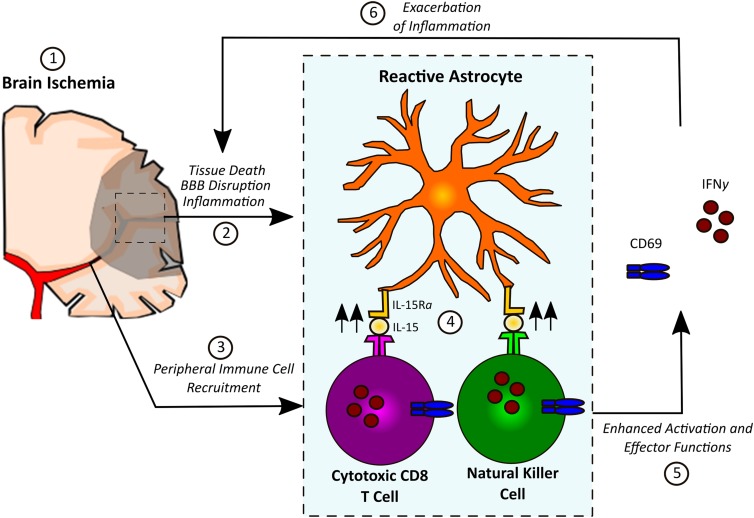
Astrocytes weave their processes throughout the central nervous system (CNS), supporting and protecting delicate neurons via the regulation of cerebral blood flow, cerebral metabolism, and neurotransmission (1, 2). Astrocytes also form the backbone of the glial limitans, the unique blood–brain barrier (BBB) that sequesters the CNS away from the rest of the body (3). The role of astrocytes after a brain injury like ischemic stroke is complex, with evidence demonstrating both beneficial and deleterious roles of astrocytes in stroke pathology (4–8). Following CNS insults, astrocytes react robustly, releasing proinflammatory mediators that recruit immune cells and initiate the formation of a glial scar to contain the area of injury and restore the integrity of the BBB (3). However, little is currently known about interactions between astrocytes and the peripheral immune system following brain injury. In PNAS, Li et al. (9) report that astrocyte-derived IL-15 is a major driver of tissue damage and poor outcome after cerebral ischemia, an effect that is dependent upon the ability of IL-15 to specifically enhance the activation and cytotoxic effector functions of natural killer (NK) cells and CD8+ T lymphocytes (Fig. 1).
Fig. 1.
In response to inflammatory signals released by dying cells after ischemia, astrocytes up-regulate IL-15. IL-15 complexes with IL-15Rα before being transported to the membrane surface, where it is recognized by infiltrating NK cells and CD8+ T lymphocytes, resulting in their activation (CD69 expression) and up-regulation of their cytotoxic effector functions (including IFN-γ production), contributing to cell-mediated immunity and further exacerbating brain inflammation.
Using proteomic profiler array analysis, Li et al. (9) identified IL-15 as one of the factors produced in large amounts by astrocytes isolated from the brains of mice 24 hours after stroke. The Li et al. (9) study is the first to demonstrate that astrocytes are a major source of IL-15 in the CNS after ischemic stroke. To determine the role of IL-15 in stroke pathology, Li et al. transgenically modified mice to specifically overexpress IL-15 in astrocytes. Although these mice showed no enhanced neuroinflammation under normal conditions, overexpression of astrocytic IL-15 in transgenic animals exacerbated tissue damage and neurological dysfunction after experimental ischemic stroke. A complementary study using in vivo lentiviral knockdown of IL-15 in the astrocytes of wild-type mice resulted in reduced tissue damage and better neurological outcomes, providing further evidence that astrocyte-derived IL-15 aggravates ischemic stroke pathology.
Until publication of this study, the mechanistic role of IL-15 in brain inflammation was not well understood (10). Peripherally, IL-15 is known to play a critical role in the maintenance and function of immune cells, including NK lymphocytes and T lymphocytes (11, 12). Li et al. (9) demonstrate that overexpression of IL-15 in astrocytes resulted in increased infiltration of NK and CD8+ T lymphocytes into the brain after stroke. Importantly, no changes in other infiltrating peripheral immune cells or altered microglial polarization were observed. In vivo depletion of CD8+ T cells, NK cells, or both ameliorated the deleterious consequences of astrocyte IL-15 overexpression in transgenic animals.
