y cells that were treated with Cas9 only or with an additional TRIM21-directed guide RNA. (D and E) Relative levels of seeding in SHSY-5Y cells after challenge with tau assemblies that were pretreated with PBS, nonspecific mouse IgG, or tau-specific antibody 5A6 in the (D) absence and (E) presence of LF. **Unpaired t test P < 0.01; ***P < 0.0001. Error denotes SEM. In A and E, n = 12 fields per condition; in B, mean of triplicate cDNA preparations; in D, n = 24 fields per condition.
Neutralization of Tau Seeding Is Proteasome- and VCP-Dependent.
To investigate whether neutralization of tau seeding follows a similar cofactor dependency as virus neutralization, we treated cells with the proteasomal inhibitor MG132. Under these conditions, neutralization of tau seeding was significantly reduced, although not completely inhibited (Fig. 6A). To examine the contribution of the molecular unfoldase VCP, we used the inhibitor N2,N4-dibenzylquinazoline-2,4-diamine (DBeQ) (27) or its derivative ML240 (28). Treatment with these compounds almost completely reversed neutralization (Fig. 6 B and C). Depletion of VCP by siRNA also reduced levels of neutralization, confirming VCP dependence (Fig. 6 D and E). These results demonstrate that neutralization of tau seeding occurs with similar cofactor dependencies as viral neutralization. However, the results suggest a more prominent role for VCP compared with the proteasome, as inhibition of neutralization was greater with inhibitors of the former. VCP has a role in both proteasomal and macroautophagy degradation pathways. We therefore tested whether TRIM21 also exerts neutralization via autophagy. We used siRNA depletion of the ubiquitin-like protein ATG12, whose conjugation to ATG5 is an upstream requirement for the formation of autophagosomes, to test the effect of impaired autophagy on intracellular neutralization. However, despite preventing the formation of GFP-LC3 puncta after starvation (Fig. S6), ATG12 depletion did not impair the neutralization of tau seeding by 5A6 (Fig. 6F). The role of VCP in neutralization of tau seeding is therefore likely to be independent of autophagy. Further supporting this finding, chemical inhibition of autophagy or lysosomal acidification did not prevent neutralization of tau seeding (Fig. 6G and Fig. S6). Overall, these results demonstrate that, similar to viruses, antibody-labeled tau aggregates are neutralized in a proteasome- and VCP-dependent manner in the intracellular environment.
Fig. 6.
Neutralization of tau seeding occurs via established TRIM21 degradative pathways. (A) Effect of proteasome inhibitor MG132 on neutralization by 5A6. (B–D) Effect of VCP inhibitors (B) DBeQ, (C) ML240, or (D) VCP knockdown on neutralization by 5A6. (E) Immunoblot for VCP and β-actin after VCP-directed or control (CTRL) siRNA treatment. (F and G) Effect of (F) ATG12 depletion by siRNA or (G) inhibitors 3-methyladenine (3mA) and chloroquine (CQ) on neutralization of tau seeding. *Unpaired t test P < 0.02; ***P < 0.0001. Error denotes SEM. In B–E, n = 12 fields per condition.
Fig. S6.
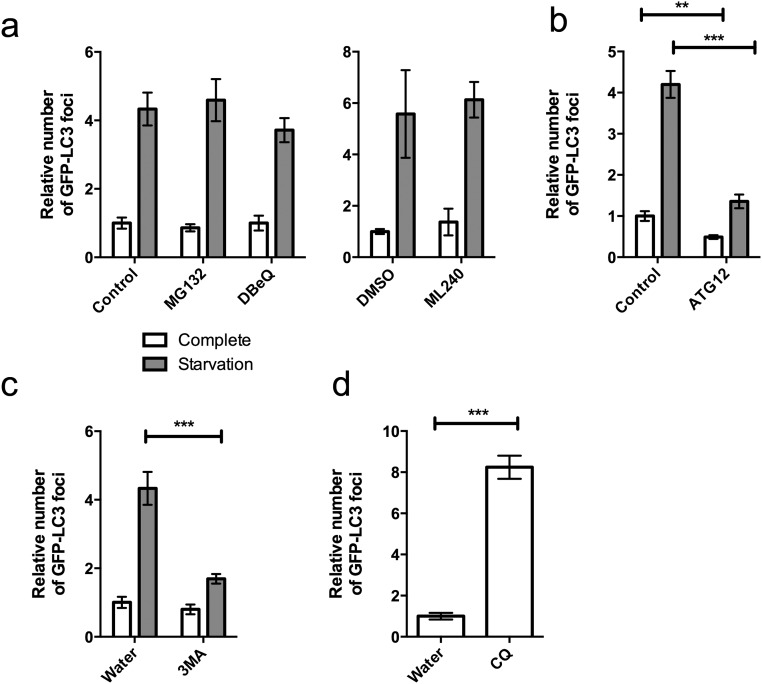
Influence of perturbations to the autophagy/lysosome pathway on GFP-LC3 puncta. HEK293 cells were stably transfected with GFP-LC3B and maintained in complete media or starvation conditions (140 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM glucose, 20 mM Hepes at pH 7.4) for 3 h before fixing and quantification of GFP puncta. Effect of (A) proteasome inhibitor MG132 and VCP inhibitor DBeQ and ML240 or solvent (DMSO), (B) control or ATG12-directed siRNA, or (C) autophagy inhibitor 3-methyladenine or solvent (water). (D) Relative levels of GFP-LC3B puncta after treatment with lysosome inhibitor chloroquine (CQ) in complete media. Error denotes SEM. **Unpaired t test P < 0.01; ***P < 0.0001.
