Significance
Tbx20 is a transcription factor whose critical role in cardiogenesis is well-established. Here we functionally analyzed the electrophysiological effects produced by a mutation (p.R311C) in Tbx20 found in some affected individuals belonging to a family with long QT syndrome (an inherited cardiac arrhythmia due to delayed ventricular repolarization). We demonstrated that Tbx20 selectively increases the expression of KCNH2, which encodes for the channel Kv11.1 (hERG) that generates the main ventricular repolarizing current. Conversely, the p.R311C mutation disables the Tbx20 protranscriptional activity over KCNH2, leading to a decrease in the hERG current and a prolongation of the action potentials recorded in human induced pluripotent stem cell-derived cardiomyocytes. Therefore, we propose that Tbx20, besides its described role, regulates KCNH2 expression.
Keywords: Tbx20, hERG channels, long QT syndrome, cardiomyocytes, human induced pluripotent stem cells
Abstract
Long QT syndrome (LQTS) exhibits great phenotype variability among family members carrying the same mutation, which can be partially attributed to genetic factors. We functionally analyzed the KCNH2 (encoding for Kv11.1 or hERG channels) and TBX20 (encoding for the transcription factor Tbx20) variants found by next-generation sequencing in two siblings with LQTS in a Spanish family of African ancestry. Affected relatives harbor a heterozygous mutation in KCNH2 that encodes for p.T152HfsX180 Kv11.1 (hERG). This peptide, by itself, failed to generate any current when transfected into Chinese hamster ovary (CHO) cells but, surprisingly, exerted “chaperone-like” effects over native hERG channels in both CHO cells and mouse atrial-derived HL-1 cells. Therefore, heterozygous transfection of native (WT) and p.T152HfsX180 hERG channels generated a current that was indistinguishable from that generated by WT channels alone. Some affected relatives also harbor the p.R311C mutation in Tbx20. In human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), Tbx20 enhanced human KCNH2 gene expression and hERG currents (IhERG) and shortened action-potential duration (APD). However, Tbx20 did not modify the expression or activity of any other channel involved in ventricular repolarization. Conversely, p.R311C Tbx20 did not increase KCNH2 expression in hiPSC-CMs, which led to decreased IhERG and increased APD. Our results suggest that Tbx20 controls the expression of hERG channels responsible for the rapid component of the delayed rectifier current. On the contrary, p.R311C Tbx20 specifically disables the Tbx20 protranscriptional activity over KCNH2. Therefore, TBX20 can be considered a KCNH2-modifying gene.
Long QT syndrome (LQTS) is characterized by abnormal prolongation of the QT interval of the electrocardiogram (ECG) and is due to delayed ventricular repolarization. LQTS increases the occurrence of ventricular tachyarrhythmias, particularly torsade de pointes, leading to recurrent syncope, seizures, ventricular fibrillation, and sudden cardiac death (SCD) (1). At least 15 genes have been reported in autosomal-dominant forms of LQTS (1). However, mutations in KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3) represent the most frequent forms of LQTS (∼90%) (1, 2).
KCNH2 encodes Kv11.1, or hERG, channels, which generate the rapid component of the delayed rectifier current (IKr) responsible for ventricular repolarization in humans (3). In a Spanish family of African ancestry suffering LQTS, we identified a frameshift and a missense mutation in KCNH2 that were assumed to be the disease-causing mutations. However, in some family members, we also identified a missense mutation in TBX20 coding for the transcription factor Tbx20, which is necessary in early stages of heart development (4). Importantly, results in flies and mice demonstrated that Tbx20 is also required for maintaining adult heart function (5, 6).
Here we have tested the KCNH2 and TBX20 mutations to establish whether they can account for prolongation of repolarization. Our results demonstrated that more than “one hit” is necessary to give rise to LQTS in the affected relatives. Moreover, data reveal that the peptide resulting from the KCNH2 frameshift mutation exerts chaperone-like effects by increasing the membrane expression of WT hERG channels. Conversely, the p.R311C Tbx20 mutation specifically and markedly decreases KCNH2 expression. Therefore, our genetic and functional studies suggest that Tbx20 controls the expression of hERG channels in human myocytes and, thus, TBX20 may be considered a KCNH2-modifying gene.
Results
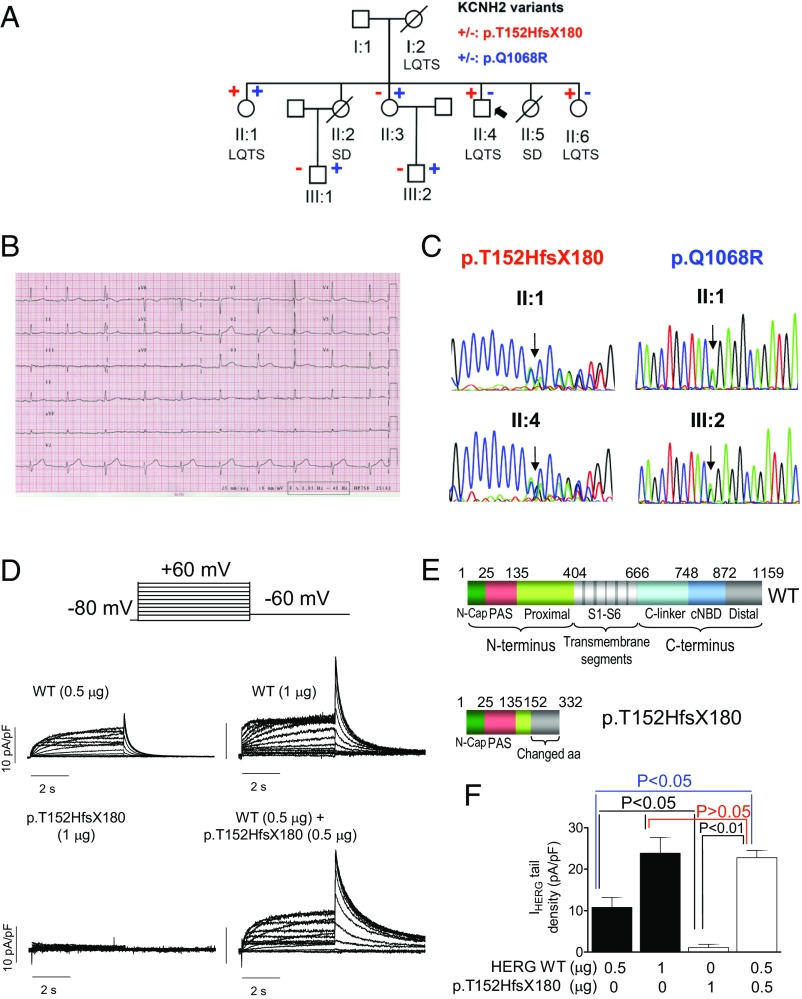
The proband (II:4; Fig. 1A) was a 41-y-old male who experienced syncope when he got out of bed. The ECG showed sinus rhythm at 68 beats per min (bpm) with normal PR (168 ms) and QRS (88 ms) but with a low-amplitude and wide T wave (Fig. 1B). Bazzett-corrected QT value (QTc) was 480 ms. Echocardiography, exercise test, and Holter were completely normal. Thereafter, bisoprolol treatment was started. No new episodes have been documented to date. Evaluation of the family identified two sisters who died suddenly: one at age 19, in the postpartum period (II:2), and another at age 17 (II:5). Both had been diagnosed with epilepsy and treated with phenobarbital until death. Interestingly, II:2, who exhibited a QTc of 440 ms, underwent an adrenaline test that was negative.
Fig. 1.
(A) Pedigree of the studied family. The arrow indicates the proband. Circles and squares represent females and males, respectively. + and – represent subjects with and without the p.T152HfsX180 and p.Q1068R hERG variants, respectively. (B) Twelve-lead electrocardiogram of the proband (paper speed 25 mm/s). (C) DNA sequence chromatograms depicting the heterozygous c.453dupC and the c.3203A>G changes of the KCNH2 gene in different family members. (D) Traces were obtained by applying the protocol (Top) for currents recorded in CHO cells transfected with WT, p.T152HfsX180, and WT/p.T152HfsX180 hERG channels. (E) Schematic representation of the WT and p.T152HfsX180 hERG protein domains. (F) hERG tail current density recorded in CHO cells transfected with WT, p.T152HfsX180, and WT/p.T152HfsX180 hERG channels after pulses to +60 mV (n ≥ 6). Each bar represents mean ± SEM of the data (n ≥ 8 cells).
Sister II:1 also presented syncopal episodes since she was 13, when she was diagnosed with epilepsy and treated with phenobarbital. After a syncopal episode at rest, the ECG showed a QTc of 560 ms, and ECG monitoring documented a polymorphic ventricular tachycardia. At age 43, she experienced an aborted SCD despite atenolol treatment (50 mg twice daily) and pacemaking at 75 bpm with a dual chamber pacemaker (DDD). Thereafter, a dual-chamber cardioverter defibrillator (ICD) was implanted. She has been asymptomatic since then.
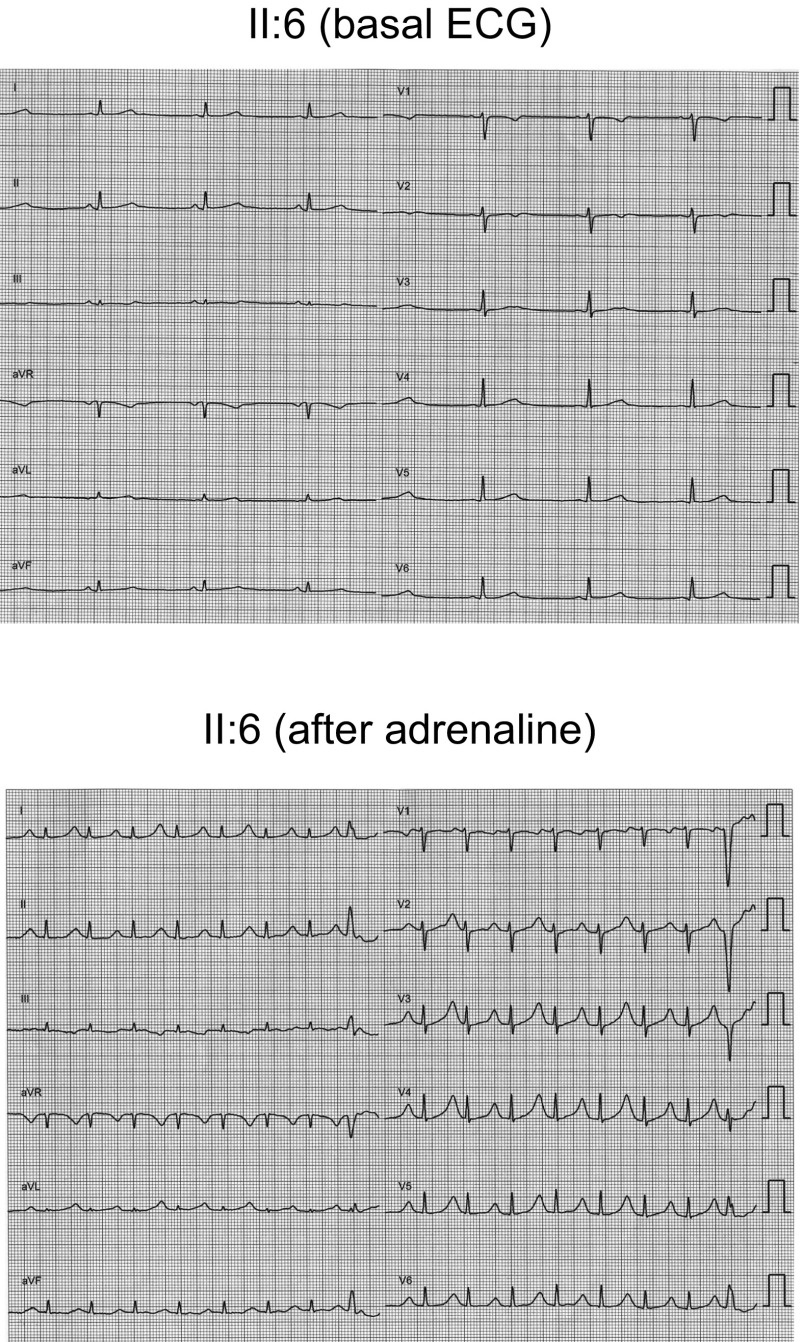
Sister II:3 has no cardiac symptoms. Her ECG showed sinus rhythm at 68 bpm with normal PR, QRS, and QTc values. Echocardiography, ergometry, Holter, and adrenaline test did not reveal any structural disease or arrhythmias. However, she suffers from lupus with a mild decrease of kidney function and is under treatment with prednisone, hydroxychloroquine, mycophenolate, and spironolactone (25 mg daily). Her most recent serum K+ concentration was 5.2 mEq per L. Sister II:6 is also asymptomatic; her ECG showed normal PR (140 ms) and QRS (90 ms) intervals but sinus bradycardia (48 bpm) and low-voltage wide QT waves (QTc 460 ms) (Fig. S1). An adrenaline test was positive: on ECG the QTc was prolonged to 618 ms, and there were T-wave amplitude alternans (Fig. S1) and polymorphic ventricular extrasystoles. Therefore, an ICD was implanted and bisoprolol treatment was started. The proband’s mother (66 y old), who had previously been asymptomatic with a normal ECG, was also diagnosed with LQTS after an aborted SCD episode during antibiotic therapy (piperacillin/tazobactam, ciprofloxacin, and tobramycin) in the context of chemotherapy (idarubicin and cytarabine) for the treatment of acute leukemia. An ICD was implanted but she died shortly thereafter from the leukemia. The proband’s father is still alive, asymptomatic, and with a normal ECG. The proband has two nephews (Fig. 1A). III:1 was studied (ECG, echocardiography, ergometry, and Holter) when he was a child; the results revealed an electrically and structurally normal heart. Afterward, an adrenaline test was conducted that was negative when he was 23 y old. Conversely, III:2 has experienced epileptic crises since he was 2 yo. He has been treated with oxcarbazepine since he was 6 y old, and no new episodes have been documented (he is 14 now). His ECG, Holter, echocardiogram, and stress test are normal.
Fig. S1.
Twelve lead ECGs of sister II:6 under basal conditions (Top) and after adrenaline bolus injection (Bottom) (paper speed 25 mm/s). Basal ECG shows normal PR (140 ms), QRS (90 ms), sinus bradycardia (48 bpm), and low-voltage wide QT waves (QTc 460 ms). As shown, the adrenaline test was positive, because QTc was prolonged to 618 ms, the amplitude of the T waves exhibited alternance, and polymorphic ventricular extrasystoles were developed. Adrenaline tests were developed in all relatives following the protocol described by Shimizu et al. (33).
KCNH2 Variants and Functional Analysis.
Next-generation sequencing of 82 genes (Table S1) demonstrated that the proband and sister II:1 carried a heterozygous frameshift mutation in the KCNH2 gene (NM_000238.3:c.453dupC) (Fig. 1C) encoding for p.T152HfsX180 hERG. This variant is also present in sister II:6. Sisters II:1 and II:3 and both nephews carry another variant in the KCNH2 gene (NM_000238.3:c.3203A>G) encoding for p.Q1068R hERG (Fig. 1C). Because recombination is a very rare event after fertilization, expression of the p.T152HfsX180 mutation in one allele and p.Q1068R in the other allele is more likely to represent the condition of sister II:1 (compound heterozygosity).
Table S1.
