Significance
Dysregulation of both vascular architecture and function is a hallmark of numerous diseases, including cancer. This dysregulation is currently largely attributed to up-regulated proangiogenic growth factors. Here, we show that the stiffness of the underlying extracellular matrix also plays a central role in promoting angiogenesis and a characteristic tumor-like vasculature both in vitro and in vivo. The matrix stiffness-mediated angiogenesis is dependent on increased matrix metalloprotease activity. In addition, increased matrix cross-linking disrupts endothelial cell–cell junctional integrity and results in leakier vasculature. These results suggest that altered tissue mechanics, which are characteristic of solid tumors, directly influence vascular phenotype and, subsequently, may impair therapeutic delivery and efficacy.
Keywords: tumor stiffness, endothelial cells, vascular permeability, glycation, extracellular matrix
Abstract
Tumor microvasculature tends to be malformed, more permeable, and more tortuous than vessels in healthy tissue, effects that have been largely attributed to up-regulated VEGF expression. However, tumor tissue tends to stiffen during solid tumor progression, and tissue stiffness is known to alter cell behaviors including proliferation, migration, and cell–cell adhesion, which are all requisite for angiogenesis. Using in vitro, in vivo, and ex ovo models, we investigated the effects of matrix stiffness on vessel growth and integrity during angiogenesis. Our data indicate that angiogenic outgrowth, invasion, and neovessel branching increase with matrix cross-linking. These effects are caused by increased matrix stiffness independent of matrix density, because increased matrix density results in decreased angiogenesis. Notably, matrix stiffness up-regulates matrix metalloproteinase (MMP) activity, and inhibiting MMPs significantly reduces angiogenic outgrowth in stiffer cross-linked gels. To investigate the functional significance of altered endothelial cell behavior in response to matrix stiffness, we measured endothelial cell barrier function on substrates mimicking the stiffness of healthy and tumor tissue. Our data indicate that barrier function is impaired and the localization of vascular endothelial cadherin is altered as function of matrix stiffness. These results demonstrate that matrix stiffness, separately from matrix density, can alter vascular growth and integrity, mimicking the changes that exist in tumor vasculature. These data suggest that therapeutically targeting tumor stiffness or the endothelial cell response to tumor stiffening may help restore vessel structure, minimize metastasis, and aid in drug delivery.
The ingrowth of newly sprouted blood vessels is necessary for solid tumor growth, and tumor vasculature is typically malformed, leakier, and more tortuous than the vasculature of normal tissues (1–3). Generally, aberrant tumor vasculature is considered to be caused by up-regulated VEGF expression resulting in chaotic vascular growth and failure to establish mature, well-regulated networks (4, 5). Here, we propose a different hypothesis, namely that extracellular matrix (ECM) mechanical properties also contribute to the aberrant vascular phenotype seen in tumors.
Solid tumor tissue is typically stiffer than native, healthy tissue (1, 6). Increased ECM stiffness within tumors is caused primarily by both increased collagen deposition and increased cross-linking within the tumor stroma (7). Increased ECM density and cross-linking are associated with poor prognosis in a number of cancers (8, 9). Many studies have investigated the role of matrix density on angiogenesis and, in both collagen and fibrin matrices, have shown that angiogenesis decreases with increasing matrix concentration (10–13). Increased matrix density appears to act as a physical barrier that restricts cell migration, and cells rely on matrix metalloproteinases (MMPs) to overcome that barrier (14, 15). Indeed, evidence points to an important role of MMP regulation in efficient angiogenesis (16, 17). Most notably, membrane-type matrix metalloproteinase 1 (MT1-MMP) appears to play a central role in regulating tumor-associated angiogenesis and vascular function (18). However, within the tumor microenvironment, ECM stiffness can increase independently of collagen density through cross-linking enzymes (7). Cross-linking can result in increased matrix stiffness without changing the ECM architecture (19). Recent work has shown that endothelial cells (ECs) are mechanosensitive to changes in matrix stiffness (20, 21), but matrix stiffening in the tumor microenvironment affects tumor angiogenesis remains less clear.
In this study, we examine the effects of collagen cross-linking and the resulting increase in matrix stiffness on the growth and integrity of angiogenic vessels. Using in vitro, in vivo, and ex ovo models, we show that increasing the extent of collagen cross-linking leads to significantly more vessel outgrowth and branching. We further show that matrix stiffness plays an important role in vessel permeability and endothelial cell–cell junctional integrity. Together, our results demonstrate that matrix cross-linking modulates the growth, structure, and integrity of neo-vessels and suggest that the phenotype of tumor vasculature is mediated in part by collagen cross-linking.
Results
Collagen Cross-Linking and Collagen Density Modulate the Mechanical Properties and Fiber Arrangements of Collagen Gels.
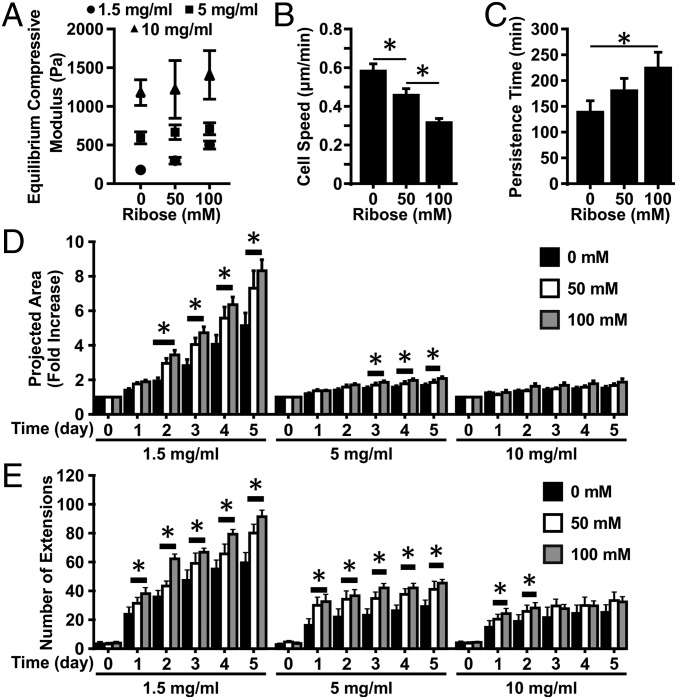
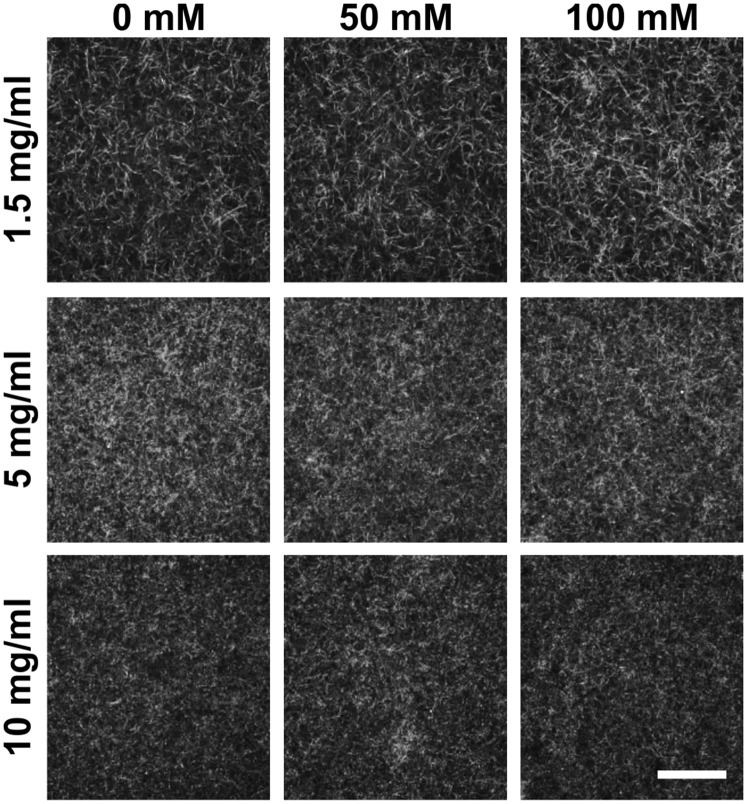
To establish an in vitro model in which collagen stiffness can be modulated, the individual and combined effects of collagen cross-linking and collagen density on the mechanical and structural properties of collagen gels were studied. Cross-linking of collagen was done through nonenzymatic glycation to form advanced glycation end product (AGE) cross-links (19), followed by confined compression testing to characterize the mechanical properties of the collagen gels. Increasing the density of the collagen gels from 1.5 to 10 mg/mL increases the equilibrium compressive modulus approximately sixfold, from ∼180 to ∼1,200 Pa (Fig. 1A). Within a given density, increasing the extent of glycation from 0 to 100 mM also increases the modulus of the gels from ∼180 to ∼500 Pa, ∼600 to ∼715 Pa, and ∼1,200 to ∼1,400 Pa for 1.5-, 5-, and 10-mg/mL collagen gels, respectively. To investigate the effects of collagen density and cross-linking on collagen gel fiber architecture further, the internal collagen fiber distributions were visualized with confocal reflectance microscopy. Increasing the density of the collagen visibly decreases the porosity and alters the size and distribution of fibers within the collagen gels (Fig. S1). However, collagen gels within a given density and glycated with 0, 50, or 100 mM ribose form gels with qualitatively similar fiber architectures. Taken together, these results indicate that nonenzymatic glycation can modulate the mechanical properties of collagen gels while only minimally altering the collagen fiber architecture.
Fig. 1.