Subsequent experiments showed that astrocyte-derived IL-15 enhances the expression of molecules in NK and CD8+ T cells necessary for their activation and cytotoxcicity, including CD69 and IFN-γ. Together with previous work demonstrating that NK and CD8+ T lymphocytes exacerbate tissue damage after ischemic stroke, these data suggest that astrocytic IL-15 is a driver of lymphocyte activation and cytotoxicity in the CNS following injury (9).
IL-15 is a unique cytokine that forms a complex with IL-15 receptor-α (IL-15Rα) before presentation on the cell surface (13). Peripherally, this membrane-bound form of IL-15 is responsible for the major effects of this cytokine on target NK and CD8+ T cells (14). Li et al. (9) determined that the expression of IL-15α, the membrane presentation of IL-15 on astrocytes, and direct cell-to-cell contact are necessary to augment the activity of NK and CD8+ T cells both in vivo and in vitro. These results suggest that targeted interruption of IL-15Rα, IL-15, or the complementary receptor on target cells may all represent viable therapeutic strategies for the suppression of lymphocyte-mediated cytotoxicity in the CNS and other tissues.
It is important to note that the use of mouse models for the identification of therapeutic targets to treat human inflammatory diseases has recently been called into question (15). This problem is particularly critical in the field of ischemic stroke research, where hundreds of successful rodent studies have failed to translate into effective clinical trials and little overlap has been found between the peripheral inflammatory transcriptome of mice and humans (16). However, recent work suggests that identifying and focusing on inflammatory mechanisms shared between mice and humans provides the greatest chances of identifying viable therapeutic targets (17). The study by Li et al. (9) elegantly demonstrated that astrocytes are a major source of IL-15 in the poststroke brains of both mice and human patients. Further supporting the translational relevance of their findings, the authors went on to show that NK and CD8+ T cells were found in close proximity to reactive astrocytes poststroke in both mice and humans.
The patient brain samples used in this study were all obtained from elderly patients, underscoring the fact that ischemic stroke is primarily an age-related disease (9). Elderly patients suffer higher mortality and morbidity after stroke, findings that have been replicated in aged mouse models of ischemic stroke, which better mimic the physiologic age of the average human stroke patient (18). Because there is evidence that the inflammatory response to stroke changes with advanced age (19, 20), Li et al. strengthened the argument for the clinical utility of their findings by performing additional experiments to demonstrate that astrocytic IL-15 also plays a deleterious role in ischemic stroke in aged animals (9).
This new evidence that astrocyte-derived IL-15 augments cell-mediated immunity after ischemic stroke has wide-reaching implications regarding our understanding of astrocytes and brain-immune cross-talk in CNS injury. As the inflammatory response has been shown to have both beneficial and deleterious functions after stroke, future studies should be expanded to examine the function of IL-15 in long-term functional outcomes and neurological repair after stroke. These results have significantly expanded our knowledge of the role of astrocyte/lymphocyte interactions following cerebral ischemia, and will likely have important implications in other neuropathologies, particularly for other traumatic brain injuries and neurodegenerative diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page E396.
References
- 1.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18(7):942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28(3):468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, et al. Rat focal cerebral ischemia induced astrocyte proliferation and delayed neuronal death are attenuated by cyclin-dependent kinase inhibition. J Clin Neurosci. 2008;15(3):278–285. doi: 10.1016/j.jocn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Fang SH, et al. Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience. 2006;140(3):969–979. doi: 10.1016/j.neuroscience.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, et al. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci USA. 2017;114:E396–E405. doi: 10.1073/pnas.1612930114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Nicola D, Valle-Argos B, Pita-Thomas DW, Nieto-Sampedro M. Interleukin 15 expression in the CNS: Blockade of its activity prevents glial activation after an inflammatory injury. Glia. 2008;56(5):494–505. doi: 10.1002/glia.20628. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Kokaji AI, Hockley DL, Kane KP. IL-15 transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. J Immunol. 2008;180(7):4391–4401. doi: 10.4049/jimmunol.180.7.4391. [DOI] [PubMed] [Google Scholar]
- 13.Tamzalit F, et al. IL-15.IL-15Rα complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci USA. 2014;111(23):8565–8570. doi: 10.1073/pnas.1405514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntington ND, et al. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell-dependent antibody responses in vivo. Proc Natl Acad Sci USA. 2011;108(15):6217–6222. doi: 10.1073/pnas.1019167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seok J, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp FR, Jickling GC. Modeling immunity and inflammation in stroke: Differences between rodents and humans? Stroke. 2014;45(9):e179–e180. doi: 10.1161/STROKEAHA.114.005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2015;112(4):1167–1172. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritzel RM, et al. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J Immunol. 2016;196(8):3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6(10):e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]