Discussion
We have developed high-content quantitative assays for measuring cytosolic tau aggregation in situ. Whereas the introduction of tau assemblies to the cytoplasm induced rapid aggregation, the presence of anti-tau antibodies diverted tau seeds to a neutralization pathway that substantially reduced their seeding capacity (diagram in Fig. S7). This inhibition relied on the detection of antibody-bound tau by the cytosolic Fc receptor TRIM21, which exerted neutralization activity in concert with known intracellular neutralization machinery, the molecular unfoldase VCP and the proteasome. Intracellular neutralization occurred rapidly, before tau hyperphosphorylation, and was observed for tau assemblies prepared from recombinant or AD brain-derived sources. These results demonstrate that, despite lacking PAMPs, pathogenic protein assemblies can be recognized and destroyed by the cell if presented in context with a danger signal, intracellular antibody. Thus, intracellular antiviral immunity can be repurposed to protect against self-propagating protein misfolding.
Fig. S7.
A model for intracellular neutralization of tau seeding. Tau assemblies are taken up into naive cells, where they are able to recruit native tau and induce its seeded aggregation (Upper). Alternatively, antibodies in complex with tau assemblies are brought into the cell and recognized by TRIM21, which diverts tau assemblies into a proteasome-dependent neutralization pathway before the initiation of seeding (Lower).
Large tau aggregates are refractory to proteasomal activity, and their turnover is thought to rely primarily on macroautophagy (29, 30). In contrast, seeds are reported to be short fibrils containing >10 copies of tau (31). Our results suggest that tau seeds are substrates for proteasomal degradation if targeted rapidly after cell entry. Thus, targeting seeds rather than established aggregates may be a more effective strategy for preventing spreading tau aggregation. Importantly, we observed that the inhibition of tau aggregation was more dependent on VCP than the proteasome. This is in contrast to adenovirus neutralization, in which proteasome and VCP activities are equally critical (1, 5). VCP has been linked to both proteasomal degradation and autophagy; however, we did not observe any reduction in the neutralization of aggregation under conditions where autophagy was prevented. Instead, it is possible that VCP exerts its effect by unfolding tau seeds to prevent them from catalyzing aggregation. This would be consistent with the enzyme’s known unfoldase activity (32), and with altered tau conformations being the drivers of seeded aggregation.
The discovery that antibodies are capable of preventing cytoplasmic tau aggregation is significant to the development of immunotherapy for AD and other tau pathologies. In transgenic mouse models, passive transfer of tau-specific antibodies can reduce tau pathology (20, 33–35). Similar approaches in humans are being widely studied as potential treatments for neurodegeneration (36). However, the mechanisms by which antibodies exert their activity is unclear. Mechanisms that have been described include the clearance by FcγRs expressed on microglia (37), the blockade of tau assemblies from entering neurons (20), the uptake of antibodies in an FcγRII/III-dependent manner and subsequent colocalization with tau in an endolysosomal compartment (24), and the uptake of uncomplexed antibodies by neurons via unknown mechanisms and degradation of soluble tau toxic precursors via TRIM21 (13). The relevance of mechanisms in which interaction with an FcγR is required has recently been questioned by the finding that an anti-tau antibody mutated to prevent FcγR binding remains protective in mice (26). This is consistent with our in vitro findings that FcγR interactions neither promote nor inhibit tau aggregation in neuronal cell lines. Use of mutated ‘“effectorless” antibodies may be favorable in therapeutic settings, as it would minimize the proinflammatory effects of extracellular tau immune complexes. Importantly, however, the mutations used to render antibody effectorless do not affect the TRIM21 binding site, and would therefore maintain full levels of TRIM21 binding. Observations of antibody protection in mouse models of tau pathology are therefore consistent with Trim21-mediated protection. In vivo experiments in a Trim21 null setting are necessary to determine the contribution of cytoplasmic degradation and neutralization to immunotherapeutic protection against tau pathology.