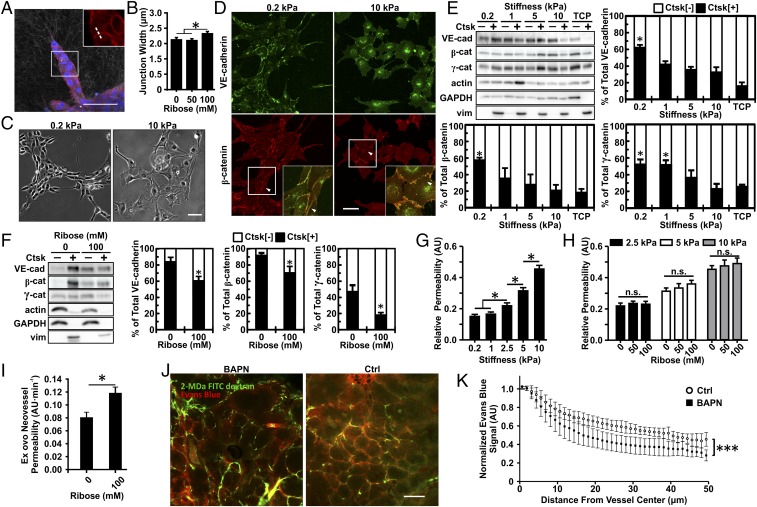
Matrix density and cross-linking alter collagen gel mechanical properties and the angiogenic sprouting response from multicellular spheroids. (A) Equilibrium compressive moduli of 1-, 5-, and 10-mg/mL collagen gel following cross-linking with 0, 50, or 100 mM ribose. (B and C) Quantification of migration speed (B) and persistence (C) for ECs embedded within 1.5-mg/mL collagen gels glycated with 0, 50, or 100 mM ribose. (D and E) The projected spheroid area (D) and the number of extensions (E) were measured over the course of 5 d. Data are presented as mean ± SEM; *P < 0.05.
Fig. S1.
Matrix density and cross-linking alter collagen fiber arrangement. Confocal reflectance microscopy images of 1-, 5-, and 10-mg/mL collagen gel following cross-linking with 0, 50, or 100 mM ribose. (Scale bar, 50 μm.)
Angiogenic Outgrowth and Branching Are Modulated by ECM Mechanical Properties.
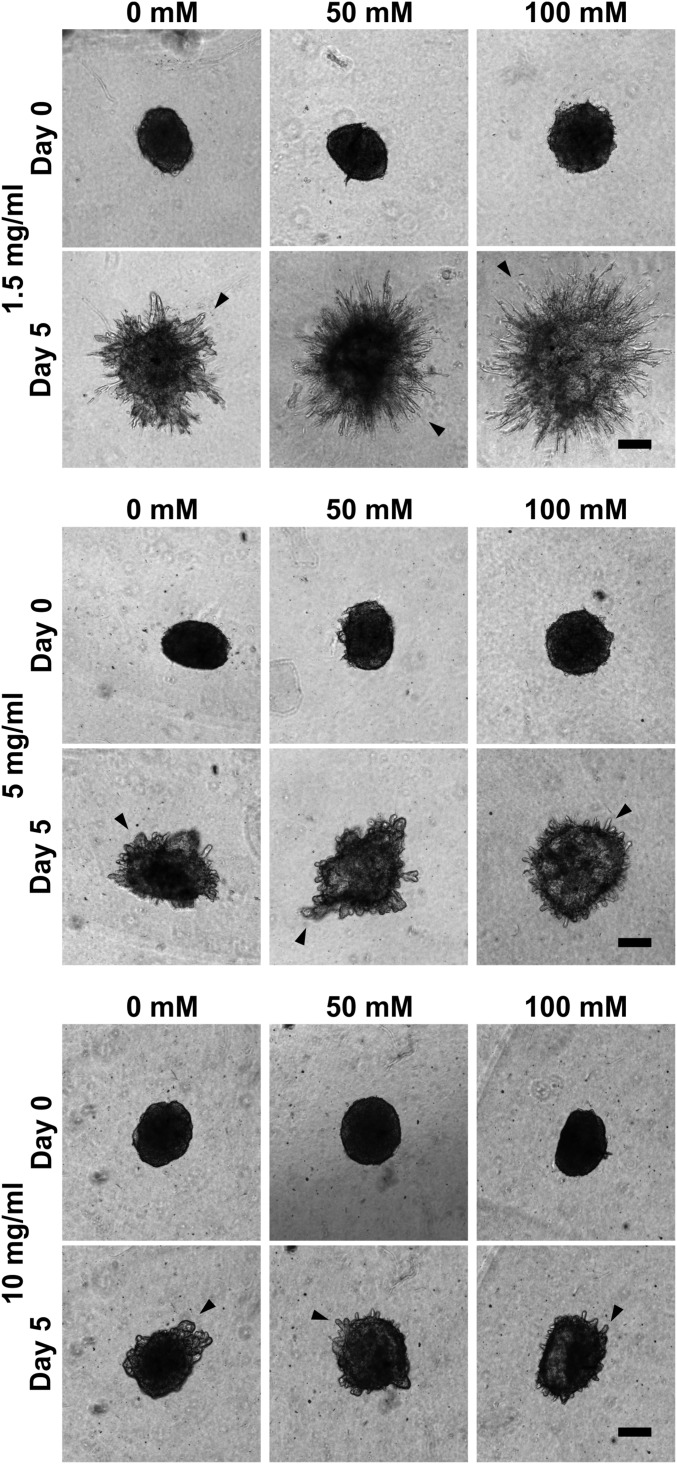
The effects of stiffening collagen gels via either cross-linking through glycation or increased density on EC migration and outgrowth were investigated. Interestingly, single-cell 3D migration speed decreased and the persistence time increased as the stiffness of the gels increased (Fig. 1 B and C). Increasing the density of the collagen matrix decreased the overall outgrowth of angiogenic sprouts from the spheroids (Fig. S2), as has been shown previously (11). Importantly, however, at all densities, increasing the stiffness of the collagen via glycation increased the outgrowth response from the spheroids. EC spheroids invaded significantly farther in collagen gels glycated with 100 mM ribose than with 0 mM ribose beginning at days 1 and 3 for 1.5-mg/mL and 5-mg/mL collagen densities, respectively (Fig. 1D). This trend is observed across all collagen densities, although it is not statistically significant at 10 mg/mL. However, there were significantly more angiogenic sprouts in glycated gels at all densities (Fig. 1E). These findings suggest that the effects of stiffness on EC sprouting are dependent on the mechanism of stiffening, i.e., cross-linking or increased density.
Fig. S2.
Matrix density and cross-linking alter the angiogenic sprouting response from multicellular spheroids. EC spheroids were embedded within 1.5-, 5-, or 10-mg/mL collagen gels glycated with 0, 50, or 100 mM ribose, and the angiogenic sprouting response (arrowheads) was monitored. (Scale bars, 200 μm.)
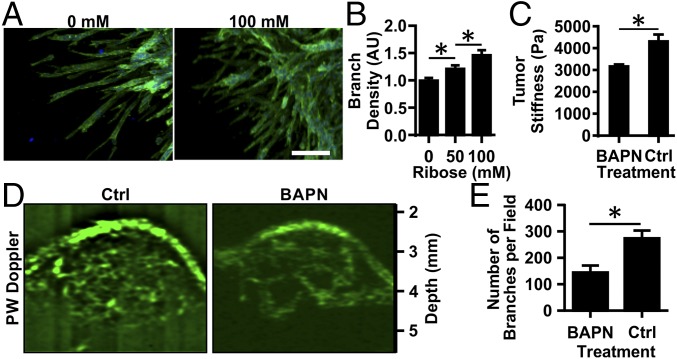
Notably, tumor angiogenesis is marked by increased branching and tortuosity (5). Our previous work indicates that matrix cross-linking alters the shape of individual cells (19), suggesting it also may alter the shape of assemblies of cells during angiogenesis. To analyze further the effects of matrix stiffness on the shape and structure of neovessels, the density of branching along vessels outgrowths was analyzed. Increasing the stiffness of the gels via nonenzymatic glycation increased the density of branching in angiogenic outgrowths from spheroids (Fig. 2A). Outgrowths from spheroids in stiffer gels (100 mM ribose) had an ∼1.5-fold increase in branching compared with those within more compliant (0 mM ribose) gels (Fig. 2B). These findings indicate that increasing matrix stiffness via cross-linking not only causes increased outgrowth from spheroids but also changes the morphology of the angiogenic sprouts that form, mimicking the changes occurring in the tumor microenvironment.
Fig. 2.
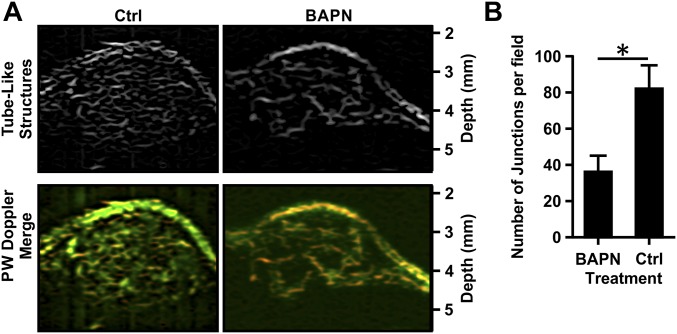
Matrix cross-linking alters angiogenic branching in vitro and in vivo. EC multicellular spheroids were embedded within 1.5-mg/mL collagen gels glycated with 0, 50, or 100 mM ribose. (A) Spheroids were fixed, stained for actin (green) and nuclei (blue), and imaged using confocal microscopy after 5 d. (Scale bar, 100 μm.) (B) The number of branches per sprout length was counted, and data were normalized to the 0-mM ribose condition. AU, arbitrary units. (C) MMTV-PyMT mice were treated with BAPN to prevent collagen cross-linking or with vehicle (controls; Ctrl), and the equilibrium compressive moduli were measured using unconfined compression testing. (D) The tumor vasculature was visualized using ultrasound. (E) The number of visible vascular branches was quantified using the ImageJ Tubeness plugin. Data are presented as mean + SEM; *P < 0.05.
