Significance
Little is known regarding nonavian dinosaur embryology. Embryological period relates to myriad aspects of development, life history, and evolution. In reptiles incubation is slow, whereas in birds it is remarkably rapid. Because birds are living dinosaurs, rapid incubation has been assumed for all dinosaurs. We discovered daily forming growth lines in teeth of embryonic nonavian dinosaurs revealing incubation times. These lines show slow reptilian-grade development spanning months. The rapid avian condition likely evolved within birds prior to the Cretaceous–Paleogene (K–Pg) mass extinction event. Prolonged incubation exposed nonavian dinosaur eggs and attending parents to destructive influences for long periods. Slow development may have affected their ability to compete with more rapidly generating populations of birds, reptiles, and mammals following the K–Pg cataclysm.
Keywords: Dinosauria, teeth, embryology, Neornithes, extinction
Abstract
Birds stand out from other egg-laying amniotes by producing relatively small numbers of large eggs with very short incubation periods (average 11–85 d). This aspect promotes high survivorship by limiting exposure to predation and environmental perturbation, allows for larger more fit young, and facilitates rapid attainment of adult size. Birds are living dinosaurs; their rapid development has been considered to reflect the primitive dinosaurian condition. Here, nonavian dinosaurian incubation periods in both small and large ornithischian taxa are empirically determined through growth-line counts in embryonic teeth. Our results show unexpectedly slow incubation (2.8 and 5.8 mo) like those of outgroup reptiles. Developmental and physiological constraints would have rendered tooth formation and incubation inherently slow in other dinosaur lineages and basal birds. The capacity to determine incubation periods in extinct egg-laying amniotes has implications for dinosaurian embryology, life history strategies, and survivorship across the Cretaceous–Paleogene mass extinction event.
Skeletal growth series afford considerable understanding about posthatchling development in nonavian dinosaurs (hereafter dinosaurs) (1, 2). Nevertheless, very little is known about their embryology. An important parameter that can provide myriad insights into dinosaurian in ovo development is incubation period. This ontogenetic measure is relevant to the timing of developmental milestones (e.g., formation of eyes, teeth, brain, and so forth) (3–5), mortality risks (6–9), egg and clutch sizes (10–12), fecundity and lifespan (13), reproductive effort (14), altricial vs. precocial developmental modes (5, 15), parental attentiveness (13, 16), environmental and genetic influences affecting development (11, 12, 17), migration (18), environmental restrictions on breeding seasons (13, 14), and so forth.
Incubation periods differ substantially among extant nonavian reptiles (Lepidosauria, Testudines, Crocodylia; hereafter reptiles) (11) and birds (Neornithes) (10, 12). Most reptiles have two functional oviducts, and the eggs of an entire clutch are formed then laid at the same time (19–21). The eggs typically hatch over considerably longer time periods than same-sized avian eggs (12, 22). Birds, on the other hand, typically have a single functional ovary and lay fewer, but relatively larger eggs than reptiles (23). In amniotes, egg size positively correlates with incubation period (10–12). This should be a negative effect because of extended exposure of eggs to destructive influences (6, 24–26). Birds generally mitigate these costs with short incubation periods made possible by elevated and relatively stable nest temperatures (through brooding or environmental heat sources) (27, 28), efficient gas conductance through the eggshell (29), and high embryonic metabolic and growth rates (22).
Birds are members of Sauria, which includes extant nonavian reptiles showing characteristically long incubation periods. However, they are also living dinosaurs (Dinosauria) (30, 31), with extant forms showing rapid incubation. Phylogenetically, either condition is plausible for their dinosaurian ancestors. A number of anatomical, behavioral and eggshell attributes of birds related to reproduction [e.g., medullary bone (32), brooding (33–36), eggshell with multiple structural layers (37, 38), pigmented eggs (39), asymmetric eggs (19, 40, 41), and monoautochronic egg production (19, 40)] trace back to their dinosaurian ancestry (42). For such reasons, rapid avian incubation has generally been assumed throughout Dinosauria (43–45).
Incubation period estimates using regressions of typical avian values relative to egg mass range from 45 to 80 d across the known 0.42- to 5.63-kg dinosaurian egg-size spectrum (43). Similar estimates have resulted using alternative methods [e.g., 31–77 d across the dinosaurian size spectrum using estimates of adult and hatchling size and assuming avian embryonic metabolism (45) and 65–82 d for large (1.62–5.08 kg) sauropod eggs using avian clutch mass to adult size ratios and avian incubation rates (46)]. [Note: The possibility that sauropods showed incubation rates like members of the Crocodylia (the extant sister clade to Dinosauria) was explored in the latter study. However, this also predicts rapid values (68–71 d) because the regression line for crocodilians intercepts avian rates when extrapolated out to the grossly larger sauropod egg sizes.]
Direct determination of dinosaurian incubation duration has been considered intractable (43, 47). However, in crocodilian (48) and human (49) embryonic teeth, incremental lines of von Ebner that reflect diurnal pulses of mineralization during odontogenesis are present. Counts of these markers have been used to determine tooth formation times in postparturition mammals (50, 51), and replacement rates in posthatchling crocodilians (52) and dinosaurs (53, 54). This finding prompted us to explore the possibility that these time markers exist in embryonic dinosaur teeth and could be used to determine incubation period.
Here we: (i) show that incremental lines of von Ebner are present in embryonic dinosaur teeth; (ii) use increment counts and data on tooth initiation in reptiles to reveal incubation period in two ornithischian dinosaurs (Protoceratops andrewsi and Hypacrosaurus stebingeri), whose eggs span nearly the entire size range reported for dinosaurs; (iii) test whether these dinosaurs show typical rapid avian incubation times or primitive slow reptilian development; and (iv) explore ramifications of our results with regard to the origin of the modern avian condition, dinosaurian life history, and survivorship through the Cretaceous–Paleogene (K–Pg) extinction event.
Results
Mean von Ebner incremental line width for embryonic P. andrewsi is 10.04 μm (n = 20; range 9.05–13.92 μm) and 15.26 μm (n = 15; range 9.42–16.62 μm) for H. stebingeri (Fig. 1 A and B). Mean replacement rates for embryonic P. andrewsi teeth are 30.68 d (n = 2 tooth families; 31.19 and 30.18 d) and 44.18 d for embryonic H. stebingeri teeth (n = 3 tooth families; 43.22, 43.48, and 45.83 d) (Materials and Methods and Fig. S1)
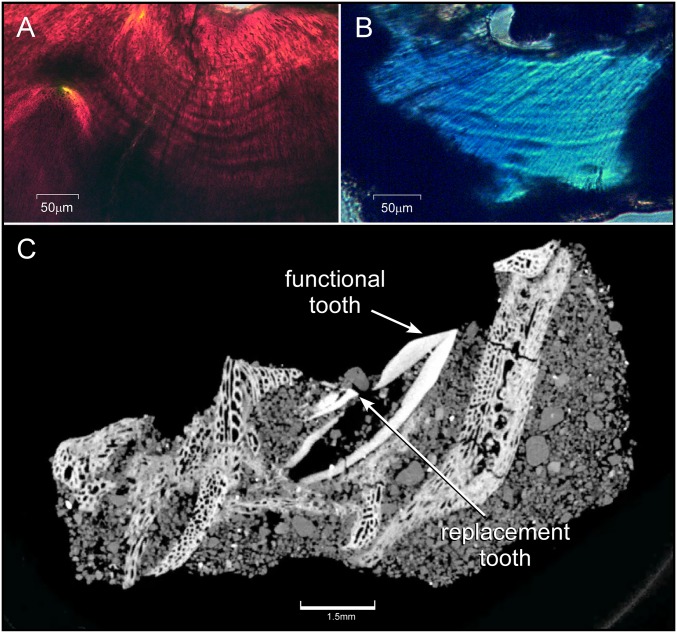
Fig. 1.