Our results demonstrate that antibodies can direct a potent TRIM21-dependent neutralizing response immediately after cytosolic entry of tau seeds. The selective targeting of pathological proteins in the intracellular domain by antibodies may provide an effective therapeutic strategy.
Materials and Methods
Seeding Assays.
A full description of experimental techniques is provided in SI Materials and Method. Recombinant, heparin-assembled tau preparations were added to HEK293 or SHSY-5Y cells expressing P301S tau-venus in 96-well plates. LF was added at 0.5 µL per well where indicated. Seeded cells were enumerated by high-content imaging.
Adenovirus Infection.
Mouse primary neurons and other cells were challenged with E1E3-deleted human adenovirus type 5 vector in the presence or absence of anti-hexon antibody 9C12, as before.
SI Materials and Methods
Cells.
HEK293, HEK293T and SHSY-5Y cells were maintained in complete DMEM (C-DMEM) with 10% (vol/vol) FCS, 100 U/mL penicillin, 100 μg/mL streptomycin at 37 °C in a 5% CO2 atmosphere. Tau bearing point mutation P301S with a C-terminal venus tag, separated by a GSGS linker, was generated by PCR amplification of human P301S 0N4R tau, using primers tauF KpnI ACTGGGTACCGCCACCATGGCTGAGCCCCGCCAGG (KpnI restriction site in bold, start codon underlined) and tauR EcoRI CCTGGCCAAGCAGGGTTTGGAATTCACGT (EcoRI site in bold). Venus was amplified using VN F EcoRI ACTGGAATTCGGATCCGGATCCATGGTGAGCAAGGGCGAGG (EcoRI site in bold, linker sequence underlined) and VC R XhoI CAGTCTCGAGTTACTTGTACAGCTCGTCC (XhoI site in bold, stop codon underlined). The resulting PCR products were cloned into pcDNA3.1(+) (Life Technologies), using the indicated restriction enzymes (Life Technologies). To generate tau without a tag, a stop codon was introduced at the end of tau, using the QuikChange protocol (Stratagene). The resulting plasmids were transfected into HEK293 cells using LF, and subjected to selection under G418 (Life Technologies) at 500 µg/mL. A single-cell clone expressing tau-venus (3B2) was isolated that displayed long-term stable expression of tau-venus. TRIM21 was disrupted by CrispR, using gRNA sequences T21-1 TCTTCTTCAGCCCTGGCACA or T21-4 ATGCTCACAGGCTCCACGAA expressed in LentiCRISPR v2, which was a gift from Feng Zhang (Addgene plasmid #52961). To generate lentiviral-expressed tau-venus, the construct was subcloned into HIV vector pSMPP2. Lentiviral particles were produced using HIV-1 GagPol expressor pcRV1, a gift from Stuart Neil, and VSV-G glycoprotein expressor pMD2.G, which was a gift from Didier Trono (Addgene plasmid #12259). Plasmids were cotransfected into HEK293T cells, using Fugene-6. After 3 d, supernatant was filtered at 0.45 µm and used to transduce SHSY-5Y cells. Cells were maintained in the presence of puromycin at 2.5 µg/mL before clonal selection and Western blotting for TRIM21, using antibody D-12 (Santa Cruz Biotechnology).
Preparation of P301S Tau Assemblies.