List of 82 genes included in the custom sequencing panel
| No. | Gene | Protein name | Location |
| 1 | ABCC9 | ATP-binding cassette, subfamily C (CFTR/MRP), member 9 (SUR2) | 12p12.1 |
| 2 | ACE | Angiotensin II converting enzyme | 17q23.3 |
| 3 | ADRB1 | Beta-1 adrenoreceptor | 10q24-q26 |
| 4 | ADRB2 | Beta-2 adrenoreceptor | 5q31-q32 |
| 5 | AKAP9 | A kinase (PRKA) anchor protein (yotiao) 9 | 7q21-q22 |
| 6 | ANK2 | Ankyrin 2 | 4q25-q27 |
| 7 | CACNA1C | Calcium channel, voltage-dependent, L type, alpha 1C subunit | 12p13.3 |
| 8 | CACNA1D | Calcium channel, voltage-dependent, L type, alpha 1D subunit | 3p14.3 |
| 9 | CACNA1G | Calcium channel, voltage-dependent, T type, alpha 1G subunit | 17q22 |
| 10 | CACNA1H | Calcium channel, voltage-dependent, T type, alpha 1H subunit | 16p13.3 |
| 11 | CACNA2D1 | Calcium channel, voltage-dependent, alpha 2/delta subunit 1 | 7q21-q22 |
| 12 | CACNB2 | Calcium channel, voltage-dependent, beta 2 subunit | 10p12 |
| 13 | CALM1 | Calmodulin 1 (phosphorylase kinase, delta) | 14q32.11 |
| 14 | CALM2 | Calmodulin 2 (phosphorylase kinase, delta) | 2p21 |
| 15 | CASQ2 | Calsequestrin 2 (cardiac muscle) | 1p13.3-p11 |
| 16 | Cav3 | Caveolin 3 | 3p25 |
| 17 | CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | 6p21.1 |
| 18 | CDKN1C | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | 11p15.5 |
| 19 | CHRM2 | Cholinergic receptor, muscarinic 2 | 7q35-q36 |
| 20 | DLG1 | Discs, large homolog 1 (SAP97) | 3q29 |
| 21 | DPP6 | Dipeptidyl-peptidase 6 | 7q36.2 |
| 22 | FGF13 | Fibroblast growth factor 13 | Xq26.3 |
| 23 | GATA4 | GATA binding protein 4 | 8p23.1-p22 |
| 24 | GJA1 | Gap junction protein, alpha 1, 43 kDa (Cx43) | 6q22-q23 |
| 25 | GJA5 | Gap junction protein, alpha 5, 40 kDa (Cx40) | 1q21.1 |
| 26 | GPC5 | Glypican 5 | 13q32 |
| 27 | GPD1L | Glycerol-3-phosphate dehydrogenase 1-like | 3p22.3 |
| 28 | HAND1 | Heart and neural crest derivatives expressed 1 | 5q33 |
| 29 | HCN1 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 1 | 5p12 |
| 30 | HCN2 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 2 | 19p13 |
| 31 | HCN3 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 3 | 1q21.2 |
| 32 | HCN4 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 4 | 15q24.1 |
| 33 | IRX5 | Iroquois homeobox 5 | 16q12.2 |
| 34 | KCNA4 | Potassium voltage-gated channel, shaker-related subfamily, member 4 | 11p14 |
| 35 | KCNA5 | Potassium voltage-gated channel, shaker-related subfamily, member 5 | 12p13 |
| 36 | KCNA7 | Potassium voltage-gated channel, shaker-related subfamily, member 7 | 19q13.3 |
| 37 | KCNB1 | Potassium voltage-gated channel, Shab-related subfamily, member 1 | 20q13.2 |
| 38 | KCNB2 | Potassium voltage-gated channel, Shab-related subfamily, member 2 | 8q13.2 |
| 39 | KCND2 | Potassium voltage-gated channel, Shal-related subfamily, member 2 | 7q31 |
| 40 | KCND3 | Potassium voltage-gated channel, Shal-related subfamily, member 3 | 1p13.2 |
| 41 | KCNE1 | Potassium voltage-gated channel, Isk-related family, member 1 | 21q22.1-q22.2 |
| 42 | KCNE1L | Potassium voltage-gated channel, Isk-related family, member 1-like | Xq22.3 |
| 43 | KCNE2 | Potassium voltage-gated channel, Isk-related family, member 2 | 21q22.1 |
| 44 | KCNE3 | Potassium voltage-gated channel, Isk-related family, member 3 | 11q13.4 |
| 45 | KCNE4 | Potassium voltage-gated channel, Isk-related family, member 4 | 2q36.1 |
| 46 | KCNH2 | Potassium voltage-gated channel, subfamily H (eag-related), member 2 | 7q36.1 |
| 47 | KCNIP2 | Kv channel interacting protein 2 | 10q24.32 |
| 48 | KCNJ11 | Potassium inwardly rectifying channel, subfamily J, member 11 (Kir6.2) | 11p15.1 |
| 49 | KCNJ12 | Potassium inwardly rectifying channel, subfamily J, member 12 (Kir2.2) | 17p11.1 |
| 50 | KCNJ2 | Potassium inwardly rectifying channel, subfamily J, member 2 (Kir2.1) | 17q24.3 |
| 51 | KCNJ3 | Potassium inwardly rectifying channel, subfamily J, member 3 (Kir3.1) | 2q24.1 |
| 52 | KCNJ4 | Potassium inwardly rectifying channel, subfamily J, member 4 (Kir2.3) | 22q13.1 |
| 53 | KCNJ5 | Potassium inwardly rectifying channel, subfamily J, member 5 (Kir3.4) | 11q24 |
| 54 | KCNJ8 | Potassium inwardly rectifying channel, subfamily J, member 8 (Kir6.1) | 12p12.1 |
| 55 | KCNN3 | Potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 3 | 1q21.3 |
| 56 | KCNQ1 | Potassium voltage-gated channel, KQT-like subfamily, member 1 | 11p15.5 |
| 57 | KCNV1 | Potassium channel, subfamily V, member 1 | 8q23.2 |
| 58 | NCS1 | Neuronal calcium sensor 1 | 9q34.11 |
| 59 | NOS1 | Nitric oxide synthase 1 (neuronal) | 12q14-qter |
| 60 | NOS1AP | Nitric oxide synthase 1 (neuronal) adaptor protein | 1q23.3 |
| 61 | PITX2 | Paired-like homeodomain 2 | 4q25 |
| 62 | PLN | Phospholamban | 6q22.1 |
| 63 | PRKAG2 | Protein kinase, AMP-activated, gamma 2 noncatalytic subunit | 7q35-q36 |
| 64 | PRKCA | Protein kinase C, alpha | 17q22-q24 |
| 65 | PRMT3 | Protein arginine methyltransferase 3 | 11p15.1 |
| 66 | PRMT5 | Protein arginine methyltransferase 5 | 14q11.2 |
| 67 | RANGRF | RAN guanine nucleotide release factor (MOG1) | 17p13 |
| 68 | SCN10A | Sodium channel, voltage-gated, type X, alpha subunit | 3p22.2 |
| 69 | SCN1B | Sodium channel, voltage-gated, type I, beta subunit | 19q13.12 |
| 70 | SCN2B | Sodium channel, voltage-gated, type II, beta subunit | 11q22-qter |
| 71 | SCN3B | Sodium channel, voltage-gated, type III, beta subunit | 11q24.1 |
| 72 | SCN4B | Sodium channel, voltage-gated, type IV, beta subunit | 11q23.3 |
| 73 | SCN5A | Sodium channel, voltage-gated, type V, alpha subunit | 3p21 |
| 74 | SCN8A | Sodium channel, voltage gated, type VIII, alpha subunit | 12q13.1 |
| 75 | SLMAP | Sarcolemma associated protein | 3p21.2-p14.3 |
| 76 | SNTA1 | Syntrophin, alpha 1 (dystrophin-associated protein A1, 59 kDa, acidic component) | 20q11.2 |
| 77 | STRN | Striatin, calmodulin binding protein | 2p22.2 |
| 78 | TBX20 | T box 20 | 7p14.3 |
| 79 | TBX3 | T box 3 | 12q24.1 |
| 80 | TBX5 | T box 5 | 12q24.1 |
| 81 | TRPM4 | Transient receptor potential cation channel, subfamily M, member 4 | 19q13.3 |
| 82 | TRDN | Triadin | 6q22.31 |
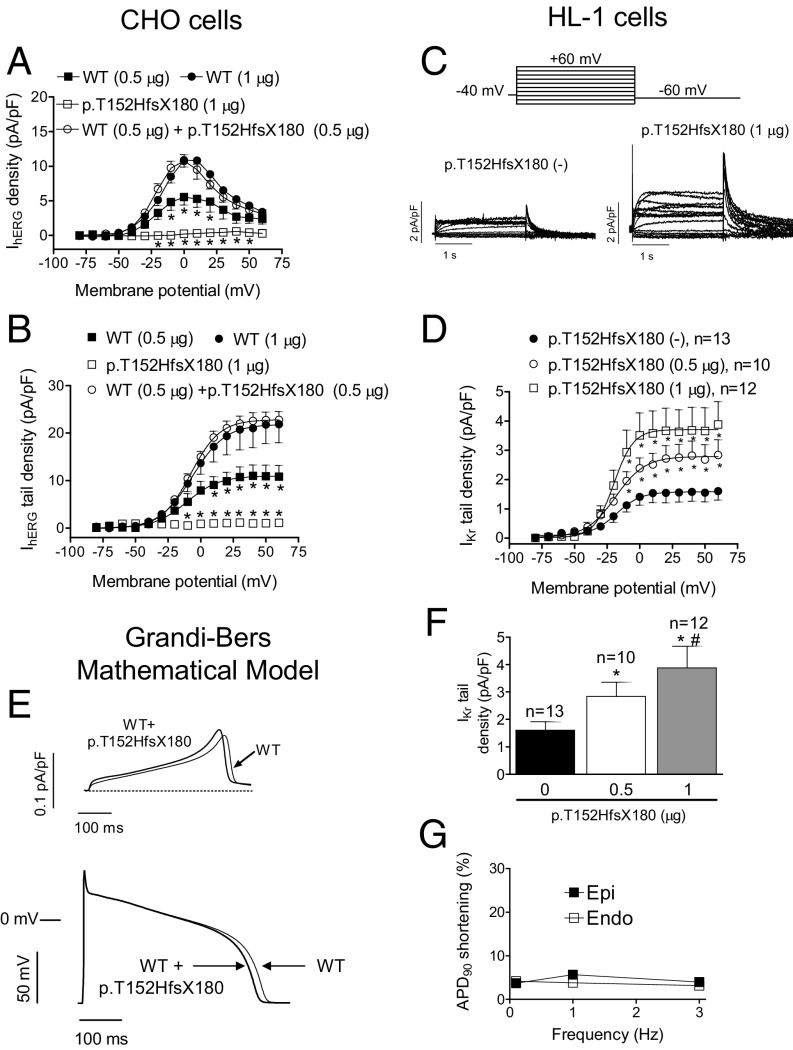
p.Q1068R is considered a “rare control” variant (7) that appears with a 0.03% frequency in the African population (Table S2). Functional analysis has demonstrated that the p.Q1068R mutation accelerates both inactivation and recovery from inactivation, whose time constants suffer ∼10- to 20-mV shifts in their voltage dependence (8). However, the p.Q1068R variant has not been considered pathogenic by itself (7). In contrast, p.T152HfsX180 hERG is a 332-aa peptide (Fig. 1E) that we considered to be highly pathogenic and responsible for the LQTS in this family. Thus, we conducted a functional analysis by transfecting CHO cells with cDNA encoding either WT (n = 7) or p.T152HfsX180 hERG (n = 6) channels (1 µg). hERG channels generated a slowly activating current whose amplitude progressively increased with pulses up to 0 mV and then progressively decreased at potentials >0 mV owing to the fast C-type inactivation (9), resulting in the bell-shaped current density–voltage curve typical of hERG channels (Fig. 2A). Fig. 1D shows that, as expected, p.T152HfsX180 hERG channels did not generate any current. To simulate the heterozygous condition of all of the mutation carriers, cells (n = 17) were transfected with WT plus p.T152HfsX180 hERG channels (0.5 + 0.5 µg). Surprisingly, maximum current amplitudes generated by depolarizing pulses (Fig. 2A) and tail currents recorded upon repolarization to −60 mV (Figs. 1F and 2B) were not statistically different from those generated by WT hERG channels (1 µg) (P > 0.05). We surmised that the p.T152HfsX180 hERG peptide could exert a “chaperone-like” effect by increasing membrane expression of WT hERG channels. In fact, Fig. 1F demonstrates that addition of the peptide (0.5 µg) to hERG WT (0.5 µg) generated significantly greater currents than those generated by hERG channels alone (P < 0.05). Furthermore, p.T152HfsX180 hERG did not modify the voltage dependence of hERG activation (Fig. 2B) but slowed deactivation (Table S3). To confirm this chaperone-like effect, we transfected mouse atrial-derived HL-1 cells with the p.T152HfsX180 hERG. Some HL-1 cells (36%) exhibited IKr as the main repolarizing current (IKr-predominant cells), whereas in other cells (36%) IKr overlapped with the slow component of the delayed rectifier current (IKs) (intermediate cells). Thus, IKr was measured in IKr-predominant and -intermediate cells as the dofetilide-sensitive current, because it was completely inhibited by this selective Kr blocker (1 µmol/L) (10). Fig. 2C demonstrates that p.T152HfsX180 hERG significantly increases both the maximum and tail amplitudes of IKr (P < 0.05). Furthermore, the tail current increase and the slowing of tail current deactivation depended on the amount of cDNA transfected (Fig. 2 D and F and Table S3).
Table S2.
Summary of all nonsynonymous variants identified in the proband
| Gene | Genotype | Ancestral allele | Variant | dbSNP_ID | MAF | Frequency in African population | Amino acid substitution | Transcript | PROVEAN prediction | SIFT prediction |
| ACE | Heterozygous | A | G | rs12709426 | 0.0050 | 0.0550 | D592G | NM_000789.3 | Neutral | Tolerated |
| ACE | Heterozygous | T | C | rs4317 | 0.0180 | 0.2010 | S32P | NM_152830.2 | Neutral | Tolerated |
| ACE | Heterozygous | A | G | rs4318 | 0.0190 | 0.2064 | S49G | NM_152830.2 | Neutral | Tolerated |
| ANK2 | Heterozygous | C | T | rs3733617 | 0.0900 | 0.3614 | P2835S | NM_001148.4 | Neutral | Tolerated |
| ANK2 | Heterozygous | C | G | rs45570339 | 0.0008 | 0.0089 | L3621V | NM_001148.4 | Neutral | Tolerated |
| CACNB2 | Heterozygous | G | C | rs149167651 | 0.0001 | 0.0012 | V158L | NM_201590.2 | Neutral | Tolerated |
| KCNB2 | Heterozygous | A | G | rs16938507 | 0.0041 | 0.0470 | E657G | NM_004770.2 | Neutral | Tolerated |
| KCND2 | Heterozygous | C | T | rs6975107 | 0.0097 | 0.1268 | Intronic | NM_012281.2 | Neutral | Damaging |
| KCNH2 | Heterozygous | T | TG | 0.0002 | 0.0011 | T152HfsX180 | NM_000238.3 | Unknown | Unknown | |
| KCNH2 | Heterozygous | T | C | rs151031345 | 0.00004 | 0.0003 | Q1068R | NM_000238.3 | Deleterious | Damaging |
| KCNN3 | Heterozygous | A | G | rs76040784 | 0.0120 | 0.1485 | 5′ UTR (−66 bp to ATG) | NM_002249.5 | Neutral | Damaging |
| TBX20 | Heterozygous | G | A | rs199774220 | 0.0002 | 0.0001 | R311C | NM_001077653.2 | Deleterious | Damaging |
Only nonsynonymous variants with a coverage >30 were included. MAF, minor allele frequency and frequency in the African population, as provided in exac.broadinstitute.org. Reference SNP numbers were obtained from https://www.ncbi.nlm.nih.gov/projects/SNP/.