Daily growth lines in embryonic dinosaur teeth and CT rendering of a P. andrewsi jaw and tooth family. (A) Von Ebner’s growth lines (alternating dark and light bands) in the orthodentine surrounding the pulp cavity (at top of graphic) of an embryonic H. stebingeri tooth (polarized microscopy, transverse view). (B) Von Ebner’s growth lines surrounding the pulp cavity (at top of the graphic) in an embryonic tooth of P. andrewsi (polarized microscopy, transverse view). (C) High-resolution CT rendition of a P. andrewsi tooth family within the jaw used to determine tooth-formation times in embryonic teeth.
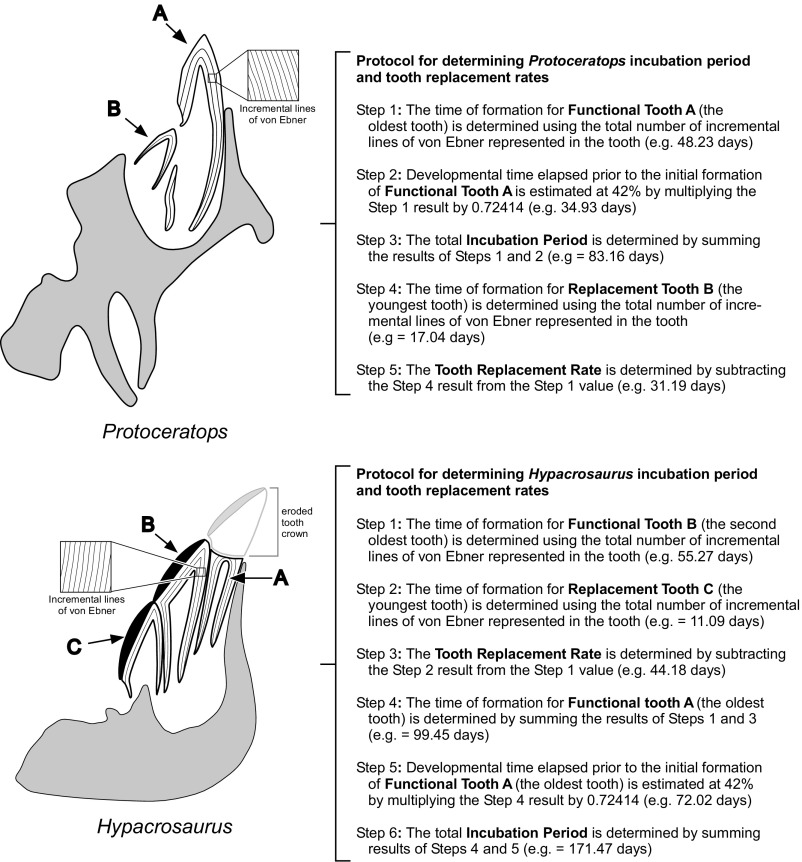
Fig. S1.
Determining incubation period and tooth replacement rates in embryonic dinosaurs. Embryonic dentitions of Protoceratops andrewsi and Hypacrosaurus stebingeri show different numbers of functional teeth in each tooth family and require different methods to determine incubation period. P. andrewsi (Upper) has a single functional tooth and a single replacement. The formation time for the most-developed functional teeth in the dentition was modeled as representing 58% of the total incubation period. In other words, the teeth destined to be the hatchling functional dental compliment were modeled as having been initiated 42% through development. (Note: The mean replacement rate, which equals the rate at which teeth were typically shed from each tooth family, is not used in the incubation period calculation, but was determined for future use in comparative dental studies.) In H. stebingeri (Lower) there are two functional teeth and a single replacement. The time elapsed to make the hatchling functional dentition similarly requires determining how many days it took to form the oldest tooth. However, the crown of that tooth is worn away from in ovo chewing. As such, a subset of the incremental lines have been effaced in the tooth and its formative time cannot be made using a total line count. However, it is a single-replacement cycle older than the next younger functional tooth. By aging that tooth and adding the tooth-replacement rate, the formative time for the oldest functional tooth is revealed. Coupling this value with consideration that that tooth began 42% through incubation provides an estimate of total incubation period.
Near-term P. andrewsi embryos (Fig. 2 A and B) show tooth families composed of two teeth: one that is functional and the other a replacement (Fig. 1C and 2C and Fig. S1). The time elapsed in forming the oldest tooth in the dentition, determined from the total number of incremental lines represented in the tooth, is 48.23 d. As described below (Materials and Methods), hatchling teeth were conservatively modeled as initiating at 42% of the incubation period (34.93 d). The sum of these values reveals a minimum incubation period of 83.16 d for the P. andrewsi embryo.
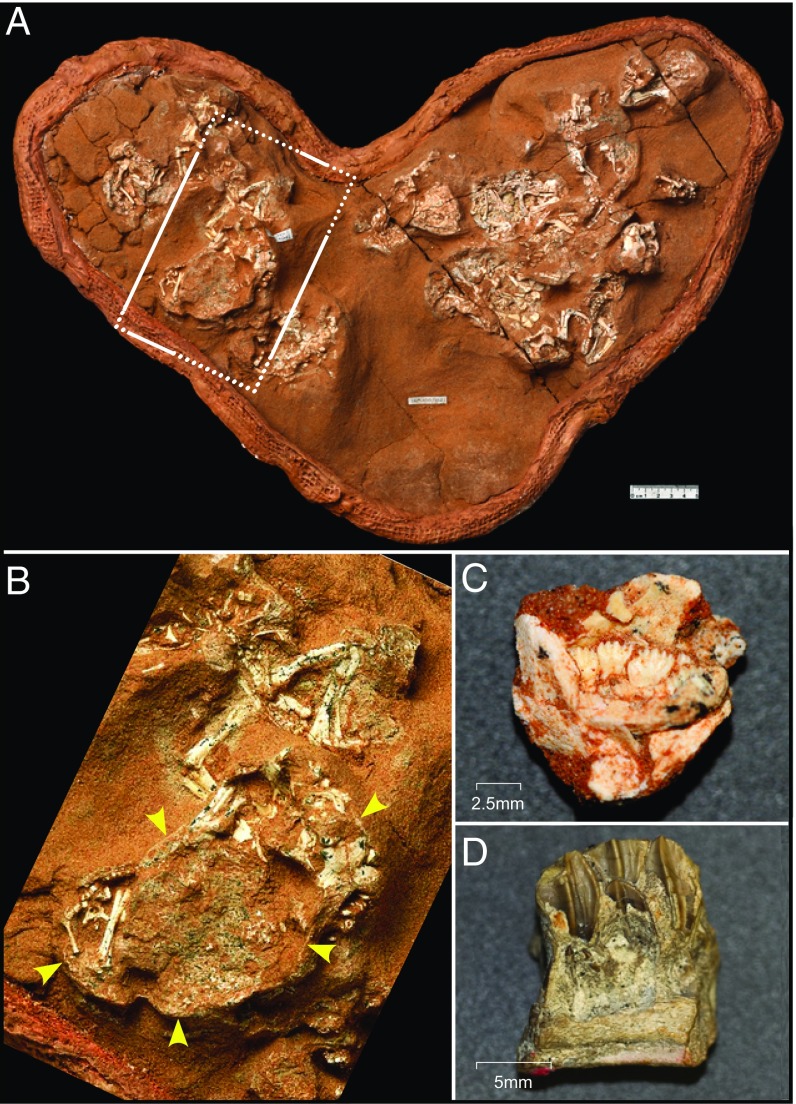
Fig. 2.