Recombinant human P301S 0N4R tau was expressed as previously described (38). Bacterial pellets were collected by centrifugation at 5,000 × g for 10 min. Lysates were prepared in 25 mM Tris⋅HCl at pH 7.4, 10 mM EDTA, 0.1 mM DTT, and 0.1 mM phenylmethanesulfonyl fluoride, using a cell disruptor (at a pressure of 25,000 psi), and cleared by centrifugation at 18,000 × g for 20 min. Clarified lysate was passed through a DE52 anion exchange column, followed by a phosphocellulose cation exchange column. Tau fractions were eluted with 500 mM NaCl, precipitated using 25% (mass/vol) ammonium sulfate, and run on a Superdex 200 HiLoad 16/60 column. The resulting fractions were passed over a Mono S 50/50 GL cation exchange column and eluted with a 50–275 mM NaCl gradient. Fractions were then pooled and dialyzed against 40 mM Hepes at pH 7.4, containing 0.1 mM DTT. Aliquots of recombinant tau were snap-frozen and stored at −20 °C. Where monomeric tau was used, purified recombinant tau was centrifuged at 100,000 × g at 4 °C for 1 h, and the supernatant was collected. Assemblies were prepared by addition of heparin as described (39), using tau at 60 µM in the presence of 400 µg/mL heparin (Sigma Aldrich) in 30 mM Mops at 37 °C for 3 d. Tau-venus assemblies were purified from HEK293 cells that had previously been subjected to seeding by heparin-assembled tau, as below. Seeded cells were homogenized with a Dounce homogenizer in H buffer [10 mM Tris at pH 7.4, 1 mM EGTA, 0.8 M NaCl, 10% sucrose, protease inhibitors (Roche) and phosphatase inhibitors (Roche)]. Homogenates were spun at 27,000 × g, and the pellet was re-extracted with H buffer and respun. Supernatants were combined and incubated with 1% sarkosyl for 1 h before centrifugation at 150,000 × g over a 20% (mass/vol) sucrose cushion. The pellet was resuspended in PBS and centrifuged at 10,000 × g for 10 min before storage of the supernatant at −80 °C.
Preparation of Sarkosyl-Insoluble Fractions.
Sarkosyl-insoluble tau was prepared from HEK293 cells and AD brain, as described (40). We used temporal cortex tissue from a 74-y-old female patient who had died with a histologically confirmed diagnosis of AD. By immunoelectron microscopy, the sarkosyl insoluble fraction contained numerous paired helical and straight tau filaments. By Western blotting with BR134, pathological tau bands of 60, 64, 68, and 72 kDa were present.
Seeding Assays.
HEK293 P301S tau-venus cells were plated at 10,000 cells per well in black 96-well plates pretreated with poly D-lysine, and allowed to adhere overnight. Cells were washed with PBS, and 50-µL recombinant tau assemblies, diluted in OptiMEM (Life Technologies), were added. LF, diluted in 50 µL OptiMEM, was subsequently added to each well at a concentration of 0.5 µL per well unless indicated otherwise. After 2 h, 100 µL C-DMEM was added to each well to stop the transfection process. Cells were incubated at 37 °C until time of harvest, typically 48 h after addition of fibrils, unless indicated otherwise. For neutralization assays, tau assemblies at 1 µM total tau were preincubated with antibody (at 1 µM or as indicated) or PBS for 1 h before dilution in OptiMEM and addition to cells as earlier. Where indicated, the concentrations of tau and antibody reflect the concentrations in the wells during the transfection period. A similar paradigm was used for seeding in SHSY-5Y cells expressing tau-venus, except that tau assemblies were coincubated with antibody in C-DMEM before addition to cells. Tau assemblies were added to cells overnight in the absence of LF or for 3 h in the presence of 0.5 µL LF per well before replacing supernatant with fresh C-DMEM. Cells were fixed at 3 d (in the absence of LF) or 24 h (in the presence of LF). For seeding experiments in the presence of inhibitors, DBeQ (5 µM), ML240 (2 µM), and MG132 (10 µM) or equivalent concentrations of DMSO in C-DMEM were added to cells for 1 h before seeding. Cells were treated with fibrils and LF for 2 h as earlier, without inhibitors. After 2h, C-DMEM containing inhibitors was added back to cells. Cells were fixed after 16 h incubation at 37 °C to limit any toxic effects of the inhibitors. VCP depletion was performed using siRNAs J-008727–11 (Thermo Fisher Scientific) and Ambion Negative Control No. 1 siRNA (Thermo Fisher Scientific) and Lipofectamine RNAiMAX (Life Technologies) in 24-well plates, using the manufacturer’s reverse transfection protocol. The next day, cells were counted and replated into 96-well plates for seeding assays as earlier. VCP knockdown was verified by immunoblot with 1:500 dilution of anti-VCP ab11433 (Abcam) and chemiluminescent detection (ECL Plus; Thermo Fisher Scientific). β-actin was detected using antibody sc47778 (Santa Cruz) at 1:5,000. Excess mouse IgG was used at 2 mg/mL, and anti-CD16/CD32 16–0161 (Affymetrix) was used at 1 in 50 dilution in culture supernatant during the seeding reaction.