Fig. 2.
(A and B) Maximum current density (current density–voltage relationships) (A) and tail currents (activation curves) (B) generated by WT and p.T152HfsX180 hERG channels alone or when they are cotransfected in CHO cells, as a function of the membrane potential. In B, solid lines represent the fit of a Boltzmann equation. *P < 0.05 vs. hERG WT (1 µg) (n ≥ 6). (C) IKr traces recorded in IKr-predominant HL-1 cells transfected or not with p.T152HfsX180 hERG. (D) IKr tail current densities recorded in HL-1 cells transfected or not with p.T152HfsX180 hERG. (E) Simulated IKr traces (Top) and APs (Bottom) obtained at 0.1 Hz by using the Grandi–Bers mathematical model of human ventricular endocardial cells by introducing the modifications produced by p.T152HfsX180 hERG on the IKr. (F) IKr tail current densities recorded in HL-1 cells transfected or not with p.T152HfsX180 hERG after pulses to +60 mV. (G) Percentage of APD90 shortening in APs simulated at different frequencies in epicardial and endocardial cells. Points/bars represent mean ± SEM of the data. In D and F, n, number of cells; *P < 0.05 vs. nontransfected cells; #P < 0.05 vs. p.T152HfsX180 0.5 μg transfected cells.
Table S3.
Time- and voltage-dependent properties of cardiac ion currents recorded in this study
| Current | τact, ms | Vhact, mV | kact | τfdeact, ms | τsdeact, ms | τfinact, ms | τsinact, ms | Vhinact, mV | kinact | τinact, ms | τdeact, ms |
| IhERG (CHO) | |||||||||||
| hERG WT (1 μg) | 1,887 ± 307 | −9.5 ± 2.5 | 12.8 ± 0.8 | 265 ± 15 | 1,229 ± 91 | ||||||
| hERG WT + p.T152HfsX180 (0.5 μg + 0.5 μg) | 1,136 ± 140* | −9.1 ± 1.6 | 11.1 ± 0.6 | 404 ± 58* | 1,571 ± 181 | ||||||
| IKr (HL-1) | |||||||||||
| p.T152HfsX180 (-) | 378 ± 61 | −15.7 ± 2.2 | 8.6 ± 1.0 | 70 ± 9 | 430 ± 49 | ||||||
| p.T152HfsX180 (0.5 μg) | 222 ± 36# | −16.9 ± 2.7 | 9.2 ± 1.3 | 112 ± 14# | 632 ± 86# | ||||||
| p.T152HfsX180 (1 μg) | 205 ± 35# | −15.5 ± 0.4.1 | 7.5 ± 0.8 | 162 ± 26# | 771 ± 98# | ||||||
| INa (HL-1) | |||||||||||
| Tbx20 (-) | 0.19 ± 0.01 | −40.6 ± 4.3 | 6.8 ± 1.1 | 1.2 ± 0.1 | 6.4 ± 1.1 | −92.9 ± 2.0 | 5.1 ± 0.3 | ||||
| Tbx20 WT | 0.21 ± 0.04 | −43.9 ± 0.7 | 5.9 ± 0.4 | 1.4 ± 0.2 | 6.4 ± 1.0 | −90.3 ± 2.0 | 5.0 ± 0.4 | ||||
| Tbx20 p.R311C | 0.16 ± 0.01 | −39.9 ± 2.1 | 6.4 ± 0.2 | 1.5 ± 0.3 | 7.4 ± 1.4 | −92.3 ± 3.1 | 5.6 ± 0.3 | ||||
| IBa (HL-1) | |||||||||||
| Tbx20 (-) | 1.9 ± 0.4 | 8.8 ± 1.4 | 6.6 ± 0.9 | −14.7 ± 3.8 | 7.2 ± 0.9 | 218 ± 46 | |||||
| Tbx20 WT | 1.5 ± 0.1 | 10.8 ± 1.3 | 6.9 ± 0.4 | −15.6 ± 3.3 | 7.3 ± 1.9 | 187 ± 16 | |||||
| Tbx20 p.R311C | 1.6 ± 0.2 | 9.0 ± 1.5 | 6.8 ± 1.1 | −16.4 ± 3.2 | 7.6 ± 0.9 | 168 ± 13 | |||||
| IKs (HL-1) | |||||||||||
| Tbx20 (-) | 1,817 ± 487 | 35.8 ± 2.0 | 15.3 ± 2.3 | 629 ± 157 | |||||||
| Tbx20 WT | 1,497 ± 217 | 36.5 ± 5.2 | 16.8 ± 3.6 | 630 ± 173 | |||||||
| Tbx20 p.R311C | 1,558 ± 404 | 36.8 ± 5.5 | 18.4 ± 3.4 | 467 ± 131 | |||||||
| IKr (HL-1) | |||||||||||
| Tbx20 (-) | 518 ± 77 | −10.7 ± 3.6 | 7.7 ± 1.7 | 179 ± 29 | 1,168 ± 169 | ||||||
| Tbx20 WT | 486 ± 75 | −12.2 ± 3.8 | 7.8 ± 0.7 | 150 ± 19 | 1,142 ± 157 | ||||||
| Tbx20 p.R311C | 574 ± 98 | −9.2 ± 1.8 | 9.2 ± 1.2 | 177 ± 20 | 1,106 ± 126 | ||||||
| IKr (hiPSC) | |||||||||||
| Tbx20 (-) | 2.4 ± 1.9 | 7.2 ± 3.6 | 357 ± 80 | ||||||||
| Tbx20 WT | 4.5 ± 2.0 | 7.4 ± 1.6 | 280 ± 47 | ||||||||
| Tbx20 p.R311C | 1.7 ± 2.5 | 7.5 ± 0.6 | 398 ± 86 |
τact, time constant of activation measured at 0 mV (IhERG and IKr in HL-1), peak maximum current (INa), +20 mV (IBa), and +60 mV (IKs); Vhact and kact, midpoint and slope values of the activation (IhERG and IKr) or conductance–voltage curves (INa, IBa, and IKs); τfdeact and τsdeact, fast and slow time constants of IHERG and IKr deactivation measured at −60 mV after pulses to +60 mV; τfinact and τsinact, fast and slow time constants of INa inactivation measured at peak maximum current; τinact, time constant of IBa inactivation measured at +20 mV; Vhinact and kinact, midpoint and slope values of the inactivation curves (INa and IBa); τdeact, time constant of deactivation measured at −30 mV (IKs) or −60 mV (IKr in hiPSCs) after pulses to +60 mV. Each value represents mean ± SEM of >6 experiments in each group. *P < 0.05 vs. hERG WT (1 μg); #P < 0.05 vs. p.T152HfsX180 (-).
We used a previously validated in silico model of the human ventricular action potential (AP) (11) to test for the effects of the heterozygous p.T152HfsX180 hERG mutation. The model was run for endocardial and epicardial cells at different frequencies ranging between 0.1 and 3 Hz. The voltage- and time-dependent characteristics of currents generated by WT+p.T152HfsX180 hERG channels were incorporated into the model to simulate mutation effects. Fig. 2E shows superimposed human endocardial APs driven at 0.1 Hz generated by WT and WT+p.T152HfsX180 hERG channels. As can be observed, the duration of the heterologous mutant case AP (APD; action-potential duration) was slightly briefer. Furthermore, APD measured at 90% of repolarization (APD90) of simulated WT+p.T152HfsX180 endocardial and epicardial cells was only slightly abbreviated (∼3%) at either driving frequency (Fig. 2G).
Overall, these results suggested that the heterozygous p.T152HfsX180 hERG mutation produced subtle effects over the IKr, even when confirmation on a more physiological setting is needed.
TBX20 Mutation and Functional Analysis.
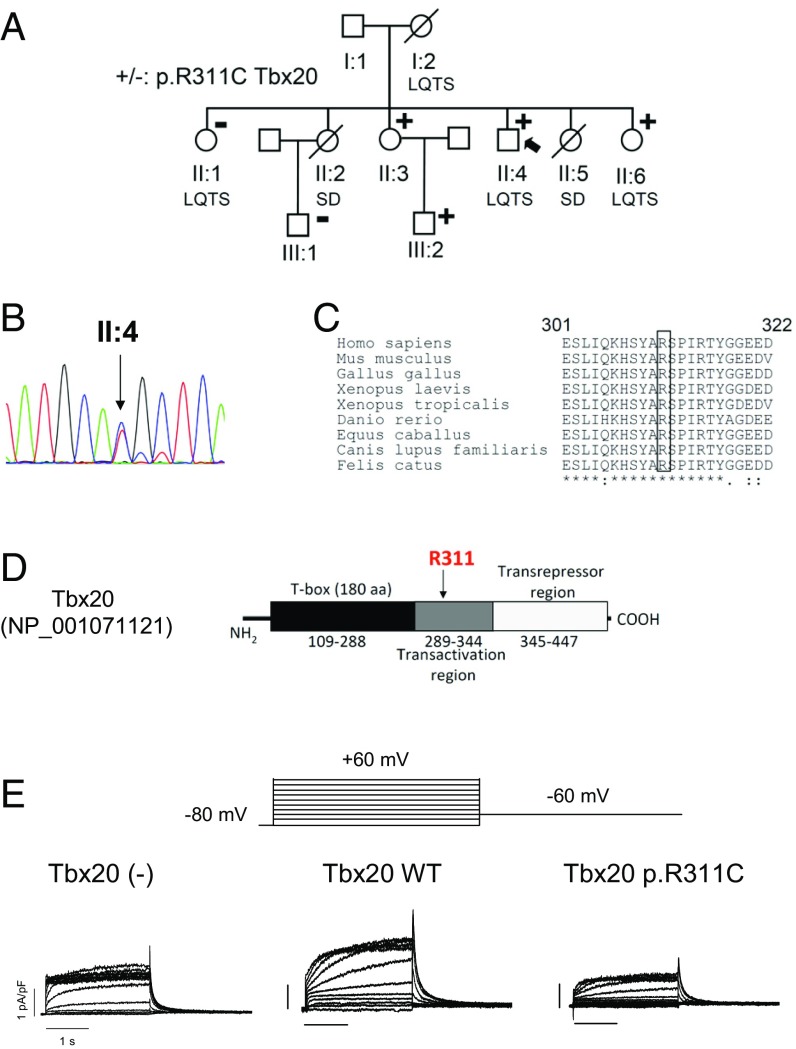
Next-generation sequencing of the proband also identified the heterozygous mutation NM_001077653.2:c.931C>T at the TBX20 gene (Table S2), which was confirmed by Sanger analysis (Fig. 3B). The mutation leads to the substitution of Arg311 by Cys (p.R311C Tbx20). Arg311, which is highly conserved among different species (Fig. 3C), is located in the transactivation region of Tbx20 (Fig. 3D). Two affected (II:6 and III:2) and another unaffected (II:3) of the proband’s relatives also carry the p.R311C Tbx20 mutation (Fig. 3A). The p.R311C Tbx20 variation was annotated with a 0.01% frequency in Africans. Other nonsynonymous variants identified in the proband are listed in Table S2.
Fig. 3.
(A) Pedigree of the studied family. The arrow indicates the proband. Circles and squares represent females and males, respectively. + and – represent subjects with and without the p.R311C Tbx20 mutation, respectively. (B) DNA sequence chromatograms of the proband depicting the heterozygous change (c.931C>T) of the TBX20 gene. (C) Sequence alignment of the region surrounding R311 in Tbx20 in several species. The box highlights the conservation of this residue. (D) Schematic representation of the Tbx20 sequence, indicating the T box, and the transactivation and transrepressor regions. (E) IKr traces recorded in IKr-predominant HL-1 cells transfected or not with either WT or p.R311C Tbx20 by applying the pulse protocol (Top).
Tbx20 binds to the consensus sequence “AGGTGTG” within the DNA of target genes (6). We hypothesized that Tbx20 might regulate the expression of cardiac ion channels involved in the control of human cardiac repolarization as it does in fly and mouse adult hearts (5, 6). Sequence analysis of mouse and human promoters of genes encoding ion-channel subunits revealed that a Tbx20 binding site appears in both KCNH2 genes (Table S4). Thus, we aimed at identifying the effects of WT and p.R311C Tbx20 on the expression of hERG in HL-1 cells by recording IKr. Transfection with WT (60.8 ± 7.2 pF, n = 72) or p.R311C Tbx20 (65.6 ± 9.4 pF, n = 65) plasmids did not modify HL-1 cell capacitance (55.2 ± 9.0 pF, n = 68) (P > 0.05).
Table S4.
Presence of the Tbx20 binding site in human and mouse promoter sequences of genes encoding ion-channel subunits and transcription factors relevant to human cardiac repolarization
Mouse and human promoter sequences were obtained from epd.vital-it.ch and switchgeargenomics.com/products/promoter-reporter-collection, respectively. Human sequences include positions ∼−800 to +200, whereas mouse sequences include positions ∼−3000 to +200. Positions were numbered with respect to the transcription start site (+1). Rat KCNJ2 promoter sequence (XM_006247567.2) does not exhibit the Tbx20 consensus binding site.
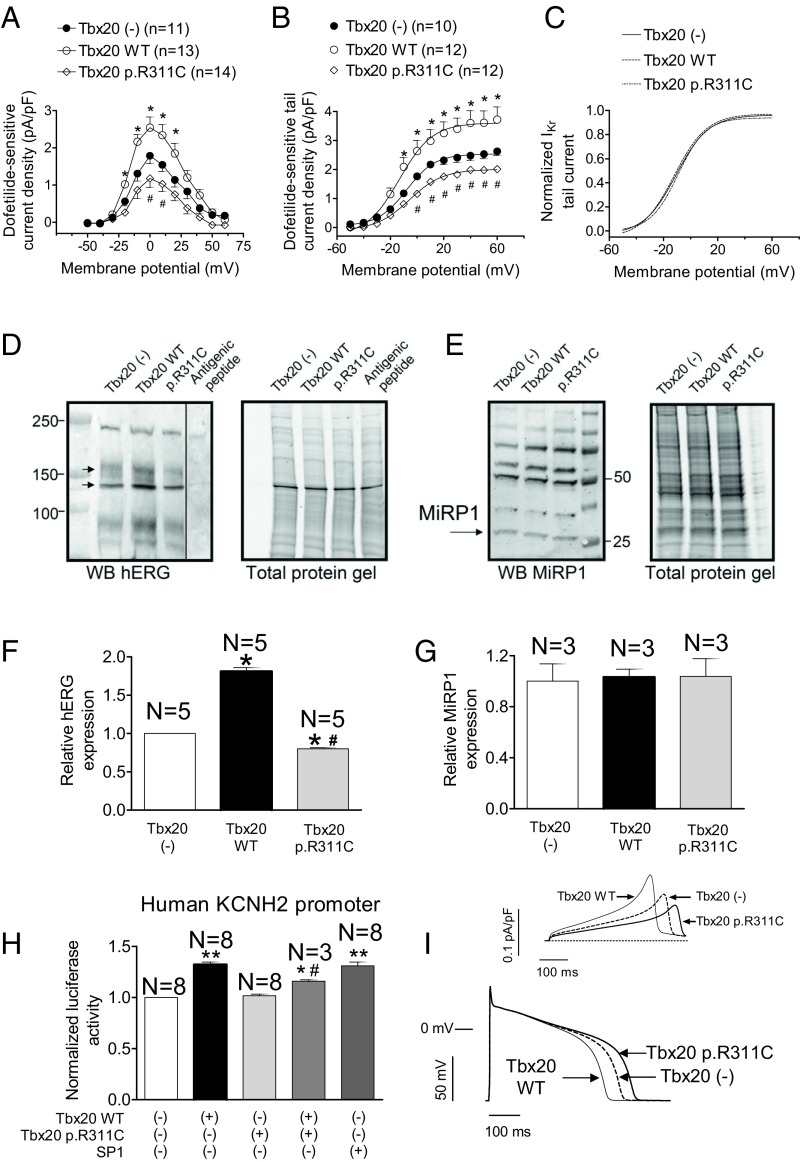
Fig. 3E shows dofetilide-sensitive currents recorded in IKr-predominant HL-1 cells. IKr was recorded again in IKr-predominant and -intermediate HL-1 cells whose distribution percentages (39% and 33%, respectively) were not modified by Tbx20. Tbx20 WT significantly increased (P < 0.05) the maximum outward current recorded upon depolarization (Figs. 3E and 4A) without modifying the activation kinetics (Table S3). Tbx20 also significantly increased the IKr tail density (Figs. 3E and 4B) (P < 0.05), whereas it did not modify tail current deactivation (Table S3). Surprisingly, p.R311C Tbx20 was unable to increase maximum outward IKr (Figs. 3E and 4A) and the tail current density elicited upon repolarization (Figs. 3E and 4B). Consistently, the mutated transcription factor did not modify either the activation or the deactivation kinetics of the current (Table S3) (P > 0.05). Importantly, p.R311C Tbx20 did not change the percentage of IKr-predominant and -intermediate HL-1 cells (39% and 28%, respectively). Fig. 4 B and C demonstrate that transfection with Tbx20, either WT or mutated, did not significantly modify the voltage dependence of Kr channel activation (Table S3). Western blot analysis in HL-1 cells showed that WT Tbx20 significantly increased (n = 5, P < 0.05), whereas p.R311C Tbx20 significantly decreased, the expression of hERG channels (Fig. 4 D and F) (n = 5, P < 0.05). It has been proposed that MiRP1 encoded by KCNE2 is also present in the channels generating the IKr in the human heart (12). Fig. 4 E and G demonstrate that Tbx20, either WT or mutated, did not modify MiRP1 expression in HL-1 cells.
