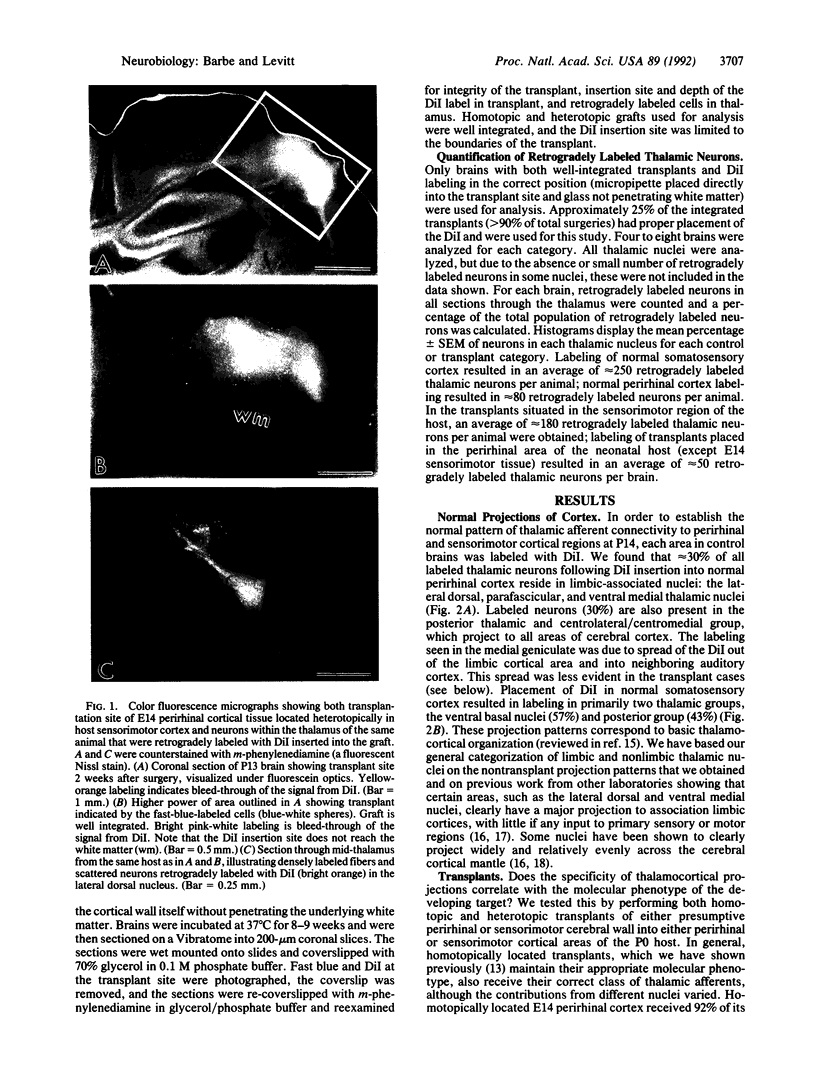
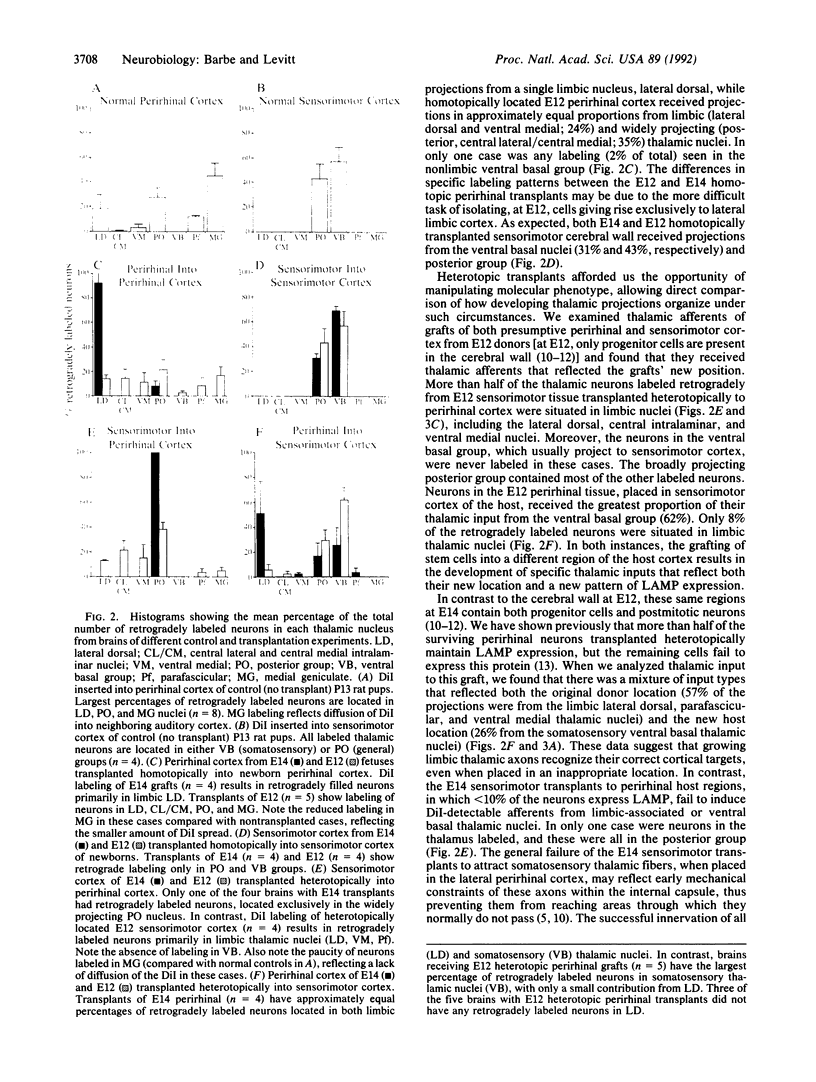
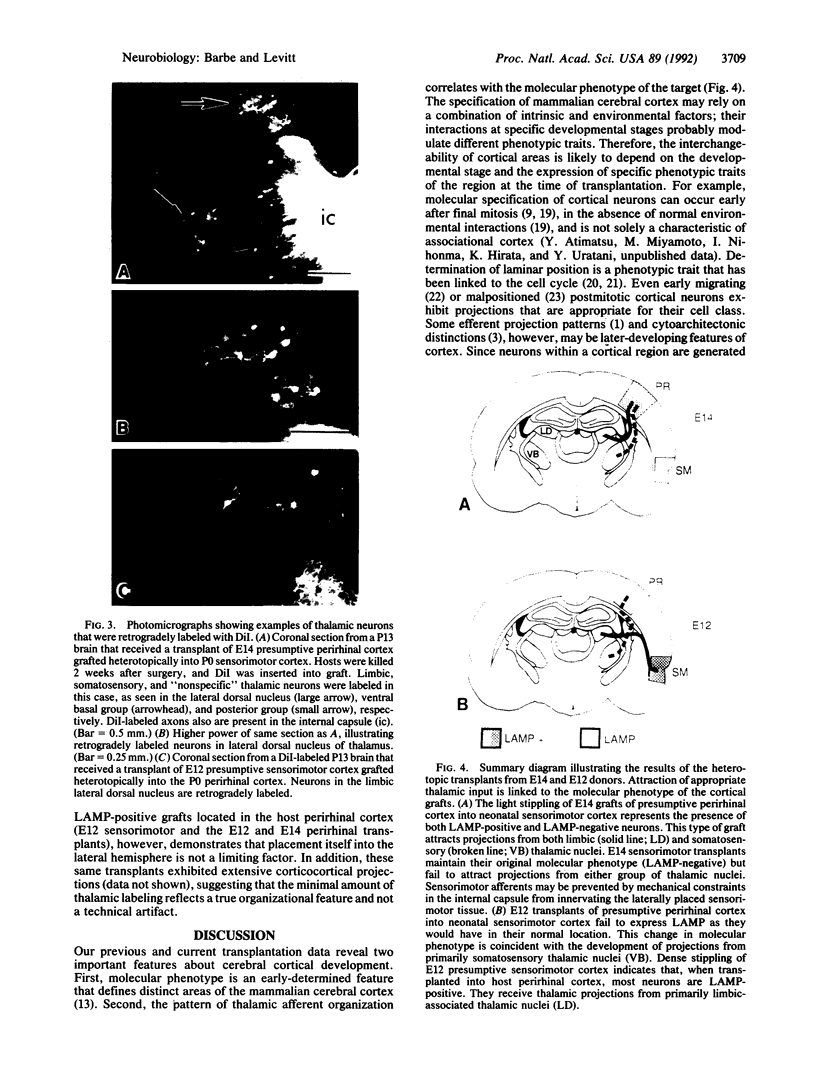
Abstract
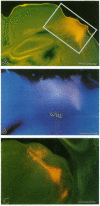
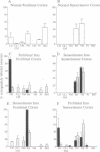
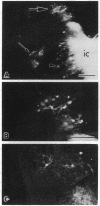
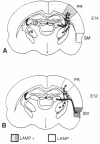
The cerebral cortex of mammals differentiates into functionally distinct areas that exhibit unique cytoarchitecture, connectivity, and molecular characteristics. Molecular specification of cells fated for limbic cortical areas, based on the expression of the limbic system-associated membrane protein (LAMP), occurs during an early period of brain development. The correlation between this early molecular commitment and formation of specific thalamocortical connections was tested by using a transplantation paradigm. We manipulated the phenotype of donor limbic and sensorimotor neurons by placing them in different cortical areas of host animals. Labeling of transplanted tissue with the lipophilic dye 1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine was used to assay host thalamic neurons projecting to the donor tissue. We found that limbic thalamic axons successfully projected into cortical transplants (i) when LAMP was expressed by early committed limbic cortical neurons, irrespective of their host location, and (ii) when LAMP was expressed by uncommitted sensorimotor progenitor cells whose fate was altered by their new host locale. Thus, the response of cortical neurons to both intrinsic and environmental cues that influence their molecular phenotype has an important anatomical correlate, the development of specific patterns of thalamocortical connectivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbe M. F., Levitt P. The early commitment of fetal neurons to the limbic cortex. J Neurosci. 