Abstract
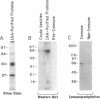
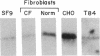
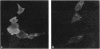
Chloride channels are present in the plasma and intracellular membranes of most cells. Previously, using the ligand indanyloxyacetic acid (IAA), we purified four major proteins from bovine kidney cortex membrane vesicles. These proteins gave rise to chloride channel activity when reconstituted into phospholipid vesicles. Two of these proteins (97 and 27 kDa) were found to be drug-binding proteins by N-terminal sequence analysis. Antibodies raised to the 64-kDa protein stained only this protein on immunoblots, and only this protein was present after purification on an immunoaffinity column. In addition, these same antibodies were able to deplete IAA-94 inhibitable chloride channel activity from solubilized kidney membranes. Of fractions obtained from the gel filtration of solubilized kidney membranes, only those containing this 64-kDa protein exhibited measurable chloride channel activity. Immunoblots of a variety of species and cell types, both epithelial and nonepithelial, revealed that this protein is ubiquitous and highly conserved. Immunocytochemistry in CFPAC-1 cells revealed staining for this protein on the apical plasma membrane and in the membranes of intracellular organelles. These results demonstrate that the integral membrane protein p64 is a component of chloride channels present in both epithelial plasma membrane and the membranes of intracellular organelles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Barasch J., Gershon M. D., Nunez E. A., Tamir H., al-Awqati Q. Thyrotropin induces the acidification of the secretory granules of parafollicular cells by increasing the chloride conductance of the granular membrane. J Cell Biol. 1988 Dec;107(6 Pt 1):2137–2147. doi: 10.1083/jcb.107.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Charnock J. S., Potter H. A., McKee D. Ethacrynic acid inhibition of (Na + +K + )-activated adenosine triphosphatase. Biochem Pharmacol. 1970 May;19(5):1637–1641. doi: 10.1016/0006-2952(70)90152-8. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. W. Inhibition of renal Na + ,K + -activated adenosine triphosphatase activity by ethacrynic acid. Biochem Pharmacol. 1970 Jun;19(6):1983–1989. doi: 10.1016/0006-2952(70)90294-7. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L., Tsai L. M., Falk R. J. Monoclonal antibodies to the apical chloride channel in Necturus gallbladder inhibit the chloride conductance. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7649–7652. doi: 10.1073/pnas.86.19.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F., Petris A. Chloride channels of biological membranes. Biochim Biophys Acta. 1990 May 7;1031(2):247–259. doi: 10.1016/0304-4157(90)90009-2. [DOI] [PubMed] [Google Scholar]
- Garty H., Rudy B., Karlish S. J. A simple and sensitive procedure for measuring isotope fluxes through ion-specific channels in heterogenous populations of membrane vesicles. J Biol Chem. 1983 Nov 10;258(21):13094–13099. [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Greger R., Kunzelmann K., Gerlach L. Mechanisms of chloride transport in secretory epithelia. Ann N Y Acad Sci. 1989;574:403–415. doi: 10.1111/j.1749-6632.1989.tb25179.x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Rector F. C., Jr, Berry C. A. Chloride transport across the basolateral membrane of rabbit proximal convoluted tubules. Am J Physiol. 1990 Jun;258(6 Pt 2):F1569–F1578. doi: 10.1152/ajprenal.1990.258.6.F1569. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990 Dec 6;348(6301):510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry D. W., Reitman M., Cragoe E. J., Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol. 1987 Dec;90(6):779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. The basic defect in cystic fibrosis. Science. 1989 Jun 23;244(4911):1423–1423. doi: 10.1126/science.2544028. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Regulation of Cl- and K+ channels in airway epithelium. Annu Rev Physiol. 1990;52:115–135. doi: 10.1146/annurev.ph.52.030190.000555. [DOI] [PubMed] [Google Scholar]
- Mulberg A. E., Tulk B. M., Forgac M. Modulation of coated vesicle chloride channel activity and acidification by reversible protein kinase A-dependent phosphorylation. J Biol Chem. 1991 Nov 5;266(31):20590–20593. [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran S., Benos D. J. Isolation and functional reconstitution of a 38-kDa chloride channel protein from bovine tracheal membranes. J Biol Chem. 1991 Mar 15;266(8):4782–4788. [PubMed] [Google Scholar]
- Reenstra W. W., Sabolic I., Bae H. R., Verkman A. S. Protein kinase A dependent membrane protein phosphorylation and chloride conductance in endosomal vesicles from kidney cortex. Biochemistry. 1992 Jan 14;31(1):175–181. doi: 10.1021/bi00116a026. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Gregory R. J., Anderson M. P., Manavalan P., Smith A. E., Welsh M. J. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991 Jul 12;253(5016):205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev. 1985 Apr;65(2):310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Ram J., Iannuzzi M. C., Bradbury N. A., Wallace R. W., Hon C. T., Kelly D. R., Schmid S. M., Gelder F. B., Rado T. A. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1990 May;87(10):4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Papazian D. M., Carretto R. C., Jan Y. N., Jan L. Y. Immunological characterization of K+ channel components from the Shaker locus and differential distribution of splicing variants in Drosophila. Neuron. 1990 Jan;4(1):119–127. doi: 10.1016/0896-6273(90)90448-o. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Jan Y. N., Jan L. Y. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988 Oct;1(8):659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Abnormal regulation of ion channels in cystic fibrosis epithelia. FASEB J. 1990 Jul;4(10):2718–2725. doi: 10.1096/fasebj.4.10.1695593. [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Butt A. G., Cliff W. H., Frizzell R. A. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989 Jun;256(6 Pt 1):C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]