Fig. 4.
(A and B) Maximum IKr density–voltage relationships (A) and activation curves (B) for currents recorded in IKr-predominant and -intermediate HL-1 cells transfected or not with either WT or p.R311C Tbx20. (C) Normalized activation curves for currents recorded in the three experimental groups. In B and C, solid lines represent the fit of a Boltzmann equation. (D and E) Western blot (WB) images and their corresponding stain-free gels showing hERG (arrows in D) and miRP1 (E) expression in HL-1 cells transfected or not with either WT or p.R311C Tbx20. In D, the sample of the last right lane was run in the same gel but was separated (continuous line) when incubating with the primary antibody together with the antigenic peptide. (F and G) Mean densitometric analysis of hERG (F) and MiRP1 (G) levels normalized to total protein. (H) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom vector carrying the human KCNH2 promoter cotransfected or not with SP1 and either WT or p.R311C Tbx20. (I) Simulated IKr traces (Top) and APs (Bottom) obtained at 0.1 Hz by using the Grandi–Bers mathematical model of human ventricular endocardial cells by introducing the modifications produced by Tbx20 WT and p.R311C on the IKr. Points/bars represent mean ± SEM of the data. n, number of cells; N, number of dishes. *P < 0.05 vs. Tbx20 (-); #P < 0.05 vs. Tbx20 WT; **P < 0.01 vs. Tbx20 (-).
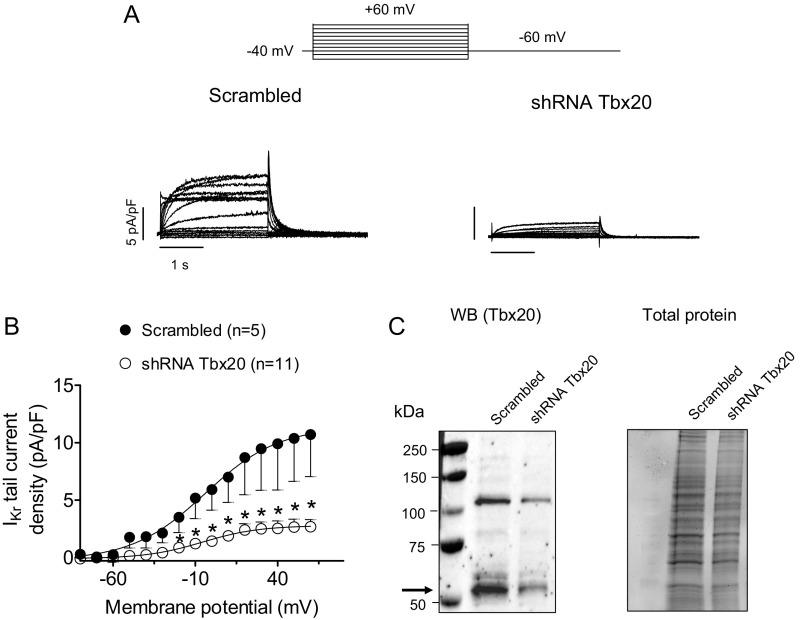
To further demonstrate the transcriptional effect of Tbx20 over the mouse KCNH2 gene, the IKr density was also assessed in HL-1 cells, in which endogenous Tbx20 was silenced using lentiviral constructs containing short hairpin RNA (shRNA) for Tbx20 together with GFP. Control cells were infected with a lentivirus containing a scrambled shRNA and GFP. At 48 h postinfection, Tbx20 expression decreased by 58% (Fig. S2). The results demonstrated that the IKr density significantly decreased in Tbx20-silenced cells (Fig. S2) (n ≥ 5, P < 0.05).
Fig. S2.
(A and B) IKr traces (A) and mean activation curves (B) recorded in IKr-predominant HL-1 cells infected with lentivirus encoding either a negative control (Scrambled) or short hairpin RNA (shRNA) Tbx20 for silencing Tbx20 by applying the pulse protocol (Top). In B, results are expressed as mean ± SEM of n experiments. *P < 0.05 vs. cells infected with scrambled shRNA. (C) Representative immunoblots after detection of Tbx20 (arrow) immunoreactivity and chemiluminescence (Left) in HL-1 cells cultured for 48 h with lentivirus-encoding scrambled or shRNA Tbx20 (17). The corresponding stain-free gel is depicted (Right) to show the total protein. To further confirm the transcriptional effect of Tbx20 on the KCNH2 gene, IKr was recorded in HL-1 cells in which endogenous Tbx20 was silenced with lentiviral constructs containing shRNA for Tbx20 together with GFP (A and B). Control cells were infected with a lentivirus containing a scrambled shRNA and GFP. Western blot analysis demonstrates that 48 h postinfection, Tbx20 expression was decreased by 58% (C). As can be observed in A and B, peak IKr tail density was markedly (by ∼75%) and significantly decreased in Tbx20-silenced cells compared with those cells infected with the scrambled shRNA (n ≥ 5, P < 0.05). However, Tbx20 silencing did not produce any modification in current kinetics or voltage dependence of activation.
To test whether Tbx20 regulates the expression of human KCNH2, we measured the luciferase activity in HL-1 cells expressing the minimum human KCNH2 promoter. The luciferase assay demonstrated that Tbx20 WT, but not p.R311C, significantly increased (P < 0.05) the transcription of the human KCNH2 gene (Fig. 4H) (n = 8 dishes per group). Similarly, SP1, a transcription factor whose binding site is also present in the minimal promoter of the human KCNH2 gene, significantly increased the transcription of this gene (Fig. 4H) (n = 8, P < 0.05). We also tested the effects of the combined action of both forms of Tbx20 (WT and mutated). Fig. 4H shows that in the joint presence of WT and p.R311C Tbx20 the protranscriptional effect was reduced (n = 3, P < 0.05 vs. Tbx20 alone), probably because of a competition between the WT and mutated forms for the Tbx20 consensus binding site in the KCNH2 minimal promoter. Interestingly, p.R311C Tbx20 alone did not decrease basal luciferase activity, probably because the sensitivity of our system is limited. The mouse KCNE2 gene promoter does not exhibit the Tbx20 binding site, whereas the human gene does (Table S3). Unfortunately, it was not possible to construct the minimal human promoter of the KCNE2 gene for a luciferase assay.
To test for the effects of the Tbx20 mutation on human ventricular AP characteristics, again the mathematical model was used. Fig. 4I shows superimposed human endocardial APs driven at 0.1 Hz in control conditions and when cells were transfected with TBX20 either WT or mutated. Tbx20 WT shortened the APD measured at 50% of repolarization (APD50) and the APD90 as a consequence of an IKr increase (Fig. 4I, Top). Conversely, p.R311C Tbx20 prolonged the APD50 (by 25% compared with Tbx20 WT) and APD90 (by 23%), due to the decrease of the IKr conductance (Fig. 4I, Top).
Effects of Tbx20 in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes.
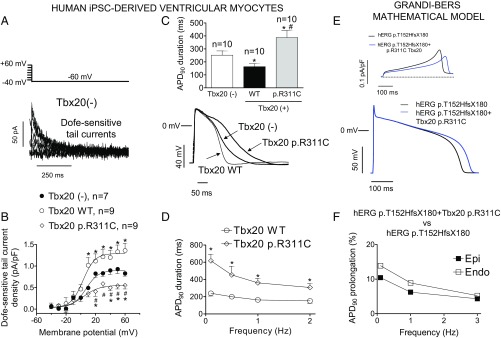
Next, we determined the effects of the Tbx20 p.R311C mutation on IKr and AP characteristics in a more physiologically relevant setting. Unfortunately, genetically modified mice were not an option, because adult mouse heart does not generate IKr (12). Therefore, we drew upon human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) infected or not with lentiviral constructs encoding Tbx20 WT or mutated. IKr was measured as the dofetilide-sensitive current (1 µmol/L). Fig. 5A shows IKr tail currents recorded at −60 mV after depolarizing pulses from −60 mV to −40 and +60 mV in a noninfected hiPSC-CM. Tbx20 WT produced a 1.6-fold increase in IKr tail current density (P < 0.05), whereas Tbx20 p.R311C reduced the tail current density by ∼60% (P < 0.05 vs. Tbx20 WT and noninfected cells; n ≥ 7) (Fig. 5B). Tbx20, either WT or p.R311C, did not modify the voltage dependence of Kr channel activation (Table S3). APs were recorded in hiPSC-CMs that exhibited automatic activity (13). In cells infected with Tbx20 WT (n = 10), maximum diastolic potential and AP amplitude averaged −68.4 ± 1.9 and 98.7 ± 14.2 mV, respectively. At 1 Hz, APD50 and APD90 were 108.1 ± 17.4 and 163.2 ± 23.2 ms, respectively, and Tbx20 WT shortened whereas Tbx20 p.R311C significantly prolonged the APD90 (Fig. 5C) (n = 10, P < 0.01). Furthermore, as shown in Fig. 5D, the prolongation of the APD90 was greater at slow- (158% at 0.1 Hz) than at fast-driving frequencies (102% at 2 Hz).
Fig. 5.
(A) Dofetilide (1 µmol/L)-sensitive (IKr) tail currents obtained by digital subtraction in a noninfected hiPSC-derived cardiomyocytes. (B) IKr density in a hiPSC-derived cardiomyocyte infected or not with either WT or p.R311C Tbx20. Solid lines represent the fit of a Boltzmann equation. (C) Superimposed APs recorded at 1 Hz in three hiPSC-derived cardiomyocytes infected or not with either WT or p.R311C Tbx20. (Top) The APD90 of each experimental group. (D) APD90 at different stimulation frequencies in cells infected with Tbx20 WT or p.R311C. (E) Simulated IKr traces (Top) and APs (Bottom) obtained at 0.1 Hz by using the Grandi–Bers mathematical model of human ventricular endocardial cells by introducing the modifications produced by heterozygous p.T152HfsX180 hERG alone or in combination with p.R311C Tbx20 on the IKr. (F) Percentage of APD90 prolongation in APs simulated at different frequencies in epicardial and endocardial cells. In B–D, points/bars represent mean ± SEM of ≥7 experiments in each group. *P < 0.05 vs. Tbx20(-); #P < 0.05 vs. Tbx20 WT.
To reproduce the genetic condition of the proband, the mathematical model was run considering both the heterozygous p.T152HfsX180 hERG mutation together with the p.R311C Tbx20 mutation. Fig. 5E demonstrated that p.R311C Tbx20 would lengthen the APD in the presence of the p.T152HfsX180 hERG mutation. Furthermore, the prolongation was greater in endocardial than in epicardial cells and also at slow- than at fast-driving frequencies (Fig. 5F).
Effects of p.R311C on the Expression of Other Cardiac K Channels.
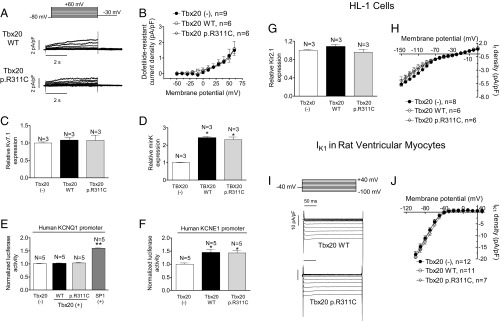
In the human ventricular myocardium Kv7.1 (encoded by KCNQ1) homotetramers associate with minK proteins (encoded by KCNE1) to form the channels that generate IKs (14). Some HL-1 cells (27%) exhibit IKs as the main repolarizing current (IKs-predominant cells). Thus, IKs was measured in IKs-predominant and -intermediate cells as dofetilide-resistant current, which was completely inhibited by a selective Ks channel blocker (HMR-1556; 1 µmol/L) (10). Fig. 6A shows IKs traces recorded in IKs-predominant cells transfected with WT or mutated Tbx20. Neither WT nor mutant Tbx20 modified the IKs density (n ≥ 6, P > 0.05) (Fig. 6B). Moreover, neither modified the percentage of IKs-predominant or -intermediate cells (28% and 33% in the presence of WT and p.R311C Tbx20, respectively). Consistently, Tbx20 did not modify either the voltage dependence of Ks channel activation or the activation and deactivation kinetics (Table S3). Western blot analysis (Fig. S3) confirmed that WT and p.R311C Tbx20 were not able to modify the expression of Kv7.1 channels (Fig. 6C) (n ≥ 3, P > 0.05). Sequence analysis of the mouse KCNQ1 gene promoter demonstrated that there are two Tbx20 consensus binding sites far away from the transcription start site (−2474 and −1992) (Table S4). Importantly, human KCNQ1 lacks the Tbx20 binding site (Table S4). Consistently, neither WT nor p.R311C Tbx20 modified the expression of human KCNQ1 measured with a luciferase assay (Fig. 6E). Conversely, SP1, whose consensus binding site is present in the minimal promoter of the human gene (16), actually increased KCNQ1 transcription significantly (Fig. 6E). Human and mouse KCNE1 gene promoters exhibit consensus Tbx20 binding sites (Table S4) and, indeed, both WT and p.R311C Tbx20 significantly increased minK expression (Fig. 6D) in HL-1 cells and transcription as measured by luciferase assay (Fig. 6F).
Fig. 6.