P. andrewsi nest and embryonic dinosaur jaws. (A) Nest of P. andrewsi eggs and embryos. The embryos were partially prepared within the eggs. (B) Expanded view of an embryo within an egg showing the thin surrounding eggshell. (C) Embryonic P. andrewsi dentary showing functional and replacement teeth. (D) Section of an embryonic H. stebingeri dentary showing functional and developing replacement teeth.
The near-term H. stebingeri embryos show tooth families composed of three teeth: two that are functional and a single replacement (Fig. 2D and Fig. S1). The oldest functional tooth is just a root remnant, so its formation time could not be directly determined from a total growth-line count because of the loss of increments present only in the tooth crown. However, the time elapsed in forming the oldest functional tooth was determined by summing the age of the younger functional tooth (55.27 d) and the tooth family replacement rate (44.18 d) (Materials and Methods and Fig. S1). This finding equates to a value of 99.45 d. Again, because the hatchling teeth conservatively began formation at 42% of incubation time (72.02 d), this reveals a minimum incubation period of 171.47 d.
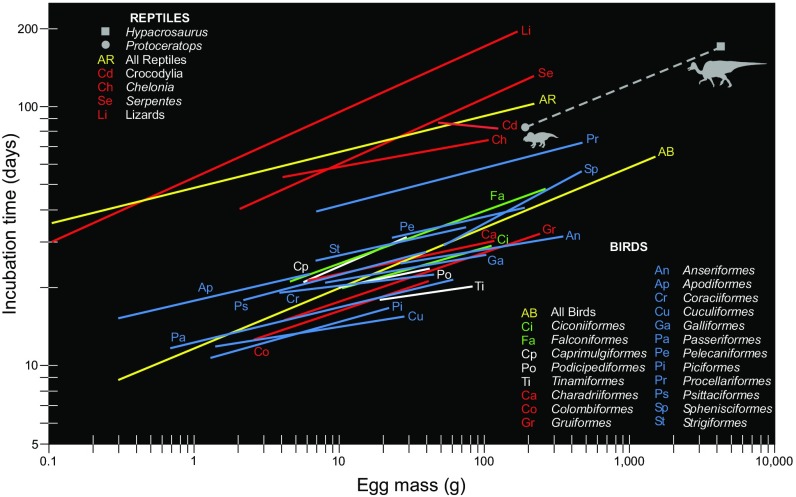
Comparison of P. andrewsi incubation period relative to that typical for birds with same-sized eggs shows greater than twofold slower values (83.16 vs. 39.72 d) and modestly faster values (∼17%) than predicted for typical reptiles (83.16 vs. 100.40 d) (Fig. 3). Among reptilian clades, the P. andrewsi incubation period is just 3.5% longer than typical for Crocodylia (83.16 vs. 80.20 d). Notably, crocodilians are the extant sister clade to Dinosauria. The results are 5.6% longer than predicted for chelonians (83.16 vs. 78.50 d) (Fig. 3). These findings support the alternative hypothesis that P. andrewsi retained the primitive reptilian incubation duration.
Fig. 3.
Dinosaur incubation periods compared with those for extant reptiles and birds. (Neontologic data from ref. 12). The dashed regression line for the two dinosaur species studied here is provided only to allow visual comparison with typical incubation periods for the other clades, Aves and reptiles in particular. Equations: All birds: incubation time = 11.64 × (egg mass)0.233. All reptiles: incubation time = 48.53 × (egg mass)0.138.
The comparison of H. stebingeri incubation period with birds with same-sized eggs also shows over twofold slower values (171.47 vs. 81.54 d) (Fig. 3). The H. stebingeri incubation period is modestly longer (∼12%) than predicted for same-sized typical reptilian eggs (171.47 vs. 153.72 d) (Fig. 3). Although it is interesting to speculate what groups H. stebingeri incubation most resembles, eggs in living birds and reptiles are grossly smaller than those from this dinosaur. Nevertheless, these results, like those data for the P. andrewsi embryo, support the alternative hypothesis that H. stebingeri retained the primitive reptilian incubation duration.
Discussion
Discovery of von Ebner incremental lines in embryonic dinosaur teeth provides direct empirical estimates for their incubation periods. This opens the door to tracing the genesis of rapid avian in ovo development and provides some of the first empirical insights into dinosaur embryology and life-history strategy.
Dinosaur incubation periods have been estimated to be very rapid, assuming typical extant avian values. Regression of incubation period versus egg size in extant birds predict 40 d for small P. andrewsi eggs and 82 d for large H. stebingeri eggs (12) (Fig. 3). Our analyses show considerably slower development, respectively 83 and 171 d. These results are more similar to slow development typical of extant reptilian embryos (12), strongly suggesting rapid avian incubation was not primitive for Dinosauria. In fact, as explained below, because tooth formation shows developmental and physiological constraints (3, 53, 55, 56), it is very likely that most if not all toothed dinosaurs and basal toothed birds showed slow reptilian-grade incubation. Hatchling compliments of teeth typically do not appear earlier than 42% through incubation in extant reptiles (3). In addition, von Ebner line widths are limited to less than 30 μm, regardless of tooth size (53, 55, 56); this is caused by the necessity for dentine calcospherites to fully congeal over a day’s time to form an increment before the next daily mineralization event (49, 57, 58). These factors suggest rapid incubation evolved near the diversification of toothless birds (Neornithes) (59) (= Aves) (60). Notably, Deeming studied eggshell thickness and porosity in eggs representing dinosaurian diversity (61) and for the basal nonneornithine bird, Gobipteryx minuta (Enantiornithes) (62). He then estimated water vapor conductance and compared the results with extant reptilian and avian (Neornithes) values and their respective nesting conditions. Deeming concluded that dinosaurs and basal lineage nonneornithine birds most likely: (i) incubated their eggs buried in substrate like most reptiles and some extant birds (e.g., Megapodiidae); and (ii) did not show contact-incubation to control the incubation environment like most extant birds (61). These factors may have contributed to the slow development we found in the dinosaurs we studied.