Image Analysis.
Cells from seeding assays were fixed in 4% (mass/vol) paraformaldehyde (Sigma Aldrich) and stained with Hoechst 33342 at 1 µg/mL in PBS. Images were taken using a 10× objective lens on a Nikon Ti-E inverted fluorescence microscope, using automated x,y positioning and autofocus. Cells were identified using thickened regions of interest surrounding nuclei, and tau-venus aggregates were detected using local contrast filters. Threshold levels for detection of aggregates were adjusted using mock-seeded images for each experiment. Levels of seeding were calculated as (cells containing aggregates)/(total cells) × 100 for individual fields. All analysis was performed using NIS Elements 4.30 (Nikon).
Fluorescent Tau Uptake Assay.
Recombinant tau assemblies were labeled with Alexa488 (Life Technologies), according to the manufacturer’s protocol, and purified by ultracentrifugation. Assemblies were incubated with antibody, as in seeding/neutralization assays, and permitted to bind to HEK293 cells in OptiMEM at 37 °C or at 4 °C for 1 h. To maintain physiological pH outside the CO2 environment, 20 mM Hepes was added to media. Cells were washed twice with PBS, harvested by scraping, and mean fluorescence intensity was measured using a BD LSR II Flow Cytometer (Beckman Coulter).
Confocal Microscopy.
HEK293 cells were plated onto poly D-lysine treated coverslips (Fisher Scientific). Cells were fixed using 4% paraformaldehyde. Recombinant tau assemblies were introduced to the cell using LF as in seeding assays, except that tau seed and antibody concentration was 3× greater, and transfection was allowed to proceed without addition of C-DMEM. For immunofluorescence, cells were permeabilized using 0.1% saponin and blocked in 5% (mass/vol) BSA in PBS with 0.1% Tween 20. mycTRIM21 was detected using rabbit anti-myc A-14 (Santa Cruz). Aggregates in the absence of antibody were stained with 5A6 after fixation. GFAP was detected using mouse antibody 1B4 (BD Biosciences). Secondary detection was via Alexa Fluor conjugated secondary antibodies (Life Technologies).
RT-qPCR.
Cellular RNA was prepared from 106 cells, using the RNeasy Mini Kit (Qiagen). cDNA was synthesized using an oligo d(T) primer and avian myeloblastosis virus reverse transcriptase (HT Biotechnology). Transcripts were quantified using a StepOnePlus Realtime PCR System (ABI), with the following TaqMan primer/probes: FCGR1B, Hs00417598; FCGR2A, Hs01017702; FCGR3A, Hs04188274; FCGR3B, Hs04334165; TRIM21, Hs00172616; and ACTB, Hs03023880.
Preparation of Primary Mouse Cells.
WT and TRIM21−/− (41) C57BL/6 embryos were harvested at E18, and cortical neurons were prepared according published protocols (42). Cytosine β-D-arabinofuranoside (araC) was added to cells at 3 d postharvest for 3 d to kill dividing cells. Neurons were matured in vitro for 2 wk before experiments. Where added, 100 IU/mL IFN was added to culture supernatants 16 h before infection with adenovirus.
Adenovirus Infection Experiments.
E1E3-deleted adenovirus vectors bearing GFP or mCherry were obtained from ViraQuest. Adenovirus infection and neutralization experiments were performed using anti-hexon mouse IgG1 9C12 as before (2).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.E. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607215114/-/DCSupplemental.