(A) Traces of dofetilide-resistant current (IKs) recorded in IKs-predominant HL-1 cells transfected with WT or p.R311C Tbx20 by applying the pulse protocol (Top). (B) Current density–voltage relationships for IKs recorded in HL-1 cells transfected or not with WT or p.R311C Tbx20. (C and D) Mean densitometric analysis of Kv7.1 (C) and minK (D) levels normalized to total protein. (E and F) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom vector carrying the human KCNQ1 (E) or KCNE1 (F) promoters cotransfected or not with WT or p.R311C Tbx20. (G) Mean densitometric analysis of Kir2.1 levels normalized to total protein. (H) Current density–voltage relationships for If recorded in HL-1 cells transfected or not with WT or p.R311C Tbx20. (I) IK1 traces recorded in two rat myocytes infected with WT and p.R311C Tbx20. (J) Mean current density–voltage curves for IK1 recorded in rat ventricular myocytes infected or not with lentiviral constructs encoding WT and p.R311C Tbx20. Each point/bar represents mean ± SEM of n cells or N dishes of cells in each group. *P < 0.05 vs. Tbx20 (-); **P < 0.01 vs. Tbx20 (-).
Fig. S3.
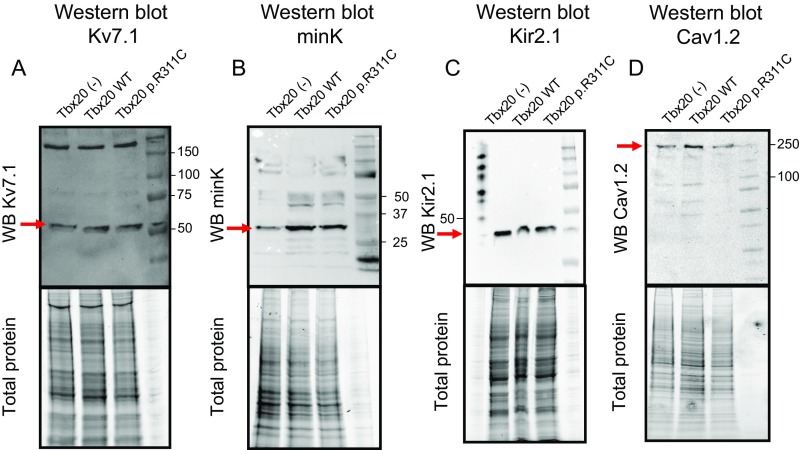
Western blot images and their corresponding total protein gels showing Kv7.1, minK, Kir2.1, and Cav1.2 expression (arrows) in HL-1 cells transfected or not with Tbx20 WT or p.R311C. As depicted in A and C, the presence of any form of Tbx20 did not modify either Kv7.1 or Kir2.1 protein expression compared with untransfected cells. On the other hand, B displays that both WT and p.R311C Tbx20 increased minK expression in HL-1 cells. Finally, D shows that both WT and p.R311C similarly increased Cav1.2 expression in accordance with the increase in the ICaL density observed in the electrophysiological experiments.
Neither human, rat, nor mouse KCNJ2 gene promoters (which encode for the inward rectifier K+ channel Kir2.1) exhibit the Tbx20 consensus binding site (Table S4). Fig. 6G and Fig. S3 demonstrate that Kir2.1 protein expression was not modified by the presence of any of the forms of Tbx20 (n = 3 dishes per group, P > 0.05). However, even when Kir2.1 channels were expressed, the inward rectifier K current (IK1) could not be recorded in HL-1 cells. Instead, the pacemaker current (If) predominated at potentials between −150 and 0 mV but its current density–voltage relation was unaltered by either WT or p.R311C Tbx20 (Fig. 6H) (n ≥ 6, P > 0.05). This result agrees with the absence of a Tbx20 consensus binding site in the promoters of the genes (HCN1, HCN3, and HCN4) encoding the channels underlying If in mice and humans (Table S4). Therefore, to assess the role of Tbx20 in Kir2.1 functional regulation, the IK1 was recorded in enzymatically dissociated rat ventricular myocytes that were transfected using a lentiviral construct (17). Fig. 6 I and J show that the IK1 density was not modified at any of the voltages tested in the presence of either WT or p.R311C Tbx20 (n ≥ 7, P > 0.05).
Effects of p.R311C on the Expression of Cardiac Na and Ca Channels.
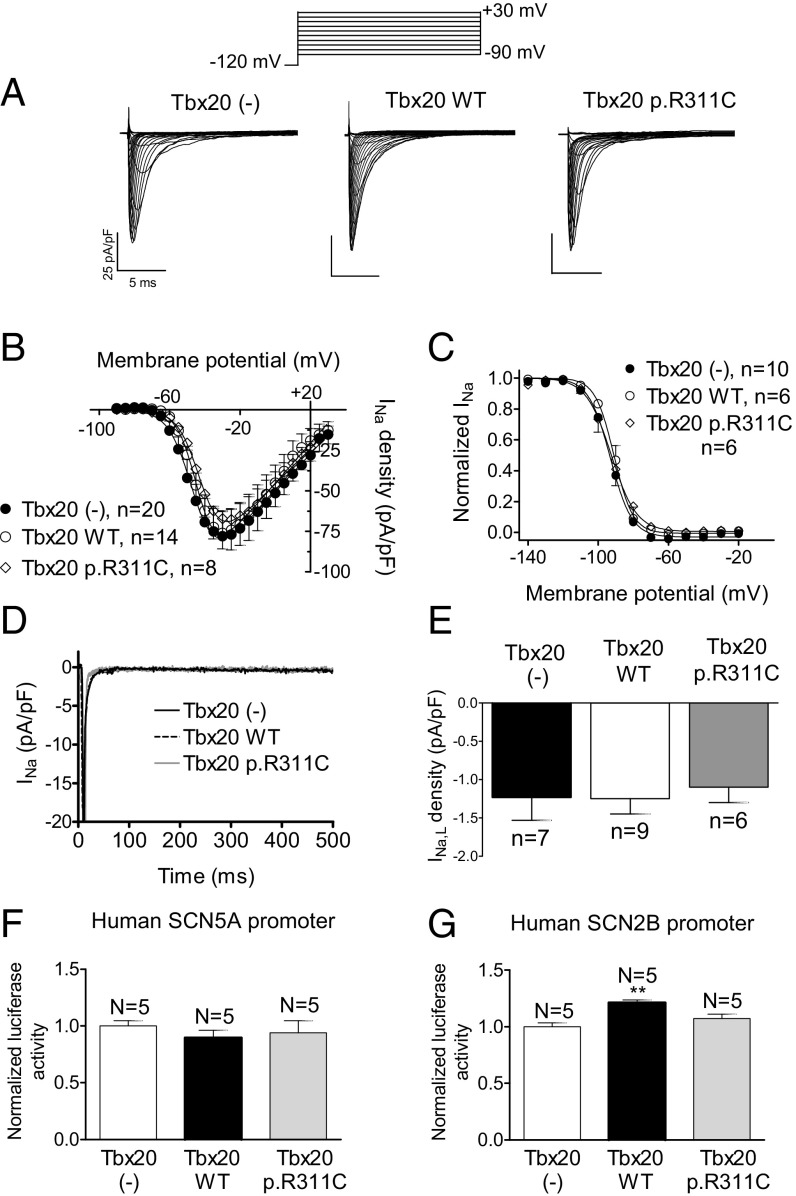
SCN5A and CACNA1C genes codify for the α-subunit of the Na+ (Nav1.5) and L-type Ca2+ (Cav1.2) channels, respectively. Mouse and human SCN5A gene promoters lack a Tbx20 binding site (Table S4). Fig. 7 A and B confirm that the Na+ current (INa) density was also not modified by WT or p.R311C Tbx20 (n ≥ 8, P > 0.05). Furthermore, transfection of WT or p.R311C Tbx20 did not modify the voltage dependence of the INa activation or inactivation or the current kinetics (Fig. 7C and Table S3). Fig. 7 D and E show that Tbx20 WT or p.R311C did not modify the amplitude of the sustained influx of Na+ measured at the end of 500-ms depolarizations to −20 mV (INaL) (n ≥ 6, P > 0.05). The human SCN2B promoter, which codifies for an Nav1.5 ancillary subunit, exhibits the Tbx20 binding site (Table S4). Luciferase assays demonstrated that Tbx20 WT and p.R311C were unable to modify human SCN5A gene expression, whereas Tbx20 WT, but not Tbx20 p.R311C, significantly increased the expression of human SCN2B (Fig. 7 F and G).
Fig. 7.
(A) INa traces recorded in HL-1 cells transfected or not with WT or p.R311C Tbx20 by applying the pulse protocol (Top). (B and C) Current density–voltage relationships (B) and steady-state inactivation (C) for INa recorded in the three experimental groups. (D and E) Superimposed INa traces (D) recorded in HL-1 cells transfected or not with WT or p.R311C Tbx20 by applying 500-ms pulses from −120 to −20 mV and bar graph (E) showing the mean INaL recorded at 500 ms. (F and G) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom vector carrying the human SCN5A (F) or SCN2B (G) promoters cotransfected or not with WT or p.R311C Tbx20. Each point/bar represents the mean ± SEM of n cells or N dishes of cells in each group. **P < 0.01 vs. Tbx20 (-).
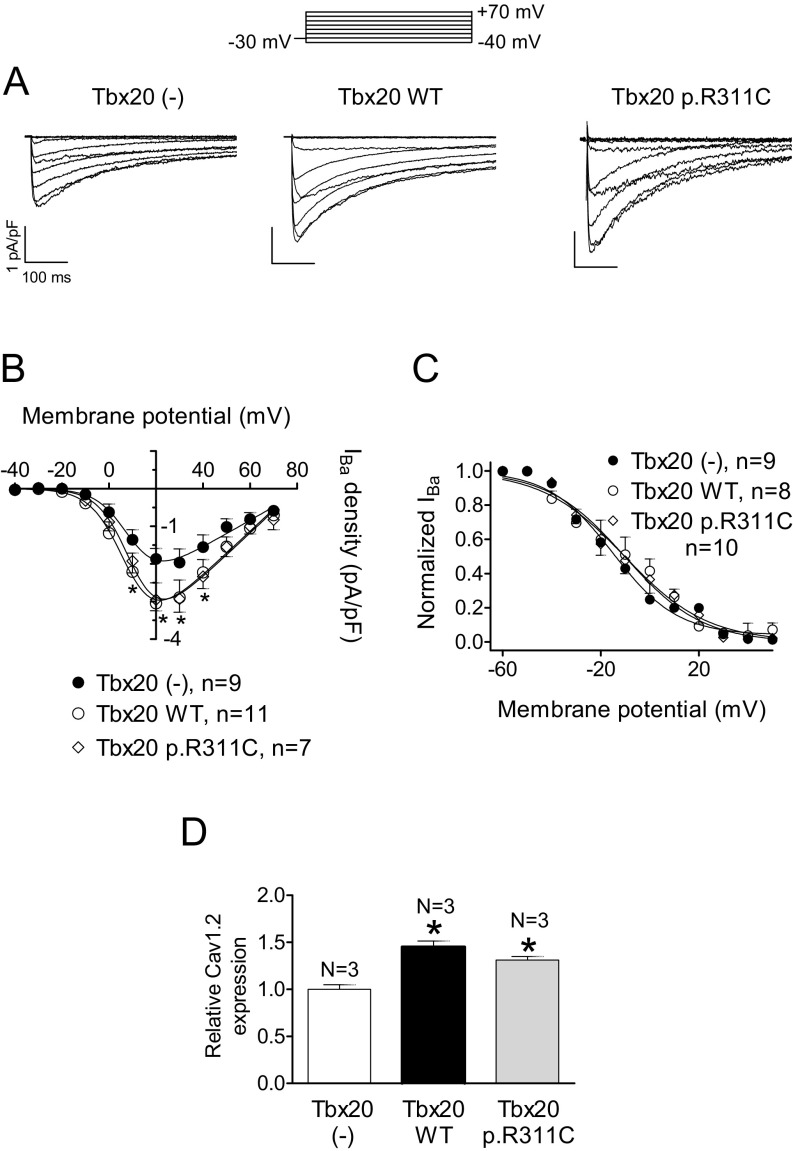
The mouse, but not the human, CACNA1C gene promoter exhibits the Tbx20 binding site (Table S4). Therefore, Tbx20 effects on the L-type Ca2+ current (ICaL) were tested in both HL-1 cells and hiPSC-CMs. In HL-1 cells, ICaL was measured using Ba2+ as a charge carrier (IBa) (15, 18). Fig. S4 shows that Tbx20, both WT and mutated, significantly increased the IBa (n ≥ 9, P < 0.05). Furthermore, p.R311C Tbx20 increased the IBa density similar to Tbx20 WT (P > 0.05 vs. Tbx20 WT). However, neither WT nor mutated Tbx20 affected the voltage dependence of activation or inactivation of the channel (Fig. S4 and Table S3). Western blot analysis in HL-1 cells (Fig. S3) demonstrated that both WT and p.R311C Tbx20 significantly and similarly increase Cav1.2 expression (Fig. S4).
Fig. S4.
(A) IBa traces recorded in HL-1 cells transfected or not with WT or p.R311C Tbx20 by applying the pulse protocol (Top). (B and C) Current density–voltage relationships (B) and steady-state inactivation (C) for IBa recorded in the three experimental groups. (D) Mean densitometric analysis of Cav1.2 levels normalized to total protein. Each point/bar represents the mean ± SEM of n cells or N dishes of cells in each group. *P < 0.05 vs. Tbx20 (-). In these experiments, ICaL was measured using Ba2+ as a charge carrier (15, 18). WT Tbx20 slightly but significantly increased the IBa density (A and B) (n ≥ 9, P < 0.05). Moreover, p.R311C Tbx20 also increased the IBa density similar to Tbx20 WT (n = 7, P > 0.05 vs. Tbx20 WT). However, neither WT nor mutated Tbx20 affected the voltage dependence of activation or inactivation of the channel (C) (Table S3). Western blot analysis in HL-1 cells (D) (Fig. S3) demonstrated that both WT and p.R311C Tbx20 significantly and similarly increase Cav1.2 expression, consistent with the presence of the Tbx20 binding site in the mouse, but not the human, CACNA1C gene promoter (Table S4).
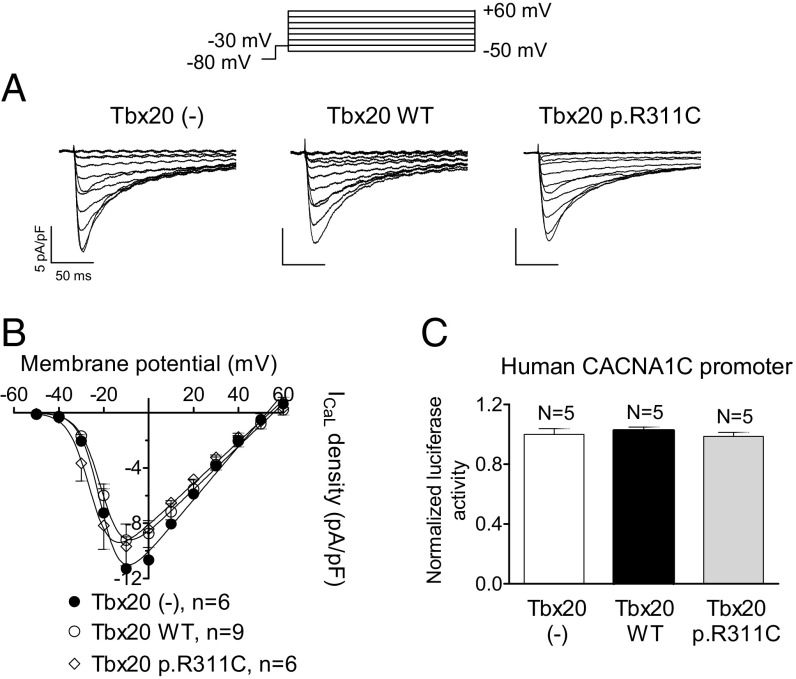
Fig. 8A shows ICaL traces recorded in hiPSC-CMs infected or not with the lentiviral constructs encoding for Tbx20, either WT or mutated. Neither WT nor p.R311C Tbx20 modified the ICaL density (Fig. 8A) at any of the voltages tested (Fig. 8B) (n ≥ 6, P > 0.05). The luciferase assay done using the minimal human CACNA1C promoter confirmed that Tbx20 WT and p.R311C were unable to modify the expression of the human CACNA1C gene (Fig. 8C), a result that explains the lack of Tbx20 effects over the human ICaL density.