Given our in vitro findings, we speculated that tumor angiogenesis is mediated in part by the matrix cross-linking that occurs in the tumor microenvironment. To investigate the relationship between tissue stiffness and tumor angiogenesis, we extended our analysis to the MMTV-PyMT mouse tumor model (6, 22). To modulate the stiffness of the spontaneous mammary tumors that develop in these mice, we used β-aminopropionitrile (BAPN), an inhibitor of the matrix cross-linking enzyme lysyl oxidase (LOX) involved in tumor stiffening (Fig. 2C) (6, 23). BAPN is a commonly used model to interrogate the mechanical properties of the ECM in tumor physiology and enables the examination of the effects of matrix cross-linking on angiogenesis when cross-linking is modulated by inhibition of LOX (6, 22). Ultrasound power wave Doppler was used to image the extensive vasculature within PyMT mouse tumors (Fig. 2D, Fig. S3A and Movie S1). The vasculature in BAPN-treated mice, in which tumor stiffness is decreased, was far less prominent (Fig. 2D, Fig. S3A, and Movie S2). Quantification of the vascular network indicates that the number of branches and junctional nodes within the vascular network is significantly reduced in mice treated with BAPN compared with the vehicle control treatment (Fig. 2E and Fig. S3B). Overall, reducing tumor stiffness by decreasing collagen cross-linking with BAPN reduced the extent of angiogenesis within the tumor.
Fig. S3.
Vasculature quantification using ImageJ skeletonization. (A) To quantify the tumor vasculature, ultrasound images obtained in the power wave (PW) Doppler mode (shown in the merged overlay) were processed using the ImageJ Tubeness plugin to obtain the tube-like structures for MMTV-PyMT mice treated with BAPN or vehicle (Ctrl). (B) Corresponding quantification of the number of junctional nodes, including the starting and ending nodes of each branch network, present within the vascular network within the field of view. Data are presented as mean + SEM; *P < 0.05.
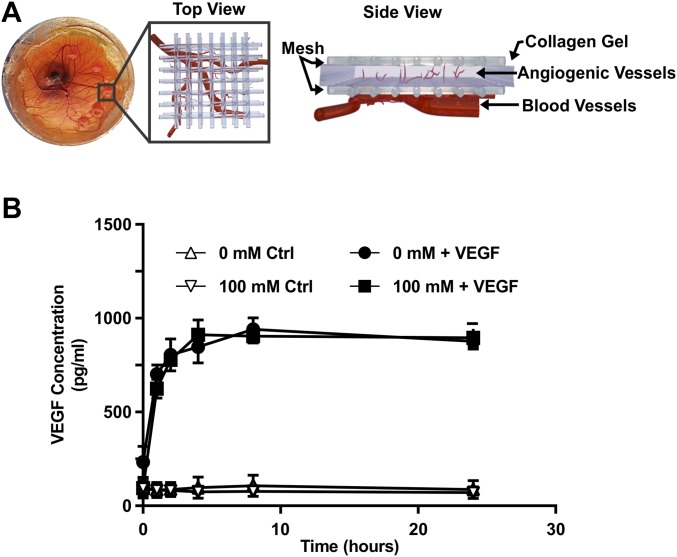
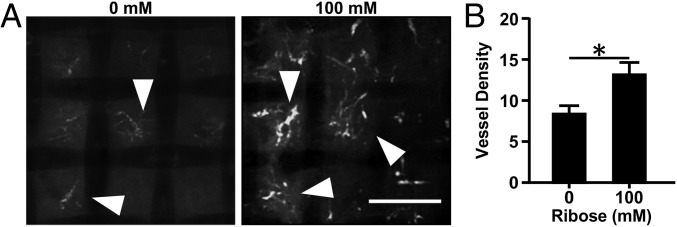
Given our in vivo results showing increased angiogenesis in cross-linked matrices, we extended our analysis to an ex ovo chick chorioallantoic membrane (CAM) angiogenesis assay. Collagen implants containing 5 μg/mL VEGF were placed on the distal region of the CAM on embryonic day (E) 10 (Fig. S4A). A VEGF ELISA was used to examine the release profile of VEGF to confirm that VEGF release is not affected by the stiffness of the gels (Fig. S4B). On E15, vessels within the collagen gels were imaged using confocal microscopy and appeared as thin fluorescent sprouts (Fig. 3A, arrowheads). Stiffer collagen gels (100 mM ribose) promoted significantly more angiogenesis than the more compliant (0 mM ribose) gels (Fig. 3B). These data support our in vitro and in vivo findings that matrix stiffness resulting from cross-linking enhances angiogenesis.
Fig. S4.
Collagen gel construct for the chick CAM model. (A) Representative picture of the collagen gel–nylon mesh constructs placed on the chick CAM along with schematic views (top and side) of the angiogenic ingrowth into the collagen gels from the vasculature underlying the CAM. (B) ELISA quantification of the VEGF released from collagen construct glycated with 0 or 100 mM ribose over a 24-h time course.
Fig. 3.
Matrix cross-linking alters angiogenic sprouting into collagen gels in the chick CAM model. Angiogenic sprouting (arrowheads) into 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose were imaged with confocal microscopy (A), and the vessel density per gel was quantified (B). (Scale bar, 200 μm.) Data are presented as mean + SEM; *P < 0.05.
Stiffness-Mediated Angiogenic Outgrowth Requires MMP Activity.
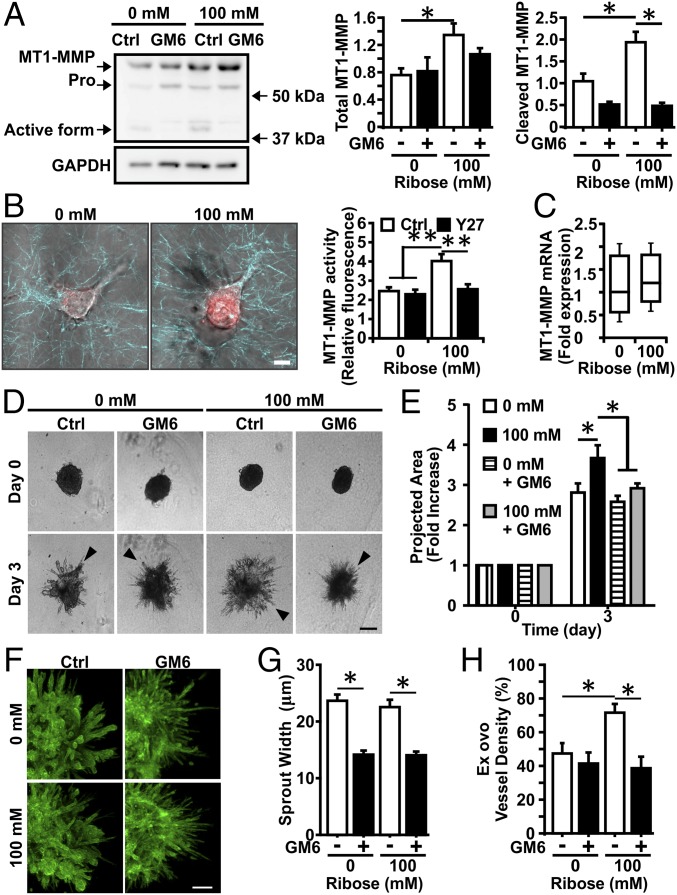
Because prior work suggests that MMP activity plays a key role in cell migration in response to increased matrix density (11, 15), we hypothesized it may serve a similar function in mediating angiogenesis in response to increased matrix cross-linking. Toward that end, we assayed for the expression and activity of the MT1-MMP in ECs in 3D cell culture because of its role in regulating tumor vasculature (18). Both MT1-MMP expression and activity, as revealed by the presence of the cleaved fragment, were increased in the stiffer gels (100 mM ribose) compared with the more compliant gels (0 mM ribose) (Fig. 4A). Treatment with the MMP inhibitor GM6001 blocked MT1-MMP cleavage in both conditions. Using a specific MT1-MMP activity probe, we confirmed that MT1-MMP activity was indeed increased in stiffer gels (Fig. 4B). Similar to observations in epithelial cells (24), the stiffness-mediated increase in EC MT1-MMP activity is dependent on Rho-associated protein kinase (ROCK)-mediated cell contractility (Fig. 4B). However, we did not observe any significant changes in MT1-MMP mRNA content in the gels of different stiffness (Fig. 4C) or any significant differences in content in vivo (Fig. S5). We then examined spheroid outgrowth when MMPs were inhibited using GM6001. Spheroids in stiffer gels (100 mM ribose) had significantly less angiogenic outgrowth in the presence of 5 μM GM6001 than did untreated control cells in the same matrix (Fig. 4 B and C); the spheroid outgrowth was similar within the more compliant gels (0 mM ribose) with or without GM6001 treatment. In all conditions, the morphologies of angiogenic sprouts were altered with GM6001 treatment (Fig. 4D). Sprouts in compliant and stiff control gels (0 and 100 mM ribose) had similar morphologies, whereas sprouts from spheroids cultured with GM6001 were much thinner than controls (Fig. 4E). Moreover, stiffness-mediated angiogenic outgrowth in the CAM angiogenesis assay was prevented upon MMP inhibition (Fig. 4F). These data suggest that MMPs play an important role in promoting the increased angiogenesis observed in stiffer matrices.