A notable implication of our capacity to estimate incubation periods using incremental line counts includes determination of them in any egg-laying fossil embryo that bears teeth, not just dinosaurs and basal birds. New embryological and comparative evolutionary insights into amniote embryology will result. For example, the potential to establish comparative developmental (aka normal) life-staging tables (the standardized metric in embryology) (63)—such as those that have been developed for chickens, mice, pigs, humans, crocodilians, and lizards (3, 63–65)—might be achieved in fossils by coupling dental and skeletal formative stages with time elapsed through incubation. Furthermore, this method can directly predict incubation period in embryos bearing teeth in any taxon, regardless of egg size. Finally, the method allows exploration of incubation time variability within and between taxa, and to test when major evolutionary shifts occurred.
Nevertheless, there are drawbacks precluding broad application of our technique for deducing dinosaurian and early avian incubation periods: namely, near-term embryos of taxa bearing teeth are rare. There are also uncertainties introduced by the method that could affect the accuracy. These uncertainties include: (i) fossil eggs are often crushed, necessitating size reconstruction (43) [various methods are used to estimate egg volume and mass, and each produces slightly different values (43)]; (ii) it is indeterminable how many days away from hatching near-term embryos were, which inherently produces underestimated incubation periods; and (iii) there is a level of uncertainty regarding when in development hatchling teeth initiate [typically 42–52% in extant sister taxon Crocodylia (3) and 51–67% in distant outgroup Squamata (64–69)], with higher percentages leading to longer incubation period estimation. Other methods for estimating dinosaur incubation periods based on regressions of typical avian or reptilian values relative to egg mass or adult body size (43–45), or alternatively using clutch mass to adult size ratios (46), also have strengths and drawbacks. The major upside is that they can be applied to any egg (43, 44), clutch (46), or adult (45), regardless of whether an egg bearing an embryo is preserved. Potential sources of error include: (i) concerns regarding egg size and mass estimation (see above) or adult size estimation (2); (ii) uncertainties about individual clutch sizes (43, 46); (iii) extrapolation well outside of the upper range of extant egg sizes for large dinosaur eggs where there is negligible statistical confidence (43); (iv) there is considerable variation in incubation rates among extant taxa for a given egg size [e.g., reptilian incubation periods can range from ∼3.5- to 13-fold among species (12)]; and finally, (v) these methods assume that incubation rates were like those seen in extant clades [e.g., reptilian versus avian-like (43–46)]. With regard to the latter, our results demonstrate that some dinosaurs showed reptilian-like incubation periods. We favor using the egg-mass methodology (12, 43, 44) based on reptilian development for predicting incubation periods in fossil and nonneornithine birds when near term embryos bearing teeth are not present.
The ecological and life-history implications of our findings that ornithischians and predictably all tooth-bearing dinosaurs (see above) had slow incubation relative to extant birds are considerable. Dinosaur eggs and attending parents (if present) (1, 33–36, 43) would have been exposed to prolonged risks (e.g., predation, starvation, and stochastic environmental events) (43, 45, 70, 71). This exposure likely contributed to their tendency to lay large clutches with relatively smaller eggs as a means to counter high-mortality rates (43, 72) within the egg (45, 46) and after hatching (73, 74). As in extant birds (13), prolonged nesting cycles may have made some environments improbable for successful reproduction and production of extra broods by some taxa, especially large dinosaurs whose incubation spanned the better part of a year. Speculation that neonates of large ornithischian dinosaurs (e.g., ceratopsians and hadrosaurids) made 2,600- to 3,200-km migrations from lower latitude nesting grounds to rich summer feeding grounds in the Arctic may have been infeasible because of unexpectedly short posthatching windows for seasonal travel (75–77). Finally, hypotheses regarding nest microenvironment (43, 61), eggshell gas conductance (61, 78, 79), embryonic physiology (45), reproductive effort, annual numbers of clutches and taxon generation times (21, 80, 81), and developmental mode (43) can be strengthened or formally tested in light of slower in ovo dinosaur development.
These results may have implications for nonavian dinosaur extinction. The end of the Cretaceous was marked by extreme catastrophe and rapid climatic change, resulting in a resource-limited environment (82). Growth-curve analyses suggest dinosaurs and basal birds were endothermic (83) or mesothermic (84) [i.e., considerably more energetically wasteful than ectothermic amphibians and reptiles (85)] but required a year or more to reach somatic and sexual maturity (35, 83). This likely required them to acquire more total resources to reproduce than surviving amphibians, reptiles, birds, and mammals. Coupled with slow generation times, augmented by slow incubation, these attributes may have put nonavian dinosaurs at a disadvantage in competing for vacated niche spaces in the post-K–Pg event world.
Materials and Methods
Specimen Acquisitions and Egg Size Estimations.
The embryonic remains of P. andrewsi (1.8-m adult total length; Ceratopsia: Protoceratopsidae) derive from a nest composed of 12 eggs discovered in Campanian sediments of the Upper Cretaceous Djadochta Formation from the Gobi Desert of Mongolia by American Museum of Natural History–Mongolian Academy of Sciences expeditions (86) (Fig. 2A). The nest is a bilobed depression in the sandstone, containing partly crushed 10.07 × 5.81 cm (±5%) elongated eggs with hemispherical ends, an egg shape previously known only in theropod dinosaurs (87). Each egg contains the skeleton of a well-ossified embryo, which occupies a substantial portion of the egg, with fully formed dentition (Fig. 2B). Notably, these are the first eggs definitively ascribed to Ceratopsia. [Eggs (ootaxon Elongatoolithidae) discovered by the Central Asiatic Expedition of 1923 were famously attributed to P. andrewsi, but were later shown to be from the theropod Oviraptor philoceratops (88). A purported egg from the neoceratopsian, Yamaceratops dorngobiensis (89) has been shown to be from an enantiornithine bird (90)]. The estimated volume of the eggs based on similarly proportioned ellipsoid reptile eggs [(π/6000)LD2 (91)] is 177.98 cc, making them the smallest eggs that can be definitively referred to a nonavian dinosaur yet discovered. [The next largest eggs containing identifiable dinosaur embryos are 9 × 7-cm therizinosaur eggs with an estimated volume of 230.79 cc (47).] For P. andrewsi, the estimated mass of the egg and embryo [vol. × 1.09 g/cc (43, 92)] at the time of hatching is 194.00 g. A left dentary (IGM 100/1021a; Mongolian Institute for Geology, Ulaanbaatar, Mongolia) containing six tooth families (each composed of a functional tooth plus a single underlying replacement tooth) was extracted from an egg for histological and computerized tomographic analyses (Fig. 2C).