1991 Feb;11(2):519–533. doi: 10.1523/JNEUROSCI.11-02-00519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Rogers A. W. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965 Oct;99(Pt 4):691–709. [PMC free article] [PubMed] [Google Scholar]
- Catalano S. M., Robertson R. T., Killackey H. P. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Antonini A., McConnell S. K., Shatz C. J. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990 Sep 13;347(6289):179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980 Feb 1;207(4430):532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Hicks S. P., D'Amato C. J. Cell migrations to the isocortex in the rat. Anat Rec. 1968 Mar;160(3):619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Horton H. L., Levitt P. A unique membrane protein is expressed on early developing limbic system axons and cortical targets. J Neurosci. 1988 Dec;8(12):4653–4661. doi: 10.1523/JNEUROSCI.08-12-04653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Subcortical projections from ectopic neocortical neurons. Proc Natl Acad Sci U S A. 1984 Feb;81(3):964–968. doi: 10.1073/pnas.81.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F., Rimvall K., Barbe M. F., Levitt P. A membrane glycoprotein associated with the limbic system mediates the formation of the septo-hippocampal pathway in vitro. Neuron. 1989 Nov;3(5):551–561. doi: 10.1016/0896-6273(89)90265-1. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977 Jan 15;171(2):157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Levitt P. A monoclonal antibody to limbic system neurons. Science. 1984 Jan 20;223(4633):299–301. doi: 10.1126/science.6199842. [DOI] [PubMed] [Google Scholar]