Fig. 4.
Stiffness-mediated angiogenic outgrowth requires MMP activity. (A, Left) Western blot for MT1-MMP in ECs embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose and fed with complete medium with (GM6) or without (Ctrl) 5 μM GM6001. (Right) The corresponding densitometric quantification normalized to actin content. GAPDH was used as loading control. (B, Left) Confocal images showing MT1-MMP activity in ECs embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose. (Right) The corresponding quantification with (Y27) or without (Ctrl) 10 μM of the ROCK inhibitor Y27632. (Scale bar, 10 μm.) (C) MT1-MMP expression determined by quantitative real-time RT-PCR does not show expression differences as a function of increased stiffness. (D) EC multicellular spheroids were embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose and fed with complete medium with (GM6) or without (Ctrl) 5 μM GM6001. (Scale bar, 200 μm.) (E) Spheroid outgrowth was quantified after 3 d of culture and normalized to the day 0 condition. (F) Spheroids were stained for actin (green) and nuclei (blue) and were imaged with confocal microscopy. (Scale bar, 100 μm.) (G) The width of angiogenic sprouts was measured by fitting the intensity profile of a line drawn perpendicular to the sprout with a two-Gaussian curve. (H) Quantification of vessel density into 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose during the CAM angiogenic sprouting assay with or without 5 μM GM6001. Data are presented as mean + SEM; *P < 0.05.
Fig. S5.
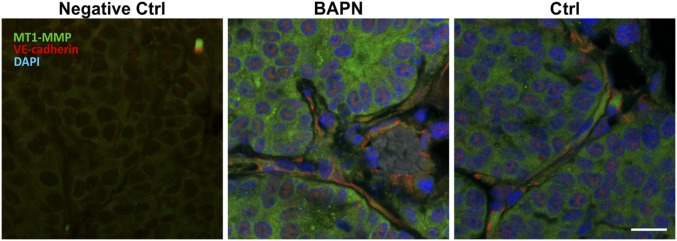
MT1-MMP expression in mammary tumors. Confocal images of MT1-MMP– and VE-cadherin–stained frozen tissue sections from mice treated with BAPN or vehicle controls (Ctrl). (Scale bar, 50 μm.)
Matrix Stiffness Influences the Localization of Vascular Endothelial Cadherin and Barrier Integrity.
We next sought to investigate the functional integrity of newly formed vessels as a function of matrix stiffness. Importantly, increased vessel permeability is a hallmark of tumor vasculature (2, 25). Our previous data indicate that increased matrix stiffness disrupts cell–cell junctions (20, 21, 26), suggesting that stiffness may result in impaired barrier integrity. Vascular endothelial cadherin (VE-cadherin) within EC–EC junctions in vascular sprouts was imaged as a function of stiffness (Fig. 5A). We found that the VE-cadherin junctions within the stiff (100 mM ribose) collagen were significantly wider than those within the more compliant (0 or 50 mM ribose) collagen gels (Fig. 5B).
Fig. 5.
Matrix stiffness alters VE-cadherin expression, junction width, and endothelial cell permeability. (A) EC spheroids were fixed 24 h after embedding within 1.5-mg/mL collagen gels glycated with 0, 50, or 100 mM ribose and were imaged with confocal microscopy to visualize VE-cadherin (red) and nuclei (blue) with confocal reflectance of the collagen fibers. The zoomed-in insert shows a representative region used to obtain the VE-cadherin junction width profiles from a line perpendicular to the junction (dotted line). (B) Corresponding quantification of the width of junctions between stalk cells of the sprouts. (C and D) Phase-contrast images showing ECs seeded on compliant (0.2 kPa) or stiff (10 kPa) PA substrates (C) along with the corresponding VE-cadherin and β-catenin localization at cell–cell junctions (arrowheads) showing a continuous distribution on compliant matrix and a punctate distribution on stiff matrix (D). Insets are magnifications of boxed regions. (E and F) Western blot and corresponding quantification of VE-cadherin, β-catenin, and γ-catenin content in the soluble fraction (Ctsk[−]) versus the cytoskeleton-associated insoluble fraction (Ctsk[+]) for ECs seeded on PA 2D substrate (E) or ECs embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose (F). Vimentin was used as the insoluble Ctsk[+] fraction control. TCP, tissue culture plastic. (G and H) Quantification of the EC monolayer permeability to 40-kDa FITC-dextran in response to matrix stiffness (G) and collagen glycation (H). (I) Quantification of the neovessel permeability in the CAM angiogenic sprouting assay in 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose. (J) Representative confocal images from MMTV-PyMT mice treated with BAPN or vehicle controls (Ctrl) showing 2-MDa FITC-dextran–labeled vasculature (green) and extravasating Evans blue (red). (K) Quantification of vessel permeability to Evans blue with BAPN or vehicle controls (Ctrl). (Scale bars, 50 μm.) Data are presented as mean ± SEM; *P < 0.05, ***P < 0.0001.
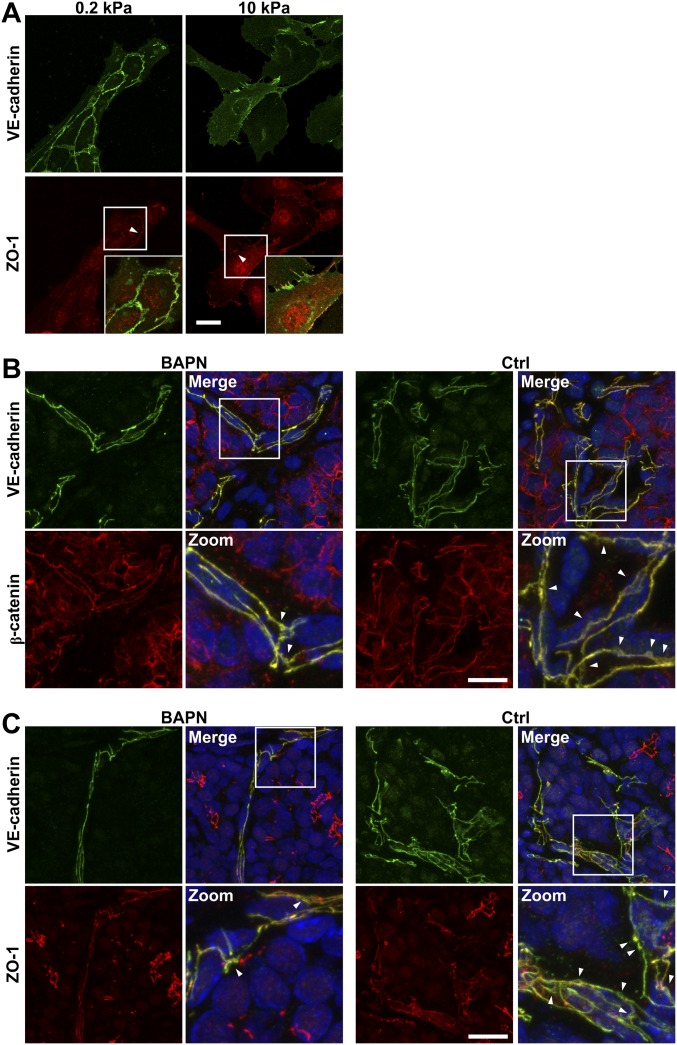
Our previous data suggest that subconfluent ECs on compliant substrates assemble spontaneously into networks reminiscent of the angiogenic process (27). Using this 2D platform, which is more amenable to imaging and manipulation than 3D cultures, we extended our analysis of VE-cadherin to EC cultured on compliant (0.2 kPa) or stiff (10 kPa) polyacrylamide (PA) substrates to visualize and characterize its localization better. Cells on compliant substrates formed VE-cadherin– and β-catenin–positive EC–EC junctions that were continuous between cells, whereas the junctions on stiff substrates were punctate (Fig. 5C). The tight junction protein zona occuldens 1 (ZO-1) shows stiffness-mediated localization that matches with VE-cadherin localization (Fig. S6A). In vivo staining of VE-cadherin, ZO-1, and β-catenin further revealed changes in junction architecture in stiffer tumors (Fig. S6 B and C). Cytoskeletal fractionation of cell lysate from ECs indicates that significantly greater proportions of total VE-cadherin, β-catenin, and γ-catenin content were found in the Triton-insoluble fraction in ECs plated on compliant gels than in ECs plated on the gels (Fig. 5D). Junction proteins of ECs embedded within glycated 3D collagen gels presented a similar stiffness-mediated insoluble/soluble dependence (Fig. 5E).
Fig. S6.
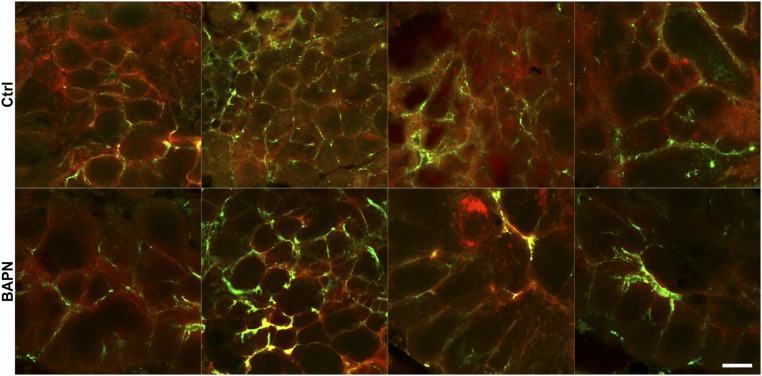
Matrix stiffness alters cell–cell junction organization. (A) Confocal images showing ECs seeded on compliant (0.2 kPa) or stiff (10 kPa) PA substrates stained for VE-cadherin and ZO-1 showing a continuous distribution on compliant matrix and a punctate distribution on stiff matrix. Insets are magnifications of the boxed regions. (Scale bars, 50 μm.) (B and C) Confocal images from MMTV-PyMT mice treated with BAPN or vehicle controls (Ctrl) showing increased diffuse staining (arrowheads) of VE-cadherin, β-catenin, and ZO-1 in stiffer tumor. Zoomed images are magnifications of the boxed regions. (Scale bars, 20 μm.)