The H. stebingeri (9.1-m adult total length; Hadrosauridae: Lambeosaurinae) eggs were found in nests containing embryonic skeletons discovered by field parties of the Royal Tyrrell Museum of Paleontology, Drumheller, Alberta, Canada, from 1987 to 1999 in fluvial overbank deposits of the late Campanian Oldman Formation at Devil’s Coulee in southernmost Alberta. The nests, eggs, and embryos were described previously in considerable detail (93, 94). These near spherical eggs have dimensions of 18.5 × 20 cm and an estimated volume of 3,900 cc (93). This volume is 76% of the upper-bound of known dinosaur egg size [5,164 cc (43)]. The estimated mass of the egg and embryo [vol. × 1.09 g/cc (43, 92)] at the time of hatching is 4,251 g. We focused on specimens from a clutch containing four broken eggs which include embryonic remains within the confines of the eggs (TMP 87.79.149; Royal Tyrrell Museum of Paleontology, Drumheller, Alberta, Canada) and associated remains of four or more individuals (93), and an isolated same-sized embryonic tooth (TMP 87.077.0099). The latter was found slightly lower in a section from the main nesting horizon (93, 94). The embryos are ∼57 cm in length and are advanced in developmental stage, having well-formed skeletal elements and dental batteries with teeth worn in ovo (93). We sampled the isolated tooth and a left dentary from the nest that preserves five tooth families (Fig. 2D).
Incubation Period and Tooth Replacement Rate Determinations.
Each jaw was digitally prepared using high-resolution CT (2010 GE phoenix v|tome|x s240 high‐resolution microfocus CT system, General Electric) at the Microscopy and Imaging Facility of the American Museum of Natural, History, NY. (The original tomography data are available on request from the authors.) The renderings were used to determine time required to form the tooth batteries from longitudinal CT sections, where the entirety of development is represented [i.e., increments represented only at the apex of the tooth, and all others extending down to the root are accounted for (52, 53)] (Fig. 1C and Fig. S1). Dentine thickness was measured on the labial sides of the teeth and divided by the mean incremental line widths (see below) to reveal time of formation (in days) for each tooth. For IGM 100/1021a (P. andrewsi) that possesses just one functional tooth, the age of the oldest tooth crown in the battery represents the time required to form the hatchling tooth battery. Although the embryonic tooth replacement rate for P. andrewsi was not used in the incubation period calculation, it was determined for future use in comparative dental studies. This was determined using the difference in total incremental line counts between functional and replacement teeth based on tooth families that were uncrushed (Fig. S1). For TMP 87.79.149 (H. stebingeri), whose tooth families are developed into a battery that is composed of a replacement tooth, an older functional tooth crown, and an even older, heavily worn functional tooth root (the nubbins of which were about to be shed), the age of the younger functional tooth crown and the replacement rate were similarly determined. The replacement rate was added to the age of the younger functional tooth crown, thereby revealing the formation time of the oldest tooth and time required to form the hatchling tooth battery (Fig. S1).
The methodology for determining tooth formation periods and replacement rates in reptiles using von Ebner’s growth lines is described by Erickson (52, 53). Specifically, the distal half of the P. andrewsi jaw was transversely sectioned in 1.2-mm segments using a slow-speed, diamond-bladed petrographic saw (Isomet 1000; Buehler). The same was then done to the remaining mesial portion of the dentary in the longitudinal plane. All sections were prepared for viewing with polarized (transmitted light) petrographic microscopy (BX-60; Olympus). The H. stebingeri tooth was serially sectioned in the transverse plane and similarly prepared for petrographic microscopic analysis. Longitudinal growth-line expression was examined using incidental light (direct illumination) dissection microscopy (SZX-12; Olympus) on fractured teeth within the jaw. Incremental lines of von Ebner were discovered in the teeth of both taxa (Fig. 1 A and B). Mean growth-line widths were determined for 15- to 20-well–delineated increments in the teeth of each taxon for use in the developmental analysis described above (Fig. S1).
Amniote teeth do not appear before skull and jaw formation; this occurs well into embryonic development in egg-laying reptiles (66). Thus, in ovo tooth formation times provide absolute minimum estimates of incubation period. Almost all toothed reptiles produce several generations of teeth before establishing the functional compliment at hatching (66). The primordial null-generation teeth are resorbed, incorporated into the jaws, or shed in ovo (3, 66, 67). For example, American alligators (Alligator mississippiensis) go through two to four tooth-replacement cycles before hatching (95–97). The timing for the establishment of amniote hatchling functional dentitions is well established. In crocodilians (extant sister taxon to Dinosauria) it typically occurs between 42% and 52% of the total incubation period (3) and at >51% in squamates (64, 65, 67–69). In chicken (Gallus gallus domesticus), living dinosaurs, teeth primordia—none of which become functional—appear at 66% through incubation (98). We conservatively adjusted the total embryonic dinosaur tooth battery formation times to accommodate 42% of the incubation period for skull and jaw formation. The actual incubation periods were likely somewhat greater because (i) the embryos had yet to hatch and (ii) it is indeterminable whether the teeth in the fossils are truly the final hatchling compliment.
Comparative Incubation Analysis.
We plotted the dinosaur incubation data on a log-transformed modern comprehensive compilation of incubation periods relative to egg mass for birds (Aves; n = 1,525 species) (12). The phylogentically corrected [comparative analysis by independent contrasts (CAIC) (99)] results were used to test the hypothesis that dinosaurs showed typical rapid avian-grade incubation periods. The results were then compared with predictions for eggs typical of extant reptiles [lizards (Squamata), n = 90 species; snakes (Squamata: Serpentes), n = 51 species; crocodilians (Crocodylia), n =12 species; turtles (Chelonia), n = 48 species] (12). The phylogentically corrected (CAIC) results were then used to test the alternative hypothesis that dinosaurs retained primitive reptilian incubation periods.
Acknowledgments
We thank James Gardner, Brandon Strilisky, Don Brinkman, François Therrien, and Carl Mehling for facilitating our access to specimens; Stephen Hendricks and Brian Inouye for discussions about the research; Mick Ellison and Ken Womble for photographic and graphics assistance; Amy Davison for preparing the Protoceratops materials; and Carolyn Merrill and Paul Gignac for conducting the CT scanning. Funding was generously provided by National Science Foundation Grant EAR 0959029 (to G.M.E. and M.A.N.); the Macaulay Family (M.A.N.); and Natural Sciences and Engineering Research Council Discovery Grant 327513-09 (to D.K.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613716114/-/DCSupplemental.