Fig. 8.
(A) ICaL traces recorded in hiPSC-CMs infected or not with WT or p.R311C Tbx20 by applying the pulse protocol (Top). (B) Current density-voltage relationships for ICaL recorded in the three experimental groups. (C) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom vector carrying the human CACNA1C promoter cotransfected or not with WT or p.R311C Tbx20. Each point/bar represents the mean ± SEM of n cells or N dishes of cells in each group.
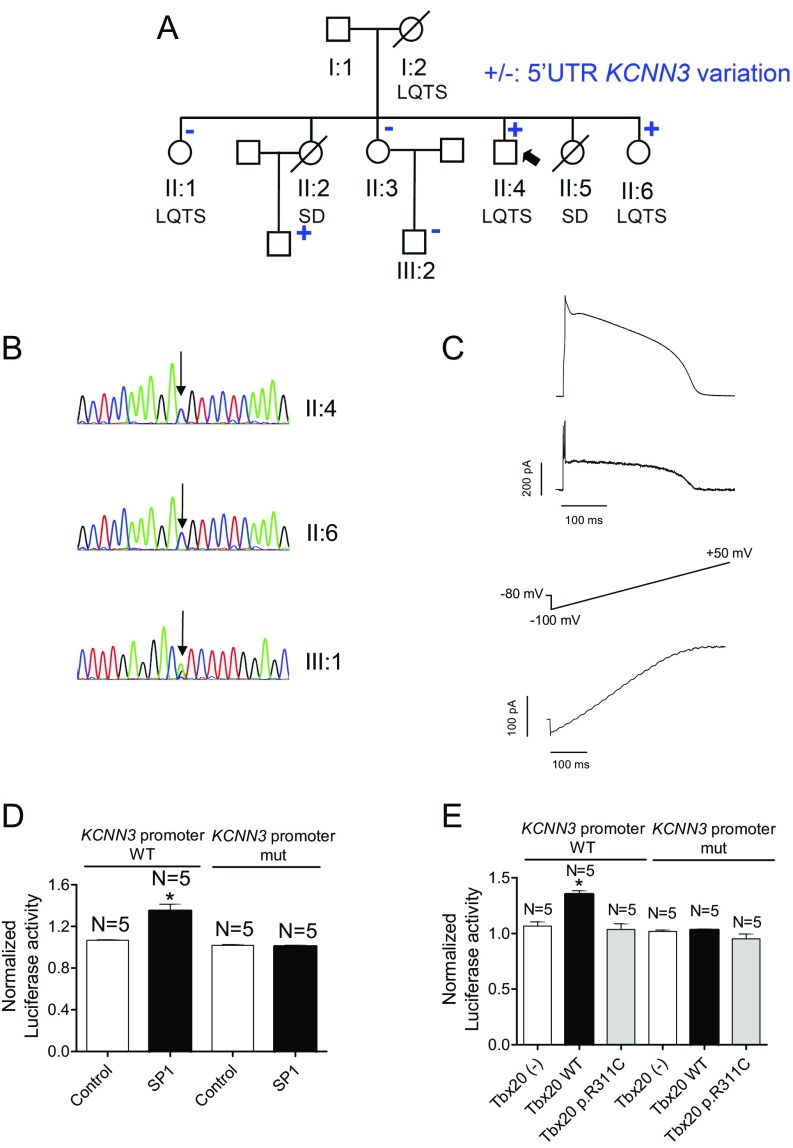
Functional Analysis of the c.-66A>G Variation of KCNN3.
The proband, sister II:6, and nephew III:1 also present a variation at the 5′ UTR of the KCNN3 gene (NM_001204087.1:c.-66A>G) that encodes the α-subunit of the small-conductance Ca2+-activated K+ channel type 3 (SK3) (19). Luciferase experiments demonstrated that expression of mutated KCNN3 cannot be activated by SP1 or Tbx20 (Fig. S5), whose binding sites are present in the human gene promoter (Table S4). Therefore, the variation completely abolished its transcription, thus leading to a KCNN3 haploinsufficiency. However, because treatment of human multicellular ventricular preparations with apamin (a selective SK blocker) does not modify the APD (20), the importance of these channels in repolarization seems to be negligible.
Fig. S5.
Functional analysis of the c.-66A>G variation of KCNN3. (A) Pedigree of the studied family. The arrow indicates the proband. Circles and squares represent females and males, respectively. + and – represent subjects with and without the 5′ UTR KCNN3 mutation, respectively. (B) DNA sequence chromatograms of the proband (II:4), sister II:6, and nephew III:1 depicting the heterozygous c.-66A>G variation at the 5′ UTR of the KCNN3 gene. (C, Top) Current trace elicited by AP command signals (Upper) as the voltage protocol recorded in CHO cells expressing SK3 channels. (C, Bottom) Current elicited in CHO cells expressing SK3 channels by applying a ramp pulse from ‒100 to +50 mV. (D) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom luciferase expression reporter vector carrying the human KCNN3 promoter in its WT or mutated form cotransfected with SP1. (E) Normalized luciferase activity in HL-1 cells expressing the pLightSwitch_Prom luciferase expression reporter vector carrying the human KCNN3 promoter in its WT or mutated form, cotransfected with Tbx20(-), Tbx20 WT, or Tbx20 p.R311C. In D and E, bars represent mean ± SEM of five independent batches of cells for each group. In D, *P < 0.05 vs. Control. In E, *P < 0.05 vs. Tbx20 (-). The proband, sister II:6, and nephew III:1 also carry a variation at the 5′ UTR of the KCNN3 gene (NM_001204087.1:c.-66A>G) that encodes the α-subunit of the small-conductance Ca2+-activated K+ channel type 3 (SK3) (19) (A and B). This variation has already been annotated (rs76040784). Luciferase experiments demonstrated that expression of mutated KCNN3 cannot be activated by SP1 or Tbx20 (D), whose binding sites are present in the human gene promoter (Table S4). (E) Tbx20 WT slightly but significantly increased the expression of KCNN3 (n = 5, P < 0.05), whereas p.R311C did not (n = 5, P < 0.05). Moreover, Tbx20, either WT or mutated, did not modify the expression of mutated KCNN3. CHO cells transiently transfected with KCNN3 cDNA were stimulated with pulses, with the morphology of endocardial APs recorded in human ventricular samples (C, Top). Time dependence of the SK3 conductance resembles the AP morphology, because it is almost linear to the voltage at this range of membrane potentials (see current elicited by ramp pulses in C, Bottom). The c.-66A>G variation completely abolished KCNN3 transcription, thus leading to haploinsufficiency. SK3 channels are detected in human ventricles, even though their presence seems to be much more abundant in human atria (31). In fact, treatment of human multicellular ventricular preparations with apamin (a selective SK blocker) does not modify APD (20). If SK3 channels actually participated in ventricular repolarization, the KCNN3 variant would further decrease the ventricular repolarization reserve in the carriers. Additionally, the proband also carries a SNP already described in KCND2 that encodes Kv4.2 channels, which are expressed in the human ventricular myocardium and contribute to the transient outward K current (Ito) (Table S2). However, this SNP is present in a deep intronic region of the gene and is suspected not to affect its expression.
Discussion
Here we functionally describe the consequences of three variants identified in a Spanish family of African ancestry with LQTS. The TBX20 mutation selectively decreased the expression of hERG channels, prolonging the AP in hiPSC-CMs. Conversely, the KCNH2 frameshift mutation did not modify IKr density. Our results strongly suggest that, in the adult heart, Tbx20 controls the expression of hERG channels, and thus TBX20 may be considered an LQTS-modifying gene.
The p.T152HfsX180 hERG mutation was found in the proband and in all of the affected relatives that were genotyped but in none of the nonaffected family members. Therefore, the mutation was considered pathogenic. Phenotypic manifestations in the family match most of the features of LQT2, and three of the proband’s sisters experienced seizures since they were children. Epilepsy has been reported to be more common with LQT2 (39%) than with other subtypes (10%), possibly because KCNH2 is also expressed in the brain (21). Interestingly, nephew III:2, who carries the TBX20 but not the KCNH2 frameshift mutation, has also experienced seizures. Additionally, one of the sisters died postpartum, which is a specific trigger of symptoms in LQT2 (22). Functional analysis of the p.T152HfsX180 mutation demonstrated that this peptide of 332 aa, of which only 152 correspond to the hERG sequence, exerts chaperone-like effects on WT hERG channels in CHO cells and on IKr recorded in HL-1 cells. Indeed, transfection of p.T152HfsX180 in HL-1 cells produces a “concentration-dependent” increase in IKr. As a consequence, current density generated by “heterozygous” transfection of WT and p.T152HfsX180 hERG channels was not different from that generated by “homozygous” transfection of WT hERG channels. This is a somewhat surprising result considering that, as expected, homozygous transfection of p.T152HfsX180 did not generate current at all. We recently demonstrated that the Nav1.5 N-terminal domain, by itself (the 132-aa peptide) (Nter), exerts a chaperone-like effect that increases INa and IK1 by enhancing the expression of Nav1.5 and Kir2.1-Kir2.2 channels in CHO cells and in rat cardiomyocytes (17). We hypothesize that the p.T152HfsX180 peptide is able to increase membrane expression of hERG channels. The molecular determinants, and the proteins involved in this effect, merit further analysis. The question now is whether this KCNH2 frameshift mutation is, by itself, responsible for the LQT phenotype of the family.
The proband and relatives II:3, II:6, and III:2 harbor a mutation in the TBX20 gene. Mutations in Tbx20 have been previously described and lead to defects in cardiac septation, valvulogenesis, and chamber growth (23). Indeed, Tbx20 is necessary for proper organogenesis, because it carries strong transcriptional activation and repression domains and physically interacts with other transcription factors involved in cardiac development (4). It is noteworthy that the R311 residue lies in the transcriptional activator domain but none of the mutation carriers presented any structural cardiac defect.
Functional analysis demonstrated that Tbx20 does not directly control the expression of the channels that underlie INa, If, IK1, and IKs. Regarding the IKs, the results demonstrated that both WT and mutated Tbx20 increased minK expression. Therefore, in HL-1 cells, an IKs augmentation would have been expected, because minK increases Kv7.1 conductance (24, 25). However, simultaneously, minK acts as an endocytic chaperone favoring the internalization of the Kv7.1–minK complexes expressed in the membrane (26), an effect that would decrease IKs density. Therefore, the balance between these two opposite actions could explain why the minK augmentation was not accompanied by a change in IKs density. Our results confirm previous data demonstrating that, in mice, Tbx20 increases ICaL (6). However, Tbx20 did not increase the expression of human CACNA1C, because the canonical Tbx20 binding site is not present in the gene promoter. Accordingly, Tbx20, either WT or mutated, did not modify the ICaL in hiPSC-CMs. Our results demonstrated that Tbx20 significantly increases the expression of hERG and thus IKr in HL-1 cells and hiPSC-CMs. Conversely, in adult flies, neuromancer (the invertebrate ortholog of Tbx20) negatively regulates the expression of the invertebrate homolog of the ERG channel (eag-like K+ channel) (5). Moreover, functional analysis developed in HL-1 cells and hiPSC-CMs strongly suggested that the p.R311C mutation specifically disables the protranscriptional activity of Tbx20 on the KCNH2 gene. Therefore, we propose that in the human adult myocardium, this Tbx20 mutation leads to a prolongation of ventricular repolarization.
Results in flies and mice demonstrated that Tbx20 is a key determinant of adult cardiac function (5, 6). Indeed, heart-specific knockdown of the gene that encodes neuromancer in flies (nmr-2) interferes with cardiac performance and disrupts contractile myofibrillar patterning (5). In adult mice, heterozygous loss of TBX20 leads to dilated cardiomyopathy (27), and the conditional homozygous loss of Tbx20 results in severe cardiomyopathy with associated arrhythmias and death (6). It has been proposed that, in the adult heart, Tbx20 is the pivotal element of a transcriptional cohort (also constituted by Mef2A, Tead1, Esrr, and Creb1) that fine-tunes expression of continuously required proteins in response to the current myocyte state, availability of resources, and contractile requirements (6). Therefore, even when Tbx20 mutant carriers apparently exhibit a mild phenotype under basal conditions, we cannot rule out that their myocardium adapts poorly to more demanding situations (e.g., sympathetic tone increase or even hormone- or drug-induced decrease of the repolarization reserve), because probably the p.R311C mutation affects Tbx20 ability to coordinate adaptive responses of the transcriptional cohort. Therefore, the simultaneous presence of KCNH2 and TBX20 mutations probably contributes to the LQTS phenotype in this family.
As in other families (28), expressivity of the LQTS phenotype in this family ranged from the mild phenotype of the proband to the high symptomatic phenotype of sister II:1. Besides demographic variables such as gender and age, variable expressivity may be attributed to the concurrence of additional genetic modifiers (29), including the presence of two or more variants, either in the same gene (compound heterozygosity) or in different genes (digenic heterozygosity), and the presence of nonsynonymous single-nucleotide polymorphism (28, 29). All such conditions converge in this Spanish family. The proband and sister II:6 present digenic heterozygosity (the KCNH2 frameshift and the TBX20 mutations), whereas sister II:1 presents a compound heterozygosity (the frameshift and the variant in KCNH2). Interestingly, nephew III:2, who has seizures, carries the benign p.Q1068R hERG variant and the TBX20 mutation.
We are aware of the potential limitations of this study, including that a better experimental approach would have been to analyze in hiPSC-CMs the effects produced by WT and mutated Tbx20 over all of the cardiac currents responsible for the AP morphology. It would have been even better to analyze the effects produced by Tbx20 over cardiomyocytes differentiated from hiPSCs derived from each family member. The latter would have allowed directly testing the impact of the variants (and their connection) in a constant genetic background, weighting their respective involvement in the phenotypic expression of the LQTS. Despite such a limitation, the results strongly suggest that TBX20 is a potential LQTS modifier gene because WT Tbx20 increases hERG channel expression. The data show also that some mutations, such as p.R311C, can disable Tbx20 protranscriptional activity over the KCNH2 gene. Therefore, the putative effects of Tbx20 variants on penetrance, expressivity, and outcome among LQTS patients merit further analysis.
Methods
Clinical Evaluation.
Patients were evaluated by the Arrhythmia Unit of the Hospital Universitario La Paz. The study was approved by the Investigation Committee of the hospital and conforms to the principles outlined in the Declaration of Helsinki. Each participant gave written informed consent.
DNA Sequencing.
Genomic DNA was sequenced by means of a HaloPlex custom panel including coding regions and untranslated boundaries of the 82 genes listed in Table S1. Sequencing using the Ion Torrent Personal Genome Machine was carried out at NIMGenetics. Variants identified in KCNH2, TBX20, and KCNN3 were confirmed by the Sanger method.
Cell Culture and Transfection.
HL-1 and Chinese hamster ovary cells were transiently transfected by using Lipofectamine 2000 and FuGENE X-tremeGENE, respectively, and cultured as described (15, 17, 18, 30).