We further proceeded to investigate the effects of matrix stiffness and altered VE-cadherin localization on ECs barrier integrity. Because AGEs are found in tumors (28, 29) and have been shown before to perturb EC barrier integrity (30), we proceeded to evaluate the permeability of EC monolayers on glycated collagen-coated PA gels of varying stiffness. Although increasing the matrix stiffness significantly increased the permeability of EC monolayers, the extent of collagen glycation had no significant effect (Fig. 5 F and G), suggesting that increased permeability is caused by matrix stiffness, not by EC interaction with AGEs. Moreover, we observed an increase in vessel permeability as a function of stiffness using the CAM angiogenesis assay (Fig. 5H). We then extended our analysis to the BAPN-treated MMTV-PyMT mouse tumor model, using an in vivo Evans blue extravasation assay to confirm our in vitro permeability findings. Notably, the higher matrix stiffness found in control mice disrupts EC barrier function of the tumor-associated vasculature to a greater extent than in the BAPN-treated mice (Fig. 5 I and J and Fig. S7). Of note, we did not observe any BAPN-mediated effects on EC permeability or mRNA expression in vitro, suggesting that BAPN does not affect EC integrity directly (Fig. S8). Together, these data indicate that the stiffness of the matrix modulates the integrity of VE-cadherin junctions, with stiff substrates preventing strong cell–cell adhesion and disrupting barrier integrity.
Fig. S7.
In vivo permeability in MMTV-PyMT mice. Confocal images from MMTV-PyMT mice treated with BAPN or vehicle controls (Ctrl) showing 2 MDa FITC-dextran–labeled vasculature (green) and extravasating Evans blue (red). Each image is from a different mouse. (Scale bar, 50 μm.)
Fig. S8.
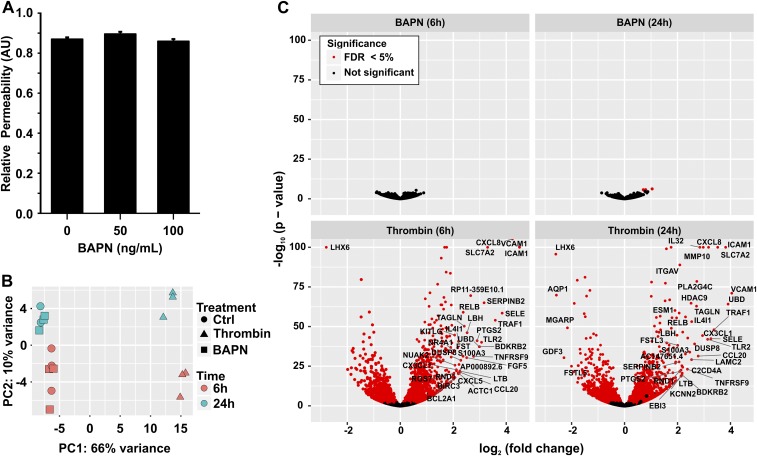
BAPN does not influence ECs in vitro. (A) Quantification of the permeability of the EC monolayer to 40-kDa FITC-dextran in the presence of 50- or 100-ng/mL BAPN. (B) 2D PCA plot of RNA-seq data indicate the clustering of gene-expression profiles arising from HUVEC cells treated with vehicle (Ctrl; circles), thrombin (4 U/mL) as positive control (triangles), or BAPN (100 ng/mL; squares) at 6 h (green) or 24 h (red). PC1 (the horizontal axis) is dominated by the treatment and accounts for 66% of variance. PC2 (the vertical axis) is dominated by the time point and accounts for 10% of variance. (C) Volcano plot indicating statistically significant changes in gene expression for BAPN at 6 h and 24 h (Upper) and thrombin at 6 h and 24 h (Lower). The horizontal axis indicates the log2-fold change for each treatment. The vertical axis indicates the −1 × log10 of the P value. Significant changes are indicated by red points (FDR <5%). Points for genes demonstrating a greater than fourfold increase or decrease in expression upon treatment are further labeled with their gene symbol.
Discussion
Matrix cross-linking is one of the main mechanisms by which the tumor stroma stiffens during solid tumor progression (6, 7). Although several studies have investigated the role of increasing 3D matrix stiffness via matrix density on angiogenesis (10–13), much less is known about the role of cross-linking on the formation of vascular structures. Here, we modulated matrix stiffness by increasing the extent of cross-linking through nonenzymatic glycation. We show that increasing matrix cross-linking increased the extent of outgrowth and branching in endothelial spheroids, in vivo mouse tumors, and in an ex ovo embryonic chick model. We demonstrate that endothelial cell–cell junctional properties are modulated by the stiffness of the matrix and that the localization of VE-cadherin and the permeability of endothelial monolayers are significantly altered by matrix stiffness. We also show that inhibiting MMP activity prevents the increased outgrowth from spheroids in cross-linked matrices and alters the resultant angiogenic sprout morphology. Together, these results show that 3D matrix stiffness plays an important role in regulating angiogenesis and vascular stability.
The vasculature within tumors is known to have disrupted cell–cell junctions and to be more permeable than the vasculature in normal tissues (2). Notably, both our current and previous work indicate that matrix stiffness can disrupt VE-cadherin cell–cell junctions and increase vessels’ leakiness (20). Interestingly, strong VE-cadherin cell–cell interaction can prevent sprouting (31). In addition, differential VE-cadherin dynamics between cell–cell junctions are required for vascular morphogenesis (32). Expression of the cytoplasmic tail of VE-cadherin can induce endothelial membrane protrusions in a process that depends on its subcellular localization (33). Taken together, these observations highlight a possible links among matrix stiffness, VE-cadherin localization, vessel leakiness, and sprouting dynamics.
Increased matrix density is known to act as a barrier to cell migration (13, 14), and any effect from increased stiffness caused by increased density cannot be decoupled from the altered matrix architecture. Others have demonstrated transient, decreased sprouting responses in matrices glycated with glucose-6-phospate but also noted increased branching and tortuosity (34, 35). Of note, direct exposure to high concentrations of glucose is known to affect cells adversely (36), whereas AGEs can have direct antiangiogenic effects on ECs (37, 38). Interestingly, it has been suggested that AGEs could contribute to ECM stiffening in tumors (29). In our experiments, increasing the collagen matrix stiffness via a preglycation step results in more angiogenic sprouting in vitro and ex ovo. Moreover, inhibition of the cross-linking in tumors in vivo resulted in reduced vasculature density and permeability. Such findings are consistent with the decreased rate of metastasis observed in BAPN-treated mice (39), suggesting lower vasculature density and permeability could reduce the ability of tumor cells to enter the blood stream. Furthermore, alternative methods of increasing collagen matrix stiffness, such as the use of microbial transglutaminase or varying the ratio of collagen monomers to oligomers, support our findings of increased angiogenic outgrowth in stiffer matrices (40, 41). Overall, when combined with our data showing the absence of increased endothelial permeability strictly resulting from collagen glycation, these findings indicate that matrix stiffness acts as an important regulator of the vascular phenotype.
Previously published work has shown that MMP activity is an important regulator of angiogenesis (10, 42, 43). GM6001 is a broad-spectrum MMP inhibitor that is known to inhibit the degradation of collagen by MMPs and, interestingly, has been shown to reduce the formation of angiogenic structures in response to the matrix density of fibrin gels (10). Prior studies have seen differences in the angiogenic sprouting response following extended incubation times (3–21 d) with different GM6001 concentrations and collagen matrix density (10, 42, 43). Similarly, we demonstrate that MMP activity is essential for the increased angiogenic response we observed within the stiffer, cross-linked collagen gels. Importantly, both the amount of active cleaved fragment of MT1-MMP and the total content were significantly higher in stiffer matrix. The GM6001 treatment resulted in a small decrease in angiogenic sprouting (along with dramatic changes in the sprout morphology) after 3 d of culture in the compliant collagen gels, whereas GM6001 treatment completely blocked the stiffness-mediated angiogenesis in the ex ovo CAM model. Taken together, these data show that MMPs are integral to the formation of angiogenic sprouts occurring in stiffer matrix.
Antiangiogenic therapies targeting VEGF are currently in therapeutic use and do provide some benefit (3, 44). However, although such therapeutics strategies are effective on a short time scale, they do not completely block the angiogenic process or provide sustained long-term normalization of the tumor vasculature (3, 45). Interestingly, these treatments induce severe tumor hypoxia (46). In turn, hypoxia has been shown to promote ECM remodeling and the expression of cross-linking proteins such as LOX (47, 48). Therefore, our current results provide a potential explanation for why increasing matrix stiffness promotes tumor revascularization. Increased matrix stiffness has been shown to potentiate cell responses to several different soluble factors present in the tumor microenvironment (49–51), and in some cases increased matrix stiffness can change the signaling pathways triggered by soluble factors (50). Thus it is possible that the interplay between matrix stiffness and soluble factors might induce or even worsen the tumor-like vascular phenotype. Overall, a combined therapeutics strategy that includes targeting matrix stiffness or the cellular response to stiffness is likely to improve the outcome of primary treatments targeting angiogenic factors or other soluble factors.