References
- 1.Horner JR, De Ricqlès A, Padian K. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: Growth dynamics and physiology based on an ontogenetic series of skeletal elements. J Vertebr Paleontol. 2000;20(1):115–129. [Google Scholar]
- 2.Erickson GM. On dinosaur growth. Annu Rev Earth Planet Sci. 2014;42:675–697. [Google Scholar]
- 3.Ferguson MW. Reproductive biology and embryology of the crocodilians. In: Gans C, Billet FS, Manderson PFA, editors. Biology of the Reptilia. Vol 14. Wiley and Sons; New York: 1985. pp. 329–491. [Google Scholar]
- 4.Miller JD. Criteria for staging reptilian embryos. In: Grigg G, Shine R, Ehmann H, editors. Biology of Australasian Frogs and Reptiles. Surrey Beatty and Sons; Sydney: 1985. pp. 305–310. [Google Scholar]
- 5.Ricklefs RE, Starck JM. Embryonic growth and development. In: Starck JM, Ricklefs RE, editors. Avian Growth and Development. Evolution Within the Alricial-Precocial Spectrum. Oxford Univ Press; New York: 1998. pp. 31–58. [Google Scholar]
- 6.Mayfield H. Nesting success calculated from exposure. Wilson Bull. 1961;73:255–261. [Google Scholar]
- 7.Martin TE. A new view of avian life-history evolution tested on an incubation paradox. Proc Biol Sci. 2002;269(1488):309–316. doi: 10.1098/rspb.2001.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinsmore SJ, White GC, Knopf FL. Advanced techniques for modeling avian nest survival. Ecology. 2002;83(12):3476–3488. [Google Scholar]
- 9.Grant TA, Shaffer TL, Madden EM, Pietz PJ. Time-specific variation in passerine nest survival: New insights into old questions. Auk. 2005;122(2):661–672. [Google Scholar]
- 10.Rahn H, Ar A. The avian egg: Incubation time and water loss. Condor. 1974;76(2):147–152. [Google Scholar]
- 11.Birchard GF, Marcellini D. Incubation time in reptilian eggs. J Zool. 1996;240(4):621–635. [Google Scholar]
- 12.Deeming DC, Birchard GF, Crafer R, Eady PE. Egg mass and incubation period allometry in birds and reptiles: Effects of phylogeny. J Zool. 2006;270(2):209–218. [Google Scholar]
- 13.Gill FB. Ornithology 3rd. WH Freeman Company; New York: 2007. [Google Scholar]
- 14.Martin TE. Avian life-history evolution has an eminent past: Does it have a bright future? Auk. 2004;121(2):289–301. [Google Scholar]
- 15.Vleck D, Vleck CM, Hoyt DF. Metabolism of avian embryos: Ontogeny of oxygen consumption in the rhea and emu. Physiol Zool. 1980;53(2):125–135. [Google Scholar]
- 16.Drent R. Incubation. In: Farner DS, Kind JR, Parkes KC, editors. Avian Biology. Vol 5. Academic; New York: 1975. pp. 333–420. [Google Scholar]
- 17.Drent RH. Adaptive aspects of the physiology of incubation. In: Voous KH, editor. Proceedings of the Fifteenth International Ornithological Congress. E. J. Brill, Leiden; The Netherlands: 1972. pp. 255–280. [Google Scholar]
- 18.Gauthreaux SA., Jr The ecology and evolution of avian migration systems. Avian Biol. 1982;6:93–168. [Google Scholar]
- 19.Varricchio D, Jackson F, Borkowski J, Horner J. Nest and egg clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature. 1997;385(6613):247–250. [Google Scholar]
- 20.Thompson MB, Speake BK, Deeming DC. Egg morphology and composition. In: Deeming DC, editor. Reptilian Incubation: Environment, Evolution and Behaviour. Nottingham Univ Press; Nottingham, UK: 2004. pp. 45–74. [Google Scholar]
- 21.Werner J, Griebeler EM. New insights into non-avian dinosaur reproduction and their evolutionary and ecological implications: Linking fossil evidence to allometries of extant close relatives. PLoS One. 2013;8(8):e72862. doi: 10.1371/journal.pone.0072862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vleck CM, Hoyt DF. Metabolism and energetics of reptilian and avian embryos. In: Deeming DC, Ferguson MWJ, editors. Egg Incubation; Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ Press; Cambridge, UK: 1991. pp. 285–306. [Google Scholar]
- 23.Varricchio DJ, Jackson FD. Two eggs sunny-side up: Reproductive physiology in the dinosaur Troodon formosus. In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Feathered Dragons: Studies on the Transition from Dinosaurs to Birds. Indian Univ Press; Bloomington: 2004. pp. 215–233. [Google Scholar]
- 24.Ricklefs RE. An analysis of nesting mortality in birds. Smithson Contrib Zool. 1969;9:1–48. [Google Scholar]
- 25.Rand AS. Iguana egg mortality within the nest. Copeia. 1980;1980(3):531–534. [Google Scholar]
- 26.Zug GR, Vitt LJ, Caldwell JP. Reproductive ecology and life histories. In: Zug GR, Vitt LJ, Caldwell JP, editors. Herpetology: An Introductory Biology of Amphibians and Reptiles. 2nd Ed. Academic; San Diego: 2001. pp. 135–153. [Google Scholar]
- 27.Deeming DC, Ferguson MWJ. Physiological effects of incubation temperature on embryonic development in reptiles and birds. In: Deeming DC, Ferguson MWJ, editors. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ Press; Cambridge, UK: 1991. pp. 147–171. [Google Scholar]
- 28.Booth DT, Thompson MB. A comparison of reptilian eggs with those of megapode birds. In: Deeming DC, Ferguson MWJ, editors. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ Press; Cambridge, UK: 1991. pp. 325–344. [Google Scholar]
- 29.Paganelli CV. The avian eggshell as a mediating barrier: Respiratory gas fluxes and pressures during development. In: Deeming DC, Ferguson MWJ, editors. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ Press; Cambridge, UK: 1991. pp. 261–275. [Google Scholar]
- 30.Huxley TH. On the animals which are most nearly intermediate between birds and reptiles. Annals Mag Nat Hist. 1868;4(2):66–75. [Google Scholar]
- 31.Ostrom JH. The ancestry of birds. Nature. 1973;242:136. [Google Scholar]
- 32.Schweitzer MH, Wittmeyer JL, Horner JR. Gender-specific reproductive tissue in ratites and Tyrannosaurus rex. Science. 2005;308(5727):1456–1460. doi: 10.1126/science.1112158. [DOI] [PubMed] [Google Scholar]
- 33.Norell MA, Clark JM, Chiappe LM, Dashzeveg D. A nesting dinosaur. Nature. 1995;378(6559):774–776. [Google Scholar]
- 34.Dong ZM, Currie PJ. On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People’s Republic of China. Can J Earth Sci. 1996;33(4):631–636. [Google Scholar]
- 35.Erickson GM, Curry Rogers K, Varricchio DJ, Norell MA, Xu X. Growth patterns in brooding dinosaurs reveals the timing of sexual maturity in non-avian dinosaurs and genesis of the avian condition. Biol Lett. 2007;3(5):558–561. doi: 10.1098/rsbl.2007.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelenitsky DK. Reproductive traits of non-avian theropods. J Paleontol Soc Korea. 2006;22:209–216. [Google Scholar]
- 37.Mikhailov KE. 1992. The microstructure of avian and dinosaurian eggshell; phylogenetic implications. Papers in Avian Paleontology; Honoring Pierce Brodkorb, ed Campbell, Jr KE (Scripta Publishing, Silver Spring, MD), pp 141–159.
- 38.Zelenitsky DK, Hills LV, Currie PJ. Parataxonomic classification of ornithoid eggshell fragments from the Oldman Formation (Judith River Group; upper Cretaceous), southern Alberta. Can J Earth Sci. 1996;33(12):1655–1667. [Google Scholar]
- 39.Wiemann J, et al. 2015 The blue-green eggs of dinosaurs: How fossil metabolites provide insights into the evolution of bird reproduction. PeerJ PrePrints (No. e1323). Available at https://peerj.com/preprints/1080v1/. Accessed December 12, 2016.