Rat Ventricular Myocyte Isolation.
Animal studies were approved by the Committee on the Use and Care of Animals at Complutense University and conformed to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Single ventricular myocytes isolated from male Sprague–Dawley rats by enzymatic dissociation (17) were infected with lentiviral constructs encoding for human Tbx20 WT or p.R311C.
Patch Clamping.
Currents were recorded using the whole-cell patch-clamp technique (15, 17, 18, 30). Series resistance was compensated manually using the compensation unit of the Axopatch amplifier; ≥80% compensation was achieved. No significant voltage errors (<5 mV) due to series resistance were expected with the micropipettes used.
IKr, ICaL, and AP Recordings in hiPSC-CMs.
Enriched and mature DF19-9-11T hiPSC-CMs were generated as described elsewhere (13) and infected with the lentiviral constructs coding WT or p.R311C Tbx20. Currents were recorded at 21 to 23 °C, and APs were recorded at 35 °C using the whole-cell patch-clamp technique.
Western Blot Analysis.
Cav1.2, Kir2.1, Kv7.1, minK, hERG, MiRP1, and Tbx20 proteins were detected in HL-1 cells transfected or not with Tbx20 WT or p.R311C by Western blot following previously described procedures (17, 18).
Luciferase Gene Expression Reporter Assay.
Luciferase reporter assays were conducted in HL-1 cells transfected with pLightSwitch_Prom luciferase expression reporter vectors carrying the minimal promoters of human SCN5A, SCN2B, CACNA1C, KCNQ1, KCNE1, KCNH2, or KCNN3 (15, 18).
Tbx20 Silencing.
For analysis of Tbx20 silencing, HL-1 cells were infected with lentivirus-encoding shRNA Tbx20 or scrambled shRNA (17).
Statistical Analysis.
Results are expressed as mean ± SEM. Unpaired t test or one-way ANOVA followed by Newman–Keuls test was used where appropriate. In small-size samples (n < 15), statistical significance was confirmed by using nonparametric tests. Comparisons between categorical variables were done using Z test. To take into account repeated sample assessments, data were analyzed with multilevel mixed-effects models. A value of P < 0.05 was considered significant. Additional details are presented in SI Methods.
SI Methods
Clinical Evaluation.
The proband and his relatives were evaluated by the Arrhythmia Unit of the Hospital Universitario La Paz. A complete clinical evaluation, including electrocardiogram (ECG), transthoracic echocardiogram, and exercise test, was performed for each. The study was approved by the Investigation Committee of the Hospital Universitario La Paz and conforms to the principles outlined in the Declaration of Helsinki. Each participant gave written informed consent.
Custom Sequencing Panel.
We selected the 82 genes listed in Table S1. In addition to genes already associated with primary arrhythmogenic syndromes, we included other genes encoding (i) constitutive proteins of cardiac ion channels, (ii) proteins that participate in cardiac channelosomes, and (iii) proteins whose involvement in the modulation of ion-channel activity has been demonstrated (functional studies) or suggested (genome-wide association studies). Most of the genes associated with inherited structural cardiomyopathy diseases were excluded. Genes underlying catecholaminergic polymorphic ventricular tachycardia were also excluded, because patients with this condition were not genotyped. A HaloPlex custom panel (216,105 kb) was designed using SureDesign (Agilent Technologies) and comprised the coding regions and untranslated boundaries (5′ and 3′ UTRs and 10-bp extensions from the 5′ and 3′ ends) of the 82 genes selected. The coordinates of the sequence data are based on the hg19 GRCh37 human genome reference.
Sample Preparation.
Genomic DNA was extracted from whole blood by using the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer’s protocol. DNA quantity and quality were measured using a NanoDrop 2000 spectrophotometer and Qubit 2.0 fluorometer (Thermo Fisher Scientific). Library construction, quantification, template preparation, and sequencing were carried out at NIMGenetics. The DNA libraries were generated following the HaloPlex HS target enrichment protocol, starting from 3 μg of genomic DNA. Enriched libraries were quantified by microfluidic analysis using the 2100 Bioanalyzer (Agilent Technologies) and templated by an emulsion PCR (OneTouch 2; Life Technologies) over Ion Sphere particles. Finally, DNA libraries were sequenced on an Ion Torrent Personal Genome Machine (Life Technologies).
Bioinformatics.
Signal processing, base calling, alignment, and variant calling were performed on a PGM Torrent Server using Torrent Suite software version 3.6.2 and the Genome Analysis Toolkit (GATK).
Variants were annotated using Ion Reporter software. Variant filtering and prioritization were performed with an in-house software program and a local database. For each genetic variation identified, minor allelic frequency was consulted in the Exome Variant Server (evs.gs.washington.edu/EVS/) and 1000 Genomes database (www.internationalgenome.org/). In addition, the Human Gene Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) was also consulted to identify pathogenic mutations previously reported. To predict pathogenicity, all variants were subjected to in silico analysis including prediction programs such as SIFT (sift.jcvi.org), PolyPhen-2 (genetics.bwh.harvard.edu/pph2), and PROVEAN (provean.jcvi.org/index.php). Alamut software was used for splicing prediction (www.interactive-biosoftware.com). Candidate variants were visualized using the Integrative Genomics Viewer (software.broadinstitute.org/software/igv/).
Sanger Sequencing.
Variants found in the proband and sister II:1 were confirmed by Sanger and identified in their relatives by the same method (11). PCR products were purified using the illustra ExoProStar 1-Step (GE Healthcare Life Sciences), and the analysis was performed by direct sequencing using the Applied Biosystems ABI Prism 3730 DNA sequencer (Secugen). The results were compared with the reference sequence from hg19 by means of Chromas Lite software (chromas.software.informer.com/2.4/).
Site-Directed Mutagenesis.
Human TBX20 cDNA subcloned in pCMV6-AC-GFP and the minimal promoter of human KCNN3 (WT and with the A-to-G substitution at −66 bp to the ATG) were purchased from OriGene and Active Motif, respectively. The p.R311C mutation in TBX20 and p.T152HfsX180 mutation in KCNH2 were introduced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) as previously described (11, 17, 30). The mutations were confirmed by direct DNA sequencing.
Cell Culture and Transfection.
HL-1 cells were cultured in 60-mm gelatin/fibronectin-coated dishes at 37 °C in an atmosphere of 5% CO2/95% O2, with a humidity of ∼95%, as previously described (15, 18). HL-1 cells were transfected with p.T152HfsX180 KCNH2 (0.5 or 1 μg) or with Tbx20 WT or p.R311C (2 μg) by using Lipofectamine 2000 according to the manufacturer’s instructions (15, 18). Forty-eight hours after transfection, cells were removed from the dish with a trypsin (1%) treatment (37 °C for 5 min). Chinese hamster ovary (CHO) cells were cultured as previously described and transiently transfected with the cDNA encoding WT and p.T152HfsX180 KCNH2 (0.5 to 1 μg) or KCNN3 channels (1.6 µg) plus the cDNA encoding the CD8 antigen (0.5 µg) by using FuGENE X-tremeGENE (Roche Diagnostics) following the manufacturer’s instructions (11, 15, 17, 18, 30). Forty-eight hours after transfection, cells were incubated with polystyrene microbeads precoated with anti-CD8 antibody (Dynabeads M-450; Life Technologies). Most of the cells that were beaded also had channel expression. To minimize the influence of the expression variability of transiently transfected mammalian cell lines, each construct was tested in a large number of cells obtained from at least three different transfection batches.
Rat Ventricular Myocyte Isolation.
Single ventricular myocytes were isolated from hearts of three male Sprague–Dawley rats (225–250 g) by enzymatic dissociation with collagenase type II (Worthington) following previously described methods (17, 30). Procedures conformed with the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. In addition, the study was approved by the University Committee on the Use and Care of Animals at Complutense University. Rats were heparinized (1,000 U/kg i.p.) and anesthetized with sodium pentobarbital (50 mg/kg i.p.). Isolated myocytes were seeded on laminin-coated glass coverslips and infected with lentiviral constructs (in pLenti-C-mGFP vector; OriGene) encoding for human Tbx20 WT or p.R311C 2 h after plating (17). A multiplicity of infection (MOI) of 40 and 70 was used for Tbx20 WT and Tbx20 p.R311C, respectively. The same stock of lentivirus was used for all of the experiments. In all cases, currents were recorded 48 h after the infection, and infected myocytes were identified by GFP fluorescence under fluorescence microscopy (Eclipse TE2000S; Nikon).
Patch Clamping.
Currents were recorded at room temperature (21 to 23 °C) using the whole-cell patch-clamp technique using an Axopatch 200B patch-clamp amplifier (Molecular Devices) (11, 15, 17, 18, 30). Recording pipettes were pulled from 1.0-mm o.d. borosilicate capillary tubes (GD1; Narishige) using a programmable patch micropipette puller (P-2000 Flaming/Brown; Sutter Instruments) and heat-polished with a microforge (MF-83; Narishige). Micropipette resistance was kept below 3.5 MΩ when filled with the internal solution and immersed in the external solution. In all of the experiments, series resistance was compensated manually by using the series resistance compensation unit of the Axopatch amplifier, and ≥80% compensation was achieved. Uncompensated access resistance and cell capacitance were 1.7 ± 0.8 MΩ and 62.1 ± 8.1 pF (n = 75), 1.5 ± 0.6 MΩ and 12.2 ± 1.0 pF (n = 55), and 2.0 ± 0.9 MΩ and 122.0 ± 6.0 pF (n = 33) in HL-1, CHO, and rat ventricular myocardial cells, respectively. Under our experimental conditions, no significant voltage errors (<5 mV) due to series resistance were expected with the micropipettes used.
To record hERG currents (IhERG), CHO cells were perfused with an external solution containing 136 mmol/L NaCl, 4 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, and 10 mmol/L glucose (pH 7.4 with NaOH). Recording pipettes were filled with an internal solution containing 80 mmol/L K-aspartate, 42 mmol/L KCl, 10 mmol/L KH2PO4, 5 mmol/L MgATP, 3 mmol/L phosphocreatine, 5 mmol/L Hepes, and 5 mmol/L EGTA (pH 7.2 with KOH). To record KCNN3 currents (IKCNN3), CHO cells were perfused with an external solution containing 136 mmol/L NaCl, 4 mmol/L KCl, 1.8 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, and 10 mmol/L glucose (pH 7.4 with NaOH). Recording pipettes were filled with an internal solution containing 130 mmol/L KCl, 1.08 mmol/L MgCl2, 10 mmol/L Hepes, 10 mmol/L EGTA, and 8.751 mmol/L CaCl2, yielding a free (unchelated) [Ca2+] of 1 μmol/L (pH 7.2 with KOH) (19, 20, 31).
To record INa in HL-1 cells, the external solution contained (17) 100 mmol/L NaCl, 50 mmol/L CsCl, 1.5 mmol/L MgCl2, 1 mmol/L CaCl2, 5 mmol/L Hepes, 5 mmol/L glucose, and 1 µmol/L nifedipine (pH 7.35 with CsOH). Recording pipettes were filled with an internal solution containing 10 mmol/L NaF, 110 mmol/L CsF, 20 mmol/L CsCl, 10 mmol/L Hepes, and 10 mmol/L EGTA (pH 7.35 with CsOH). To record ICaL in HL-1 cells, Ba2+ was used as a charge carrier (IBa) (15, 18). In these experiments, the external solution contained 137 mmol/L N-methyl-d-glucamin, 12 mmol/L CsCl, 20 mmol/L BaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, and 10 mmol/L glucose (pH 7.4 with HCl), whereas the internal solution contained 125 mmol/L CsCl, 20 mmol/L tetraethylammonium chloride (TEA⋅Cl), 5 mmol/L MgATP, 3.6 mmol/L phosphocreatine, 10 mmol/L Hepes, and 10 mmol/L EGTA (pH 7.2 with CsOH). To record If in HL-1 cells and IK1 in cultured adult rat ventricular myocytes, the external solution contained 120 mmol/L NaCl, 20 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, 2 mmol/L 4-aminopyridine, 10 mmol/L glucose, 1 µmol/L nifedipine, 1 µmol/L atropine, and 10 µmol/L glibenclamide (pH 7.4 with NaOH). Recording pipettes were filled with an internal solution containing 80 mmol/L K-aspartate, 42 mmol/L KCl, 10 mmol/L KH2PO4, 5 mmol/L MgATP, 3 mmol/L phosphocreatine, 5 mmol/L Hepes, and 5 mmol/L EGTA (pH 7.2 with KOH). For If recordings, the external solution was supplemented with 100 μmol/L BaCl2.
To record outward K+ currents in HL-1 cells, the external solution contained (15, 18) 140 mmol/L NaCl, 4 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, 10 mmol/L glucose, 2 mmol/L 4-aminopyridine, 1 µmol/L nifedipine, and 0.1 µmol/L atropine (pH 7.4 with NaOH). The internal solution contained 80 mmol/L K-aspartate, 42 mmol/L KCl, 10 mmol/L KH2PO4, 5 mmol/L MgATP, 3 mmol/L phosphocreatine, 5 mmol/L Hepes, and 5 mmol/L EGTA (pH 7.2 with KOH).
To record IK1 in cultured rat ventricular myocytes, the external solution contained (17) 120 mmol/L NaCl, 20 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L Hepes, 2 mmol/L 4-aminopyridine, 10 mmol/L glucose, 1 μmol/L nifedipine, 1 µmol/L atropine, and 10 µmol/L glibenclamide (pH 7.4 with NaOH). Recording pipettes were filled with an internal solution containing 80 mmol/L K-aspartate, 42 mmol/L KCl, 10 mmol/L KH2PO4, 5 mmol/L MgATP, 3 mmol/L phosphocreatine, 5 mmol/L Hepes, and 5 mmol/L EGTA (pH 7.2 with KOH).
IhERG was recorded by applying 5-s pulses in 10-mV increments from a holding potential of −80 mV to potentials between −80 and +60 mV (11, 18). Between −80 and −40 mV, only passive linear leak was observed, and least-squares fits to these data were used for passive leak correction. Current–voltage curves were constructed by plotting the current amplitude measured at the end of the depolarizing pulses as a function of membrane potential. Deactivating tail currents were recorded at −60 mV, and the activation curves were constructed by plotting peak tail current amplitudes as a function of the voltage of the preceding pulse. IKCNN3 was recorded by applying a human ventricular endocardial action-potential (AP) waveform (19, 20, 31). INa was recorded by applying 20-ms pulses in 5-mV increments from a holding potential of −120 mV to potentials ranging between −90 and +30 mV (17). Inactivation curves were recorded by applying 500-ms pulses from −120 mV to potentials between −130 and −30 mV in 5-mV increments followed by a test pulse to −20 mV. Availability curves were constructed by plotting the current amplitude recorded with the test pulse at −20 mV as a function of the membrane potential of the preceding pulse. To record persistent INa (INaL), 500-ms pulses from −120 to −20 mV were applied.
To obtain current–voltage relationships for IBa, 500-ms pulses in 10-mV increments from −30 mV to potentials between −40 and +70 mV were applied (15, 18). Activation curves for IBa were constructed by plotting the normalized conductance as a function of the membrane potential. To obtain the inactivation curves for IBa, a two-step protocol was used consisting of a first 500-ms conditioning pulse from −30 mV to potentials between −50 and +20 mV, followed by a 500-ms test pulse to +20 mV. Inactivation curves were constructed by plotting the current amplitude obtained with the test pulse normalized to the largest current, as a function of the voltage command of the conditioning pulse. To record If in HL-1 cells, 2-s pulses from −30 to potentials between −150 and 0 mV in 10-mV steps were applied.