Overall, increasing matrix stiffness promotes a tumor-like vascular phenotype, most notably by disrupting vessel architecture and integrity and promoting outgrowth. The altered vascular phenotype promoted by altered stiffness most likely involves the interplay between cell contractility and matrix stiffness. Notably, similar to our findings in ECs, activation of MT1-MMP in epithelial cells depends on both matrix stiffness and cell contractility (24). In addition, both the angiogenic process and tumor permeability are regulated by VEGF receptors, which in turn are known to be transactivated by integrin receptors (52, 53). Interestingly, alternative splicing of protein isoforms, including VEGF and proangiogenic signaling proteins, is regulated by matrix stiffness (22). Therefore, our results show that a further understanding of the underlying mechanism governing the relationship between matrix stiffness and tumor vascular phenotype is needed to design therapeutics targeting either tumor stiffness or the EC response to tumor stiffening.
Materials and Methods
All mice were maintained following a protocol approved by the Cornell University Institutional Animal Care and Use Committee. A detailed description of the protocols used in this study, including mice and chicks studies, cell culture, spheroid generation and studies, Western blotting, immunofluorescence, permeability assays, confocal reflectance imaging, single-cell migration, PA gel synthesis, and the preparation of collagen scaffolds are described in SI Materials and Methods.
SI Materials and Methods
MMTV-PyMT Mouse Studies.
All mice were maintained following a protocol approved by the Cornell University Institutional Animal Care and Use Committee. MMTV-PyMT mice on the FVB strain background (Jackson Laboratory) were treated with BAPN (3 mg/kg body weight) (Sigma-Aldrich) in the drinking water (n = 12 per group) beginning at 4 wk of age (22). Tumor perfusion was evaluated using a VisualSonic Vevo 2100 Ultrasound system in power wave Doppler mode when the mice reached 8 wk of age. Mice were first anesthetized with 3.5% (vol/vol) isoflurane and transferred to a heated stage on the Vevo 2100 system. The isoflurane concentration then was reduced to 1–2% (vol/vol) to achieve optimal heart and breathing rates (500 beats/min and ∼1 Hz). The fur covering the mammary glands was removed using a commercial depilatory cream. Mammary tumors were scanned with a MS700 transducer and a manual actuator. Ultrasound images were acquired using the power Doppler imaging mode.
Chicken Embryo Culture.
Ex ovo chicken embryo culture was performed as previously described (54). Briefly, fertilized white Leghorn chicken eggs were cultured for 72 h at 37.5 °C and 60% humidity in a rocking incubator. The chicken embryos then were cracked into humidified hammocks and cultured ex ovo in a static incubator. At E10, constructs consisting of two 5- × 5-mm squares of nylon mesh (Amazon Supply) sandwiched around 30 μL of 1.5-mg/mL collagen that had been glycated with 0 or 100 mM ribose and contained 5-μg/mL VEGF (R&D Systems) and 5-μg/mL basic FGF (PeproTech) were polymerized (55). Constructs were placed onto the distal region of the CAM and returned to the incubator. At E15, the embryos were injected with 0.25% fixable 70-kDa Texas Red-dextran (Invitrogen) in Dulbecco’s phosphate-buffered saline (DPBS) into the vitelline veins, and the dye was allowed to perfuse for 45 min. The collagen constructs were fixed for 1 h in 3.7 vol% formaldehyde in PBS and then were removed from the CAM and washed with PBS. The vasculature within the collagen constructs was imaged using a Zeiss LSM700 confocal laser-scanning microscope. Vascular density was scored by counting the number of mesh squares within a 6 × 6 region that had angiogenic vessels within the collagen (n = 40) (55).
Cell Culture and Spheroid Generation.
Bovine aortic endothelial cells (BAECs) and human umbilical vein endothelial cells (HUVECs) were maintained as described previously (22). BAECs were maintained in medium supplemented with 10% (vol/vol) FetalClone III (Fisher), and 1% each of penicillin–streptomycin, minimum essential medium (MEM) amino acids (Invitrogen), and MEM vitamins (Mediatech). HUVECs were maintained and plated at 37 °C and 5% CO2 in Medium 200 (Invitrogen) with 5% (vol/vol) FBS and 2% (vol/vol) low-serum growth supplement (Invitrogen). For MMP inhibition studies, ECs were treated with GM6001 (EMD Millipore) at a concentration of 5 μM. Cells were fed every other day and grown to confluence in an incubator at 37 °C and 5% CO2.
Preparation of Collagen Gels.
Type I collagen was isolated from rat tail tendons (Rockland Immunochemicals) as described previously (19). Briefly, type I collagen was solubilized in 0.1% sterile acetic acid (J.T.Baker) to obtain 10- or 20-mg/mL stock solutions. Glycated collagen solutions were prepared by mixing collagen stock solutions with 0.5 M ribose to form solutions containing 0, 50, or 100 mM ribose in 0.1% sterile acetic acid on ice. Solutions were incubated for 5 d at 4 °C and then were neutralized with 1N sodium hydroxide in 10× DPBS (Invitrogen) and were mixed with Hepes (EMD Millipore) and sodium bicarbonate (J.T.Baker) to form 1.5-, 5-, or 10-mg/mL collagen gels with final concentrations of 1× DPBS, 25 mM Hepes, and 44 mM sodium bicarbonate.
The equilibrium compressive moduli of collagen gels were quantified on an Enduratec ELF 2100 frame (Bose) with a 250 × g load cell measuring the resultant forces of 5% stepwise displacements on collagen gels in confined compression (19). Briefly, a standard linear solid model of viscoelastic behavior was used to fit the relaxation data from 1.5-, 5-, or 10-mg/mL collagen gels, and the equilibrium modulus was calculated as the slope of the stress–strain curve (12).
PA Gel Synthesis.
PA hydrogels were synthesized as described previously (20, 26). Briefly, the amounts of acrylamide [40% (wt/vol) solution] (Bio-Rad) and N,N′-methylene-bis-acrylamide [2% (wt/vol) solution] (Bio-Rad) were varied to tune the Young’s moduli of the gels from 0.2 to 10 kPa. Substrates were functionalized with N-6-((acryloyl)amido)hexanoic acid, succinimidyl ester that was synthesized in the C.A.R.-K. laboratory and covalently bound to 0.1-mg/mL type I rat tail collagen (Becton Dickinson) or 0.1-mg/mL glycated collagen.
Confocal Reflectance Imaging.
The fiber structure of collagen gels was visualized using a Zeiss LSM 700 inverted laser-scanning microscope equipped with a 405-nm laser and a 40×/1.1 N.A. water-immersion objective (Carl Zeiss) (19).
Immunocytochemistry.
Cells and spheroids were fixed in 3.7 vol.% formaldehyde in 1× DPBS, permeabilized with 1 vol% Triton X-100 (J.T.Baker) in 1× DPBS, and blocked with 3% (wt/vol) BSA (Sigma-Aldrich). Actin was stained with Alexa Fluor 488 or Alexa Fluor 568 phalloidin (Invitrogen), and nuclei were stained with DAPI (Sigma-Aldrich). ECs were immunostained with a goat polyclonal VE-cadherin primary antibody (C-19), rabbit polyclonal ZO-1 primary antibody (40-2200; Thermo Fisher Scientific); rabbit polyclonal β-catenin primary antibody (sc-7199; Santa Cruz Biotechnology), Alexa Fluor 568 donkey anti-rabbit, and Alexa Fluor 488 donkey anti-goat or Alexa Fluor 568 donkey anti-goat secondary antibody (Invitrogen).
Mechanical Testing of Tumors.
Following the ultrasound imaging session, the mice were killed by CO2 asphyxiation and were necropsied. The freshly isolated mammary gland tumor samples were snap-frozen and were allowed to thaw in PBS only immediately before mechanical testing was performed (23). Tumor mechanical properties were measured using the system described above for the mechanical testing of collagen gels with the following modifications: The tissue sample was rehydrated in PBS for 15 min; then a 3-mm cylindrical tissue section was excised using a biopsy punch. The height of the tissue section was measured, and then the tissue section was subjected to a 3–15% strain with 3% stepwise displacement in unconfined compression. The elastic modulus was computed from the stress–strain relaxation curves using a poroviscoelastic model (56).
Vasculature Quantification.
Ultrasound images were postprocessed in ImageJ to quantify tumor vasculature. Briefly, the ultrasound images were cropped to match the tumor boundaries, and a section at the approximate center of the tumor was chosen. The ImageJ Tubeness plugin then was used on the Doppler channel of the image. The resulting tube-like network was smoothed using a Gaussian filter and skeletonized using the ImageJ plugin. The resulting vascular network skeleton was quantified based on the number of junctional nodes and branches in the network. These metrics indicate the interconnectedness and density of the vascular network, respectively.
In Vivo Permeability Assay.