- McConnell S. K., Kaznowski C. E. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991 Oct 11;254(5029):282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Miller M. W. Effects of prenatal exposure to ethanol on neocortical development: II. Cell proliferation in the ventricular and subventricular zones of the rat. J Comp Neurol. 1989 Sep 15;287(3):326–338. doi: 10.1002/cne.902870305. [DOI] [PubMed] [Google Scholar]
- Molnár Z., Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991 Jun 6;351(6326):475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- O'Leary D. D. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989 Oct;12(10):400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- O'Leary D. D., Stanfield B. B. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: experiments utilizing fetal cortical transplants. J Neurosci. 1989 Jul;9(7):2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P., Suñer I., Williams R. W. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., O'Leary D. D. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991 Jun 14;252(5012):1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Rakic P., Goldman-Rakic P. S. Early phenotype expression of cortical neurons: evidence that a subclass of migrating neurons have callosal axons. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1354–1358. doi: 10.1073/pnas.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zacco A., Cooper V., Chantler P. D., Fisher-Hyland S., Horton H. L., Levitt P. Isolation, biochemical characterization and ultrastructural analysis of the limbic system-associated membrane protein (LAMP), a protein expressed by neurons comprising functional neural circuits. J Neurosci. 1990 Jan;10(1):73–90. doi: 10.1523/JNEUROSCI.10-01-00073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]