In HL-1 cells, IKr was recorded by applying 5-s pulses from −80 to potentials between −80 and +60 mV, followed by repolarizing pulses to −60 mV to record the tail currents (18). After control recordings were obtained, cells were perfused with dofetilide (1 μmol/L) (10) and currents in the presence of dofetilide were subtracted from the control currents. Therefore, IKr was measured as the dofetilide-sensitive current. Activation curves for IKr were constructed by plotting the peak density of tail currents recorded at −60 mV, as a function of the voltage of the preceding pulse. Dofetilide-resistant current (IKs) was recorded by applying 5-s pulses from −80 to potentials ranging between −80 and +60 mV, followed by repolarizing pulses to −30 mV to record the tail currents (18). Voltage-dependent IKs activation was assessed by constructing conductance–voltage curves plotting the normalized conductance as a function of the membrane potential.
The protocol to record rat ventricular IK1 consisted of 250-ms steps that were imposed in 10-mV increments from −40 mV to potentials ranging between −100 and +30 mV (17, 30).
A Boltzmann function was fitted to activation/conductance–voltage and inactivation curves to obtain the midpoint (Vh) and slope (k) values of the conductance. In each experiment, current amplitudes were normalized to membrane capacitance to obtain current densities.
In another group of experiments, HL-1 cells were infected with two lentiviral constructs encoding for mouse Tbx20 (NM_194263)-silencing shRNA (A: 5′-GCCAATGCCTTCTCCATCGCCGCGCTTAT-3′; B: 5′-GGAGTGGATCCTGAGTCCAAGTATATAGT-3′) together with GFP or with a scrambled shRNA (OriGene). Lentivirus-encoding shRNA constructs were amplified, purified, and titered using previously described protocols (17). For Tbx20 silencing, an MOI of 15 and 80 was used for shRNA Tbx20 and scrambled lentiviral constructs, respectively. Silencing of Tbx20 expression was confirmed by Western blot analysis following the procedures described below. The same stock of shRNA Tbx20 constructs and scrambled shRNA was used for all of the experiments. In all cases, currents were recorded 48 h after the infection; infected cells were identified by GFP fluorescence under fluorescence microscopy (Eclipse TE2000S; Nikon).
Currents and Action Potential Recordings in hiPSC-Derived Cardiomyocytes.
The DF19-9-11T human induced pluripotent stem cell (hiPSC) line was derived from foreskin fibroblasts without integration of vector and transgene sequences (13). DF19-9-11T hiPSCs were plated on Matrigel-coated (500 μg/mL; BD Biosciences) transparent polydimethylsiloxane membranes for cardiac-directed differentiation as previously described (13). Differentiation medium (EB-20) consisted of 80% (vol/vol) DMEM/F12, 0.1 mmol/L β-mercaptoethanol, 20% (vol/vol) FBS, and 10 μmol/L blebbistatin (13). Once differentiated, cells were dissociated from the culture using gentle mechanical agitation and seeded on laminin-coated glass coverslips and infected with the lentiviral constructs encoding Tbx20 WT or Tbx20 p.R311C (MOI 40 and 70, respectively). In each case, the same stock of lentivirus was used for all of the experiments. Currents and action potentials were recorded 48 h after the infection; infected myocytes were identified by GFP fluorescence under fluorescence microscopy (Eclipse TE2000S; Nikon). Ion currents and action potentials were recorded at room temperature (21 to 23 °C) and at 35 °C, respectively, using the whole-cell patch-clamp technique and a MultiClamp 700B amplifier (Molecular Devices). Recording pipettes were pulled using a programmable patch micropipette puller (P-97 Flaming/Brown; Sutter Instruments) and heat-polished with a microforge (MF-83; Narishige). Micropipette resistance was kept below 3.5 MΩ for current recordings or between 4 and 6 MΩ for action-potential recordings. In all experiments, series resistance and cell capacitance were compensated automatically. The external solution for IKr recordings contained (6, 8, 10) 140 mmol/L NaCl, 5.4 mmol/L KCl, 1 mmol/L MgCl2, 15 mmol/L Hepes, 5 mmol/L 4-aminopyridine, 5 mmol/L glucose, 0.2 mmol/L CdCl2, 0.5 mmol/L BaCl2, and 1.8 mmol/L CaCl2 (pH 7.4 with NaOH). Recording pipettes were filled with an internal solution containing 150 mmol/L KCl, 4.46 mmol/L K2ATP, 5 mmol/L phosphocreatine, 5 mmol/L Hepes, 5 mmol/L EGTA, 1 mmol/L MgCl2, and 2 mmol/L β-hydroxybutyric acid (pH 7.2 with KOH). The external solution for ICaL recordings contained 137 mmol/L NaCl, 5.4 mmol/L CsCl, 1.25 mmol/L MgCl2, 10 mmol/L Hepes, 1.2 mmol/L CaCl2, and 5.5 mmol/L glucose (pH 7.35 with NaOH). Recording pipettes were filled with an internal solution containing 120 mmol/L CsCl, 20 mmol/L TEA⋅Cl, 1 mmol/L MgCl2, 5.2 mmol/L MgATP, 10 mmol/L Hepes, and 10 mmol/L EGTA (pH 7.2 with CsOH).
The protocol to record IKr consisted of 3-s pulses from −60 mV to potentials between −40 and +60 mV in 10-mV increments, followed by a repolarizing pulse to −60 mV to record tail currents (11, 18). IKr was measured as current sensitive to dofetilide (1 μmol/L). To record ICaL, 200-ms pulses from −50 to +60 mV in 10-mV steps were applied (15, 18). A 50-ms prepulse from −80 to −30 mV was applied before each test pulse to activate and inactivate INa.
To record action potentials, the external solution contained (13, 32) 148 mmol/L NaCl, 0.4 mmol/L NaH2PO4, 1 mmol/L MgCl2, 5.5 mmol/L glucose, 5.4 mmol/L KCl, 15 mmol/L Hepes, and 1 mmol/L CaCl2 (pH 7.4 with NaOH), whereas the internal solution contained 150 mmol/L KCl, 4.46 mmol/L K2ATP, 5 mmol/L phosphocreatine, 5 mmol/L Hepes, 1 mmol/L EGTA, 1 mmol/L MgCl2, and 2 mmol/L β-hydroxybutyric acid (pH 7.2 with KOH). Cells were stimulated at 0.1 to 2 Hz in current-clamp configuration, using square wave pulses (amplitude 30 to 50 pA; duration 4 ms) generated by a DS8000 digital stimulator (World Precision Instruments).
Western Blot Analysis.
Detection of Cav1.2, Kir2.1, Kv7.1, minK, hERG, and MiRP1 proteins was carried out in HL-1 cells transfected or not with Tbx20 WT or p.R311C by Western blot following previously described procedures (15, 17, 18, 30). HL-1 cells were collected in RIPA buffer containing 50 mmol/L Tris⋅HCl, 500 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and protease inhibitor mixture (Sigma). Nuclei and cell debris were removed by centrifugation at 9,164 × g for 20 min at 4 °C. The total protein amount of the extracts was calculated with the bicinchoninic acid method (Pierce). Afterward, samples were run on 10% Mini-PROTEAN TGX stain-free gels (Bio-Rad) and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with 5% nonfat dried milk in PBS with Tween 20 (0.05%) for 1 h at room temperature. Membranes were then incubated with anti-Cav1.2 (1:1,000; clone L57/46; NeuroMab), anti-Kir2.1 (1:500; Alomone), anti-Kv7.1 (1:250; Sigma), anti-minK (1:250; Abcam), anti-hERG (1:3,000; Alomone), anti-MiRP1 (1:600; Sigma), and anti-Tbx20 (1:200; Abcam) antibodies overnight at 4 °C and then for 1 h with peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:10,000; Jackson ImmunoResearch). Membranes were washed three times with PBS/Tween after adding the primary and secondary antibodies. Protein expression was detected by chemiluminescence (ECL; GE Healthcare) and visualized using the ChemiDoc MP System and Image Lab 5.2.1 software (Bio-Rad). As loading control, the total protein was used by means of stain-free gels (Bio-Rad) and Image Lab software (15, 17, 18, 30). Expression of the proteins in the immunoblot was then normalized to total protein.
Luciferase Gene Expression Reporter Assay.
For luciferase reporter assays (15, 18), HL-1 cells were seeded in 96-well plates and cultured as mentioned above. Cells were transfected with pLightSwitch_Prom luciferase expression reporter vectors (Active Motif) carrying the minimal promoters of human SCN5A, SCN2B, CACNA1C, KCNQ1, KCNE1, KCNH2, or KCNN3 (WT or mutated). In these experiments, cells were cotransfected with 100 ng of an empty vector, Tbx20 WT, or p.R311C. The effects of the cotransfection of Tbx20 WT and p.R311C (100 ng each) in cells expressing the pLightSwitch_Prom luciferase expression reporter vector carrying the KCNH2 promoter were also determined. In some experiments, SP1 was used as positive control. Forty-eight hours after transfection, luciferase assays were conducted using the LightSwitch Luciferase Assay Reagent (Active Motif) and a Berthold luminometer (15, 18). Luciferase activity was normalized to sample protein concentration. All reporter assays were performed in triplicate.
Mathematical Modeling of Ventricular Action Potential.
For simulating the shapes of ventricular action potentials, we used the Grandi–Bers model of a human ventricular AP previously validated and used for similar purposes (11, 32). Simulated APs were implemented with MATLAB 6.5 (MathWorks) using the ode15s integration algorithm. Epicardial and endocardial model cells were paced at different frequencies ranging from 0.1 to 3 Hz, and currents and ionic concentrations were allowed to stabilize for at least 200 cycles. Under these conditions the model was stable.
The model was run under basal conditions and by incorporating the specific changes in conductance, time constants, and activation/inactivation curves demonstrated in the functional analyses of p.T152HfsX180 KCNH2 and p.R311C Tbx20 mutations.
Statistical Analysis.
Results are expressed as mean ± SEM. Unpaired t test or one-way ANOVA followed by Newman–Keuls test was used where appropriate. In small-size samples (n < 15), statistical significance was confirmed by using nonparametric tests. Comparisons between categorical variables were done using Z test. To take into account repeated sample assessments, data were analyzed with multilevel mixed-effects models. A value of P < 0.05 was considered significant.
Acknowledgments
We thank Paloma Vaquero, Sandra Sacristán, Lorena Ondo, and Ainara Albadalejo for their invaluable technical assistance. This work was supported by grants from Comunidad Autónoma de Madrid (S2010/BMD-2374: ITACA); Ministerio de Economía y Competitividad (SAF2014-58769-P); Instituto de Salud Carlos III (PI16/00398, CB16/11/00303, and CB16/11/00504); ERA-Net for Research on Rare Diseases (AC14/00029); Mutua Madrileña and BBVA Foundations; National Heart, Lung, and Blood Institute of the US National Institutes of Health (R01-HL122352 to J.J.); and Leducq Foundation (to J.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612383114/-/DCSupplemental.
References
- 1.Nakano Y, Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2016;61(1):51–55. doi: 10.1038/jhg.2015.74. [DOI] [PubMed] [Google Scholar]
- 2.Havakuk O, Viskin S. A tale of 2 diseases: The history of long-QT syndrome and Brugada syndrome. J Am Coll Cardiol. 2016;67(1):100–108. doi: 10.1016/j.jacc.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 4.Sakabe NJ, et al. Dual transcriptional activator and repressor roles of TBX20 regulate adult cardiac structure and function. Hum Mol Genet. 2012;21(10):2194–2204. doi: 10.1093/hmg/dds034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian L, et al. Transcription factor neuromancer/TBX20 is required for cardiac function in Drosophila with implications for human heart disease. Proc Natl Acad Sci USA. 2008;105(50):19833–19838. doi: 10.1073/pnas.0808705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen T, et al. Tbx20 regulates a genetic program essential to adult mouse cardiomyocyte function. J Clin Invest. 2011;121(12):4640–4654. doi: 10.1172/JCI59472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapa S, et al. Genetic testing for long-QT syndrome: Distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752–1760. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anson BD, et al. Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol. 2004;286(6):H2434–H2441. doi: 10.1152/ajpheart.00891.2003. [DOI] [PubMed] [Google Scholar]
- 9.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107(5):611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62(1):9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Amorós I, et al. Functional effects of a missense mutation in HERG associated with type 2 long QT syndrome. Heart Rhythm. 2011;8(3):463–470. doi: 10.1016/j.hrthm.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525(Pt 2):285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herron TJ, et al. Extracellular matrix-mediated maturation of human pluripotent stem cell-derived cardiac monolayer structure and electrophysiological function. Circ Arrhythm Electrophysiol. 2016;9(4):e003638. doi: 10.1161/CIRCEP.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plant LD, Xiong D, Dai H, Goldstein SA. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proc Natl Acad Sci USA. 2014;111(14):E1438–E1446. doi: 10.1073/pnas.1323548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barana A, et al. Chronic atrial fibrillation increases microRNA-21 in human atrial myocytes decreasing L-type calcium current. Circ Arrhythm Electrophysiol. 2014;7(5):861–868. doi: 10.1161/CIRCEP.114.001709. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, et al. Genomic structure, transcriptional control, and tissue distribution of HERG1 and KCNQ1 genes. Am J Physiol Heart Circ Physiol. 2008;294(3):H1371–H1380. doi: 10.1152/ajpheart.01026.2007. [DOI] [PubMed] [Google Scholar]
- 17.Matamoros M, et al. Nav1.5 N-terminal domain binding to α1-syntrophin increases membrane density of human Kir2.1, Kir2.2 and Nav1.5 channels. Cardiovasc Res. 2016;110(2):279–290. doi: 10.1093/cvr/cvw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Hernández M, et al. Pitx2c increases in atrial myocytes from chronic atrial fibrillation patients enhancing IKs and decreasing ICa,L. Cardiovasc Res. 2016;109(3):431–441. doi: 10.1093/cvr/cvv280. [DOI] [PubMed] [Google Scholar]
- 19.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 20.Nagy N, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009;47(5):656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JN, et al. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72(3):224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khositseth A, Tester DJ, Will ML, Bell CM, Ackerman MJ. Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm. 2004;1(1):60–64. doi: 10.1016/j.hrthm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Kirk EP, et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am J Hum Genet. 2007;81(2):280–291. doi: 10.1086/519530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 25.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112(6):651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, et al. MinK-dependent internalization of the IKs potassium channel. Cardiovasc Res. 2009;82(3):430–438. doi: 10.1093/cvr/cvp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stennard FA, Harvey RP. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development. 2005;132(22):4897–4910. doi: 10.1242/dev.02099. [DOI] [PubMed] [Google Scholar]
- 28.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: Clinical impact. Circulation. 1999;99(4):529–533. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 29.Napolitano C, Novelli V, Francis MD, Priori SG. Genetic modulators of the phenotype in the long QT syndrome: State of the art and clinical impact. Curr Opin Genet Dev. 2015;33(1):17–24. doi: 10.1016/j.gde.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Caballero R, et al. Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc Natl Acad Sci USA. 2010;107(35):15631–15636. doi: 10.1073/pnas.1004021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuteja D, et al. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107(7):851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González de la Fuente M, et al. Chronic atrial fibrillation up-regulates β1-adrenoceptors affecting repolarizing currents and action potential duration. Cardiovasc Res. 2013;97(2):379–388. doi: 10.1093/cvr/cvs313. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu W, et al. Epinephrine unmasks latent mutation carriers with LQT1 form of congenital long-QT syndrome. J Am Coll Cardiol. 2003;41(4):633–642. doi: 10.1016/s0735-1097(02)02850-4. [DOI] [PubMed] [Google Scholar]