Permeability assays of tumor-associated blood vessels were performed on 10-wk-old mice (n = 4 mice per condition). A saline solution of Evans blue (50 mg/kg body weight) and 2-MDa FITC-dextran (40 mg/kg body weight) (Invitrogen) in DPBS was injected retro-orbitally, and the dyes were allowed to circulate for 1 h. The mice were killed by CO2 asphyxiation and were necropsied; the mammary tumors were processed as described (57). Briefly, mammary tumors were collected and fixed in 3.7% (wt/vol) formaldehyde for 24 h followed by sequential dehydration in 20% (wt/vol) dextrose in 10× PBS followed by 30% (wt/vol) dextrose in 10× PBS. Tumors then were embedded within optimum cutting temperature (O.C.T.) (Electron Microscopy Sciences) compound and were processed in a cryostat to obtain 50-μm-thick sections, air-dried, and mounted in Vectashield mounting medium (Vector Laboratories). Fluorescent images were acquired using a 10×/0.3 N.A. objective on a Zeiss LSM 700 laser-scanning microscope. Permeability quantification was performed by measuring the signal intensity of both the FITC-dextran and Evans blue dyes along a line eight pixels wide and perpendicular to the blood vessels present in the images. The center of each vessel was found by fitting the FITC-dextran dye signal with a Gaussian curve. The extravasated Evans blue signal then was normalized to the Evans blue present at the center of the vessel.
In Vitro Permeability Assay.
ECs on PA substrates with Young’s moduli from 0.2 to 10 kPa were used to measure endothelial cell permeability as described previously (20). Briefly, 2-d postconfluent EC monolayers were immersed in a 10-μM solution of 40-kDa FITC-dextran (Sigma-Aldrich) for 5 min. In some cases, the EC monolayer was pretreated for 24 h with 50 or 100 ng/mL BAPN. The in vitro BAPN concentrations were based on the upper limit of blood concentration values measured in vivo (58). Confocal z-slices were acquired using a 40×/1.1 N.A. water-immersion objective on a Zeiss LSM700 microscope. To calculate relative permeability, the intensity of the dextran accumulation within the gel was divided by the fluorescent intensity signal of the solution above the gel. These values then were normalized by the relative accumulation of dextran within gels without cells (n = 21–56).
Ex Ovo Permeability Assay.
At E15, chicks with collagen constructs were injected in the proximal vein with 100 µL of dye solution containing 2.5-mg/mL 65- to 85-kDa TRITC-dextran and 2-MDa FITC-dextran. Confocal z-slices time lapse of the collagen constructs were acquired using a 10×/0.3 N.A. objective on a Zeiss LSM u880 laser-scanning confocal microscope for 60 min postinjection. The FITC signal was used to align 20-pixel-thick regions of interest across neovessels in ImageJ, and the TRITC signal was used to measure the extravasation of dye. The TRITC signal was normalized to the peak value at each time point, and neovessel permeability was defined as the rate of increase of the integral of the normalized signal. (n = 18–20).
Single-Cell Migration.
Individual EC migration was monitored beginning 24 h after cells were embedded within collagen gels using a Zeiss Axio Observer Z1m microscope equipped with a motorized stage and an environmental chamber maintained at 37 °C and 5% CO2. Images were acquired every 10 min for 15 h. Cells were traced in ImageJ (n = 46–68), and centroid positions were used to calculate the mean-square displacement (d2) and the cell migration speed (S) using the persistent random walk equation: , where t is time interval and P is the persistence time using a nonlinear least-squares regression analysis as previously described (21).
MT1-MMP Activity Assay.
MT1-MMP activity was monitored using the selective EnSens 3D Live Cell Imaging Kit for MMP-14 Activity Tracking (Enzium). ECs were embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose and were kept in culture for 24 h. The cells then were pretreated with the EnSens substrate (25 nM final concentration) (Enzium) and the supplied FRF dye (25 nM final concentration) with or without 10 μM of the ROCK inhibitor Y27632 (Calbiochem) for 1 h. The cells then were imaged with a 405-nm laser for confocal reflectance and a 633-nm laser for the EnSens probe using a 40×/1.1 N.A. water-immersion objective on a Zeiss LSM i880 microscope. The far-red fluorescent signal from the EnSens probe was quantified by tracing the cell contour and measuring the average signal using ImageJ (n = 30).
Western Blot.
Subconfluent ECs on PA substrates ranging from 0.2 to 10 kPa or on polystyrene were lysed with buffers to fractionate the Triton-soluble and -insoluble proteins (59). Triton-soluble fractions were extracted with 1% (vol/vol) Nonidet P-40 and 1 Triton X-100 in Tris-buffered saline (TBS; 10 mM Tris⋅HCl, 150 mM NaCl) with 2 mM CaCl2 (J.T.Baker) (pH 7.5) and protease inhibitor mixture (1:500; Sigma-Aldrich). The Triton-insoluble fractions were extracted with 0.5% SDS and 1% Nonidet P-40 (J.T.Baker) in TBS. The supernatants were analyzed with a protein assay (Bio-Rad) and subjected to gel electrophoresis [15 μg per sample; 8% (wt/vol) acrylamide gel] and Western blotting. Alternatively, collagen gels with embedded EC were snap-frozen in liquid nitrogen and then were ground with a mortar and pestle. Proteins were extracted as described before and were subjected to gel electrophoresis (22) or using the Triton-soluble and Triton-insoluble fractionation buffers and were subjected to gel electrophoresis.
Antibodies to GAPDH (MAB374) and MT1-MMP (AB6004; EMD Millipore), VE-cadherin (C-19), β-catenin (sc-7199; Santa Cruz Biotechnology), γ-catenin (sc-7900; Santa Cruz Biotechnology), vimentin (V6389; Sigma-Aldrich), and β-actin (AC-15; Sigma-Aldrich) were detected by chemiluminescence on a Bio-Rad ChemiDoc imaging system. Densitometry of VE-cadherin was performed with Quantity One version 4.6.5 (Bio-Rad) and was expressed as a ratio to β-actin. Total cell VE-cadherin was calculated by adding the VE-cadherin/β-actin ratios for Triton-soluble and Triton-insoluble fractions, and the VE-cadherin in each fraction was determined as a percent of the total VE-cadherin (n = 3 for 2D samples and n = 4 for 3D samples).
Quantitative Real-Time PCR.
ECs embedded within 1.5-mg/mL collagen gels glycated with 0 or 100 mM ribose were kept in culture for 24 h, snap-frozen in liquid nitrogen, and then ground with a mortar and pestle. Total RNA was collected and purified with an RNeasy Plus Mini Kit (Qiagen). To generate cDNA, 1 μg of total RNA per sample was mixed with 80-μM random primers (Invitrogen), 10 mM deoxynucleotide solution mix (New England Biolabs), and nuclease-free water and was heated for 5 min at 75 °C. After the addition of 40 U/μL RNase inhibitor and 200 U/μL M-MuLV Reverse Transcriptase in M-MuLV reaction buffer (New England Biolabs), cDNA was synthesized in an iCycler thermal cycler (Bio-Rad). Quantitative RT-PCR was performed with 1 μg of cDNA and 0.4 μM of specific primers against MMP14 (forward: 5′-TGTGACGGGAACTTTGACACCG-3′; reverse: 5′-ACGCTGCCCTTGAAACTGTGGC-3′) and GAPDH (forward: 5′-CATGAGAAGTATGACAACAGCCT-3′; reverse: 5′-AGTCCTTCCACGATACCAAAGT-3′) (Integrated DNA Technologies) using 1× iQ SYBR Green Supermix (Bio-Rad) on a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad).
RNA-Sequencing Studies.
ECs seeded on plastic were treated with vehicle, 100 ng/mL BAPN, or 4 U/mL thrombin (605157; EMD Millipore) (as a positive control) for 6 or 24 h. Total RNA was collected and purified with an RNeasy Plus Mini Kit. RNA-sequencing (RNA-seq) library preparation reagents were purchased from Kapa Biosystems. Preparation of strand-specific cDNA libraries was performed with dUTP incorporation during second-strand synthesis followed by enrichment for mRNA using magnetic oligo-dT capture beads. Sequencing of cDNA libraries was performed using an Illumina HiSeq. 2500 sequencing system producing an average of 44 million 101-bp paired-end reads per sample (range: 36–52 million reads). Quantification of transcript abundances was performed by pseudoalignment of reads to the human reference transcriptome (Gencode v21) using Kallisto (Pachter Lab) with sequence-dependent bias correction. Read counts per gene were normalized, and differentially expressed genes were identified using generalized linear models fitted to a negative binomial distribution by DESeq2 software (BioConductor). Surrogate variable analysis was used to estimate covariates for modeling and correction of batch effects. Significantly altered genes were identified controlling the false discovery rate (FDR) at 5% using the Benjamini–Hochberg procedure. Principal component analysis (PCA) was performed in R/BioConductor on corrected log-transformed counts. Plots were produced using ggplot2.
Spheroid Studies.
Multicellular spheroids were prepared through aggregation as described previously (19). Briefly, ECs were suspended in growth medium supplemented with 0.25% MethoCult (Stem Cell Technologies) and were seeded into nonadherent 96-well round-bottomed plates (Corning Inc.) at 10,000 cells per well. Cells were pelleted by centrifugation, and spheroids were formed by placing the plate on an orbital shaker for 2 h and subsequently incubating the spheroids for 2 d. Spheroids then were embedded within neutralized collagen solutions, allowed to polymerize at 37 °C and 5% CO2, and then were overlaid with complete medium. The medium was exchanged after 1 h and then was exchanged every other day during experiments. Spheroids were imaged daily with bright-field imaging on a Zeiss Axio Observer Z1.m microscope and with fluorescence on a Zeiss LSM700 laser-scanning microscope. Spheroid outgrowth was compared across conditions by measuring the area of the spheroids with the resultant extensions and normalizing to the area of the spheroid immediately after the spheroid was embedded in the collagen gel (for collagen concentration experiments, n = 7–14; for GM6001 experiments, n = 19–28).