- 40.Sato T, Cheng YN, Wu XC, Zelenitsky DK, Hsiao YF. A pair of shelled eggs inside a female dinosaur. Science. 2005;308(5720):375. doi: 10.1126/science.1110578. [DOI] [PubMed] [Google Scholar]
- 41.Zelenitsky DK, Therrien F. Unique maniraptoran egg clutch from the Upper Cretaceous Two Medicine Formation of Montana reveals theropod nesting behavior. Palaeontology. 2008;51(6):1253–1259. [Google Scholar]
- 42.Varricchio DJ, Jackson FD. Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode. Auk. 2016;133(4):654–684. [Google Scholar]
- 43.Carpenter K. 1999. Eggs, Nests, and Baby Dinosaurs: A Look at Dinosaur Reproduction (Indiana Univ Press, Bloomington), 341 pp.
- 44.Werner J, Griebeler EM. Reproductive biology and its impact on body size: Comparative analysis of mammalian, avian and dinosaurian reproduction. PLoS One. 2011;6(12):e28442. doi: 10.1371/journal.pone.0028442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SA. Incubation times of dinosaur eggs via embryonic metabolism. Phys Rev E Stat Nonlin Soft Matter Phys. 2016;94(2-1):022402. doi: 10.1103/PhysRevE.94.022402. [DOI] [PubMed] [Google Scholar]
- 46.Ruxton GD, Birchard GF, Deeming DC. Incubation time as an important influence on egg production and distribution into clutches for sauropod dinosaurs. Paleobiology. 2014;40(3):323–330. [Google Scholar]
- 47.Kundrát M, Cruickshank AR, Manning TW, Nudds J. Embryos of therizinosauroid theropods from the Upper Cretaceous of China: Diagnosis and analysis of ossification patterns. Acta Zool. 2008;89(3):231–251. [Google Scholar]
- 48.Ozaki T, Mishima H, Elsey RM. Incremental lines in the dentin of alligator teeth before and after hatching. Nihon Univ J Oral Sci. 2002;28(2):143–151. [Google Scholar]
- 49.Massler M, Schour I. Growth of the child and the calcification pattern of the teeth. Am J Orthod Oral Surg. 1946;32(9):495–517. doi: 10.1016/0096-6347(46)90067-1. [DOI] [PubMed] [Google Scholar]
- 50.Dean MC. Incremental markings in enamel and dentine: What they can tell us about the way teeth grow. In: Teaford MF, Smith MM, Ferguson MWJ, editors. Development, Function and Evolution of Teeth. Cambridge Univ Press; Cambridge, UK: 2000. pp. 119–130. [Google Scholar]
- 51.Rinaldi C, Cole TM. Environmental seasonality and incremental growth rates of beaver (Castor canadensis) incisors: Implications for palaeobiology. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;206(3):289–301. [Google Scholar]
- 52.Erickson GM. Daily deposition of dentine in juvenile Alligator and assessment of tooth replacement rates using incremental line counts. J Morphol. 1996a;228(2):189–194. doi: 10.1002/(SICI)1097-4687(199605)228:2<189::AID-JMOR7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Erickson GM. Incremental lines of von Ebner in dinosaurs and the assessment of tooth replacement rates using growth line counts. Proc Natl Acad Sci USA. 1996b;93(25):14623–14627. doi: 10.1073/pnas.93.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Emic MD, Whitlock JA, Smith KM, Fisher DC, Wilson JA. Evolution of high tooth replacement rates in sauropod dinosaurs. PLoS One. 2013;8(7):e69235. doi: 10.1371/journal.pone.0069235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schour I, Hoffman MM. Studies in tooth development. 1. The 16 microns calcification rhythm in the enamel and dentin from fish to man. J Dent Res. 1939a;18:91–102. [Google Scholar]
- 56.Schour L, Hoffman MM. Studies in tooth development. 2. The rate of apposition of enamel and dentin in man and other mammals. J Dent Res. 1939b;18:161–175. [Google Scholar]
- 57.Hillson S. Teeth. Cambridge Univ Press; Cambridge, UK: 1986. [Google Scholar]
- 58.Avery JK. Dentin. In: Reinhardt RW, Steube M, editors. Orban’s Oral Histology and Embryology. 11th Ed. Mosby-Year Book; St. Louis: 1991. pp. 106–138. [Google Scholar]
- 59.Gadow H. 1883. Catalogue of the Passeriformes, or Perching Birds, in the Collection of the British Museum, Volume 8: Cichlomorphae Part V and Certhiomorphae. (Order of the Trustees, London)
- 60.Gauthier J. Saurischian monophyly and the origin of birds. Mem California Acad Sci. 1986;8:1–55. [Google Scholar]
- 61.Deeming DC. Ultrastructural and functional morphology of eggshells supports the idea that dinosaur eggs were incubated buried in a substrate. Palaeontology. 2006;49(1):171–185. [Google Scholar]
- 62.Deeming DC. Importance and evolution of incubation in avian reproduction. In: Deeming DC, editor. Avian Incubation: Behaviour, Environment and Evolution. Oxford Univ Press; Oxford: 2002. pp. 1–7. [Google Scholar]
- 63.Hopwood N. A history of normal plates, tables and stages in vertebrate embryology. Int J Dev Biol. 2007;51(1):1–26. doi: 10.1387/ijdb.062189nh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson K. Post-ovipositional development of the monocled cobra, Naja kaouthia (Serpentes: Elapidae) Zoology (Jena) 2002;105(3):203–214. doi: 10.1078/0944-2006-00077. [DOI] [PubMed] [Google Scholar]
- 65.Boughner JC, et al. Embryonic development of Python sebae - I: Staging criteria and macroscopic skeletal morphogenesis of the head and limbs. Zoology (Jena) 2007;110(3):212–230. doi: 10.1016/j.zool.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Berkovitz BK. Tooth replacement patterns in non-mammalian vertebrates. In: Teaford MF, Smith MM, Ferguson MWJ, editors. Development, Function and Evolution of Teeth. Cambridge Univ Press; Cambridge, UK: 2000. pp. 186–200. [Google Scholar]
- 67.Zahradnicek O, Horacek I, Tucker AS. Tooth development in a model reptile: Functional and null generation teeth in the gecko Paroedura picta. J Anat. 2012;221(3):195–208. doi: 10.1111/j.1469-7580.2012.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noro M, Uejima A, Abe G, Manabe M, Tamura K. Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Dev Dyn. 2009;238(1):100–109. doi: 10.1002/dvdy.21828. [DOI] [PubMed] [Google Scholar]
- 69.Blaesild P, Granfeldt J. 2002. Statistics with Applications in Biology and Geology (Chapman and Hall/CRC, London), 568 pp.