References
- 1.Mallery DL, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci USA. 2010;107(46):19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwan WA, et al. Regulation of virus neutralization and the persistent fraction by TRIM21. J Virol. 2012;86(16):8482–8491. doi: 10.1128/JVI.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwan WA, et al. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013;14(4):327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci USA. 2007;104(15):6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauler F, Mallery DL, McEwan WA, Bidgood SR, James LC. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc Natl Acad Sci USA. 2012;109(48):19733–19738. doi: 10.1073/pnas.1210659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goedert M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 7.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284(19):12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo JL, Lee VM-Y. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286(17):15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 11.Watkinson RE, McEwan WA, Tam JCH, Vaysburd M, James LC. TRIM21 promotes cGAS and RIG-I sensing of viral genomes during infection by antibody-opsonized virus. PLoS Pathog. 2015;11(10):e1005253. doi: 10.1371/journal.ppat.1005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan W, et al. Swine TRIM21 restricts FMDV infection via an intracellular neutralization mechanism. Antiviral Res. 2016;127:32–40. doi: 10.1016/j.antiviral.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Kondo A, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523(7561):431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, et al. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 1996;384(1):25–30. doi: 10.1016/0014-5793(96)00271-2. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38delta or JNK2 in the presence of heparin generates the AT100 epitope. J Neurochem. 2006;99(1):154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson GV, et al. The tau protein in human cerebrospinal fluid in Alzheimer’s disease consists of proteolytically derived fragments. J Neurochem. 1997;68(1):430–433. doi: 10.1046/j.1471-4159.1997.68010430.x. [DOI] [PubMed] [Google Scholar]
- 19.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 20.Yanamandra K, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80(2):402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray N. Neurodegenerative diseases: A tale of two taus in traumatic brain injury. Nat Rev Drug Discov. 2015;14(9):599. doi: 10.1038/nrd4713. [DOI] [PubMed] [Google Scholar]
- 22.Falcon B, et al. Conformation determines the seeding potencies of native and recombinant Tau aggregates. J Biol Chem. 2015;290(2):1049–1065. doi: 10.1074/jbc.M114.589309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwan WA, James LC. TRIM21-dependent intracellular antibody neutralization of virus infection. Prog Mol Biol Transl Sci. 2015;129:167–187. doi: 10.1016/bs.pmbts.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Congdon EE, Gu J, Sait HBR, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013;288(49):35452–35465. doi: 10.1074/jbc.M113.491001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu J, Congdon EE, Sigurdsson EM. Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J Biol Chem. 2013;288(46):33081–33095. doi: 10.1074/jbc.M113.494922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S-H, et al. Antibody-mediated targeting of tau in vivo does not require effector function and microglial engagement. Cell Reports. 2016;16(6):1690–1700. doi: 10.1016/j.celrep.2016.06.099. [DOI] [PubMed] [Google Scholar]
- 27.Chou T-F, et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc Natl Acad Sci USA. 2011;108(12):4834–4839. doi: 10.1073/pnas.1015312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou T-F, Li K, Frankowski KJ, Schoenen FJ, Deshaies RJ. Structure-activity relationship study reveals ML240 and ML241 as potent and selective inhibitors of p97 ATPase. ChemMedChem. 2013;8(2):297–312. doi: 10.1002/cmdc.201200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2011;8(2):108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- 30.Myeku N, et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med. 2016;22(1):46–53. doi: 10.1038/nm.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson SJ, et al. Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. J Neurosci. 2016;36(3):762–772. doi: 10.1523/JNEUROSCI.3542-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barthelme D, Sauer RT. Origin and functional evolution of the Cdc48/p97/VCP AAA+ protein unfolding and remodeling machine. J Mol Biol. 2016;428(9 Pt B):1861–1869. doi: 10.1016/j.jmb.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai X, et al. Passive immunization with anti-Tau antibodies in two transgenic models: Reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286(39):34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sankaranarayanan S, et al. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One. 2015;10(5):e0125614. doi: 10.1371/journal.pone.0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends Mol Med. 2015;21(6):394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Funk KE, Mirbaha H, Jiang H, Holtzman DM, Diamond MI. Distinct therapeutic mechanisms of tau antibodies: Promoting microglial clearance versus blocking neuronal uptake. J Biol Chem. 2015;290(35):21652–21662. doi: 10.1074/jbc.M115.657924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: Correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9(13):4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goedert M, et al. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383(6600):550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 40.Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8(1):159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimi R, Ishigatsubo Y, Ozato K. Autoantigen TRIM21/Ro52 as a possible target for treatment of systemic lupus erythematosus. Int J Rheumatol. 2012;2012:718237. doi: 10.1155/2012/718237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaudoin GMJ, 3rd, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7(9):1741–1754. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 43.Varghese R, Mikyas Y, Stewart PL, Ralston R. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J Virol. 2004;78(22):12320–12332. doi: 10.1128/JVI.78.22.12320-12332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mercken M, et al. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol. 1992;84(3):265–272. doi: 10.1007/BF00227819. [DOI] [PubMed] [Google Scholar]