Spheroid branching was assessed after 5 d. Images of actin- and nuclei-stained spheroids were acquired as z-stacks with 5-μm spacing using a 10×/0.30 N.A. objective. Maximum intensity projections of the confocal z-stacks were analyzed for branching density by counting the number of branches per sprout length in five randomly chosen extensions per spheroid (n = 35–55). Data were normalized to the 0-mM ribose condition.
To investigate spheroid sprout width, EC spheroids embedded in 1.5-mg/mL collagen for 3 d with or without GM6001 treatment were stained for actin and nuclei. Images of the spheroids were acquired as z-stacks with 5-μm spacing using a 10×/0.30 N.A. objective.
To investigate VE-cadherin width, spheroids embedded for 24 h in 1.5-mg/mL collagen gels were stained for VE-cadherin and nuclei. Images of the sprouts emanating from the spheroids were acquired as z-stacks with 1-μm spacing using a 40×/1.1 N.A. water-immersion objective. The widths of spheroid sprouts or VE-cadherin junctions were analyzed as previously described (20). Briefly, a line was drawn perpendicular to the cell–cell junction in the sprout (n = 118–130), and the intensity profile was recorded and fit with a two-Gaussian curve in MATLAB (The Mathworks).
Release of VEGF from Glycated Collagen Gels.
Implants consisting of two 5- × 5-mm squares of nylon mesh and 30 μL of 1.5-mg/mL collagen that had been glycated with 0 or 100 mM ribose and containing 5 μg/mL VEGF (R&D Systems) were polymerized as described for chicken embryo culture. Individual constructs were placed in a 100-mm Petri dish (VWR) containing 10 mL TBS with 0.05% Tween (J.T.Baker) and 0.1% BSA. Petri dishes were placed in an incubator at 37 °C and 5% CO2, and 100-μL samples of the solution were obtained at 0, 1, 2, 4, 8, and 24 h. Quantification of VEGF release was performed using an ELISA (R&D Systems).
Statistics.
Data were analyzed using a one-way ANOVA followed by a post hoc Tukey’s honest significant difference test in JMP (SAS, v.10.0). To normalize the data distributions, spheroid branch density, VE-cadherin junction width, and permeability data were transformed by natural logarithm before running a Tukey’s test. Statistical significance was considered as P < 0.05. All values are expressed as the mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grant R01-HL127499 (to C.A.R.-K.), National Science Foundation (NSF) awards 1055502 and 1435755 (to C.A.R.-K.), and NIH Grant R01-CA163255 (to R.S.W.). F.B. is the recipient of a Scholarship for the Next Generation of Scientists from the Cancer Research Society. B.N.M. is the recipient of an NSF Graduate Research Fellowship in Science, Technology, Engineering and Mathematics, The Morgan Family Fellowship, and an NSF Graduate Teaching Fellows in K-12 Education Fellowship. Y.L.N.A. is the recipient of NIH Training Grant T32 GM008500. Imaging data were acquired in the Cornell Biotechnology Resource Center Imaging Facility using the shared, NIH-funded (Grant S10OD016191) VisualSonics high-resolution ultrasound platform and the New York State Stem Cell Science (Grant CO29155)- and NIH (Grant S10OD018516)-funded Zeiss LSM880 confocal/multiphoton microscope.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.L.M. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613855114/-/DCSupplemental.
References
- 1.Trédan O, Galmarini CM, Patel K, Tannock IF, Tredan O. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 2.Hashizume H, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. VEGF gene therapy: Stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6(10):1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maskarinec G, et al. Mammographic density as a predictor of breast cancer survival: The multiethnic cohort. Breast Cancer Res. 2013;15(1):R7. doi: 10.1186/bcr3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker HE, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71(5):1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12(10):2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 11.Shen CJ, et al. Decreased cell adhesion promotes angiogenesis in a Pyk2-dependent manner. Exp Cell Res. 2011;317(13):1860–1871. doi: 10.1016/j.yexcr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross VL, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgar LT, Underwood CJ, Guilkey JE, Hoying JB, Weiss JA. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS One. 2014;9(1):e85178. doi: 10.1371/journal.pone.0085178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey SP, Kraning-Rush CM, Williams RM, Reinhart-King CA. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials. 2012;33(16):4157–4165. doi: 10.1016/j.biomaterials.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf K, et al. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201(7):1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, et al. MT2-MMP expression associates with tumor progression and angiogenesis in human lung cancer. Int J Clin Exp Pathol. 2014;7(6):3469–3477. [PMC free article] [PubMed] [Google Scholar]
- 17.Seano G, et al. Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nat Cell Biol. 2014;16(10):931–941, 1–8. doi: 10.1038/ncb3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ager EI, et al. Blockade of MMP14 activity in murine breast carcinomas: Implications for macrophages, vessels, and radiotherapy. J Natl Cancer Inst. 2015;107(4):djv017. doi: 10.1093/jnci/djv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013;9(1):4635–4644. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh J, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3(112):112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95(12):6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordeleau F, et al. Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc Natl Acad Sci USA. 2015;112(27):8314–8319. doi: 10.1073/pnas.1505421112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JI, Kang I, You W-KK, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol. 2011;3(9):910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014;28(8):3589–3599. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133(1):95–109. [PMC free article] [PubMed] [Google Scholar]
- 26.Califano JP, Reinhart-King CA. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol Bioeng. 2010;3(1):68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Califano JP, Reinhart-King CA. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol Bioeng. 2008;1(2-3):122–132. [Google Scholar]
- 28.van Heijst JWJ, Niessen HWM, Hoekman K, Schalkwijk CG. Advanced glycation end products in human cancer tissues: Detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann N Y Acad Sci. 2005;1043:725–733. doi: 10.1196/annals.1333.084. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Teja M, et al. AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival. J Pathol. 2015;235(4):581–592. doi: 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- 30.Hirose A, Tanikawa T, Mori H, Okada Y, Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010;584(1):61–66. doi: 10.1016/j.febslet.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 31.Abraham S, et al. VE-cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19(8):668–674. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 32.Bentley K, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol. 2014;16(4):309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 33.Kouklis P, Konstantoulaki M, Malik AB. VE-cadherin-induced Cdc42 signaling regulates formation of membrane protrusions in endothelial cells. J Biol Chem. 2003;278(18):16230–16236. doi: 10.1074/jbc.M212591200. [DOI] [PubMed] [Google Scholar]
- 34.Francis-Sedlak ME, et al. Characterization of type I collagen gels modified by glycation. Biomaterials. 2009;30(9):1851–1856. doi: 10.1016/j.biomaterials.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Francis-Sedlak ME, et al. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. 2010;80(1):3–9. doi: 10.1016/j.mvr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Roy R, Boskey AL, Bonassar LJ. Non-enzymatic glycation of chondrocyte-seeded collagen gels for cartilage tissue engineering. J Orthop Res. 2008;26(11):1434–1439. doi: 10.1002/jor.20662. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Z, et al. The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus. Mol Cell. 2012;47(3):422–433. doi: 10.1016/j.molcel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2013;3(2):94–108. doi: 10.1016/j.molmet.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Q-T, et al. Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: Radiation Therapy Oncology Group trial 90-03. J Clin Oncol. 2009;27(26):4281–4286. doi: 10.1200/JCO.2008.20.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee P-F, Bai Y, Smith RL, Bayless KJ, Yeh AT. Angiogenic responses are enhanced in mechanically and microscopically characterized, microbial transglutaminase crosslinked collagen matrices with increased stiffness. Acta Biomater. 2013;9(7):7178–7190. doi: 10.1016/j.actbio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whittington CF, Yoder MC, Voytik-Harbin SL. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol Biosci. 2013;13(9):1135–1149. doi: 10.1002/mabi.201300128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun. 2003;312(4):903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Saunders WB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175(1):179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget. 2016;7(29):46668–46677. doi: 10.18632/oncotarget.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen E, Walters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin Cancer Res. 2011;17(16):5299–5310. doi: 10.1158/1078-0432.CCR-10-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pàez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 48.Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Huynh J, Bordeleau F, Kraning-Rush CM, Reinhart-King CA. Substrate stiffness regulates PDGF-induced circular dorsal ruffle formation through MLCK. Cell Mol Bioeng. 2013;6(2):138–147. doi: 10.1007/s12195-013-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23(5):781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124(Pt 8):1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 53.Lichtenbeld HC, Ferarra N, Jain RK, Munn LL. Effect of local anti-VEGF antibody treatment on tumor microvessel permeability. Microvasc Res. 1999;57(3):357–362. doi: 10.1006/mvre.1998.2140. [DOI] [PubMed] [Google Scholar]
- 54.Yalcin HC, Shekhar A, Rane AA, Butcher JT. An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. J Vis Exp. 2010;(44):2154. doi: 10.3791/2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen M, Shing Y, Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994;47(1):31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- 56.Galli M, Comley KSCC, Shean TAVV, Oyen ML. Viscoelastic and poroelastic mechanical characterization of hydrated gels. J Mater Res. 2009;24(3):973–979. [Google Scholar]
- 57.Hoffmann A, et al. High and low molecular weight fluorescein isothiocyanate (FITC)-dextrans to assess blood-brain barrier disruption: Technical considerations. Transl Stroke Res. 2011;2(1):106–111. doi: 10.1007/s12975-010-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machon C, et al. Quantification of β-aminopropionitrile, an inhibitor of lysyl oxidase activity, in plasma and tumor of mice by liquid chromatography tandem mass spectrometry. Biomed Chromatogr. 2014;28(7):1017–1023. doi: 10.1002/bmc.3110. [DOI] [PubMed] [Google Scholar]
- 59.Lampugnani MG, et al. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129(1):203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.