- 70.Sabath K. Upper Cretaceous amniotic eggs from Gobi Desert. Acta Palaeontol Pol. 1991;36(2):151–192. [Google Scholar]
- 71.Wilson JA, Mohabey DM, Peters SE, Head JJ. Predation upon hatchling dinosaurs by a new snake from the Late Cretaceous of India. PLoS Biol. 2010;8(3):e1000322. doi: 10.1371/journal.pbio.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu X, et al. An integrative approach to understanding bird origins. Science. 2014;346(6215):1253293. doi: 10.1126/science.1253293. [DOI] [PubMed] [Google Scholar]
- 73.Erickson GM, Currie PJ, Inouye BD, Winn AA. Tyrannosaur life tables: An example of nonavian dinosaur population biology. Science. 2006;313(5784):213–217. doi: 10.1126/science.1125721. [DOI] [PubMed] [Google Scholar]
- 74.Erickson GM, Makovicky PJ, Inouye BD, Zhou CF, Gao KQ. A life table for Psittacosaurus lujiatunensis: Initial insights into ornithischian dinosaur population biology. Anat Rec (Hoboken) 2009;292(9):1514–1521. doi: 10.1002/ar.20992. [DOI] [PubMed] [Google Scholar]
- 75.Russell DA. The environments of Canadian dinosaurs. Canadian Geog J. 1973;87:4–11. [Google Scholar]
- 76.Hotton N., III . An alternative to dinosaur endothermy: The happy wanderers. In: Thomas RDK, Olson EC, editors. A Cold Look at Warm-Blooded Dinosaurs. AAAS Selected Symposium Series. Westview Press; Boulder, CO: 1980. pp. 311–350. [Google Scholar]
- 77.Bell PR, Snively E. Polar dinosaurs on parade: A review of dinosaur migration. Alcheringa. 2008;32(3):271–284. [Google Scholar]
- 78.Seymour RS. Dinosaur eggs: Gas conductance through the shell, water loss during incubation and clutch size. Paleobiology. 1979;5(1):1–11. [Google Scholar]
- 79.Tanaka K, Zelenitsky DK, Therrien F. Eggshell porosity provides insight on evolution of nesting in dinosaurs. PLoS One. 2015;10(11):e0142829. doi: 10.1371/journal.pone.0142829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sander PM, et al. Biology of the sauropod dinosaurs: The evolution of gigantism. Biol Rev Camb Philos Soc. 2011;86(1):117–155. doi: 10.1111/j.1469-185X.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werner J, Griebeler EM. Reproductive biology and its impact on body size: Comparative analysis of mammalian, avian and dinosaurian reproduction. PLoS One. 2011;6(12):e28442. doi: 10.1371/journal.pone.0028442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brusatte SL, et al. The extinction of the dinosaurs. Biol Rev Camb Philos Soc. 2015;90(2):628–642. doi: 10.1111/brv.12128. [DOI] [PubMed] [Google Scholar]
- 83.Erickson GM, Curry Rogers K, Yerby SA. Dinosaurian growth patterns and rapid avian growth rates. Nature. 2001;412(6845):429–433. doi: 10.1038/35086558. [DOI] [PubMed] [Google Scholar]
- 84.Grady JM, Enquist BJ, Dettweiler-Robinson E, Wright NA, Smith FA. Dinosaur physiology. Evidence for mesothermy in dinosaurs. Science. 2014;344(6189):1268–1272. doi: 10.1126/science.1253143. [DOI] [PubMed] [Google Scholar]
- 85.Pough FH. The advantages of ectothermy for tetrapods. Am Nat. 1980;115:92–112. [Google Scholar]
- 86.Dingus L, et al. The geology of Ukhaa Tolgod (Djadokhta Formation, Upper Cretaceous, Nemegt Basin, Mongolia) Am Mus Novit. 2008;3616:1–40. [Google Scholar]
- 87.Grillet-Tinner G, Chiappe L, Bottjer D, Norell MA. Paleobiology of dinosaur eggs and nesting behaviors. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;232:294–321. [Google Scholar]
- 88.Norell MA, et al. A theropod dinosaur embryo and the affinities of the Flaming Cliffs dinosaur eggs. Science. 1994;266(5186):779–782. doi: 10.1126/science.266.5186.779. [DOI] [PubMed] [Google Scholar]
- 89.Balanoff AM, Norell MA, Grellet-Tinner G, Lewin MR. Digital preparation of a probable neoceratopsian preserved within an egg, with comments on microstructural anatomy of ornithischian eggshells. Naturwissenschaften. 2008;95(6):493–500. doi: 10.1007/s00114-008-0347-2. [DOI] [PubMed] [Google Scholar]
- 90.Varricchio DJ, Balanoff AM, Norell MA. Reidentification of avian embryonic remains from the Cretaceous of Mongolia. PLoS One. 2015;10(6):e0128458. doi: 10.1371/journal.pone.0128458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iverson J, Ewert MA. Physical characteristics of reptilian eggs and a comparison with avian eggs. In: Deeming DC, Ferguson MWJ, editors. Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles. Cambridge Univ Press; Cambridge, UK: 1991. pp. 87–100. [Google Scholar]
- 92.Williams DLG, Seymour RS, Kerourio P. Structure of fossil dinosaur eggshell from the Aix Basin, France. Palaeogeogr Palaeoclimatol Palaeoecol. 1984;45(1):23–37. [Google Scholar]
- 93.Horner JR, Currie PJ. Embryonic and neonatal morphology and ontogeny of a new species of Hypacrosaurus (Ornithischia, Lambeosauridae) from Montana and Alberta. In: Carpenter K, Hirsch KF, Horner JR, editors. Dinosaur Eggs and Babies. Cambridge Univ Press; Cambridge, UK: 1994. pp. 312–336. [Google Scholar]
- 94.Erickson GM, Zelenitsky DK. Osteohistology of Hypacrosaurus stebingeri teeth throughout ontogeny with comments on wear induced form and function. In: Eberth D, Evans D, editors. Hadrosaurs. Univ of Indiana Press; Bloomington: 2014. pp. 422–432. [Google Scholar]
- 95.Westergaard B, Ferguson MWJ. Development of the dentition in Alligator mississippiensis. Early embryonic development in the lower jaw. J Zool. 1986;210(4):575–597. doi: 10.1002/aja.1001870407. [DOI] [PubMed] [Google Scholar]
- 96.Westergaard B, Ferguson MWJ. Development of the dentition in Alligator mississippiensis. Later development in the lower jaws of embryos, hatchlings and young juveniles. J Zool. 1987;212(2):191–222. doi: 10.1002/aja.1001870407. [DOI] [PubMed] [Google Scholar]
- 97.Osborn JW. Relationship between growth and the pattern of tooth initiation in alligator embryos. J Dent Res. 1998;77(9):1730–1738. doi: 10.1177/00220345980770090901. [DOI] [PubMed] [Google Scholar]
- 98.Harris MP, Hasso SM, Ferguson MW, Fallon JF. The development of archosaurian first-generation teeth in a chicken mutant. Curr Biol. 2006;16(4):371–377. doi: 10.1016/j.cub.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 99.Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): An Apple Macintosh application for analysing comparative data. Comput Appl Biosci. 1995;11(3):247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]