Significance
The evolution of cooperation is a central issue in biology and the social sciences. Study of model systems of social microbes has focused on how “cooperators” and “cheats” interact but rarely accounts for the surrounding environment. We demonstrate how environmental stress in the form of antibiotics alters the evolution of public goods cooperation in a bacterium. Antibiotics accentuate the costs to cooperators, resulting in their rapid demise relative to cheats. In a more applied vein, antibiotic resistance was maximal in the presence of both producers and cheats, suggesting that knowledge about social strategies can be used to improve therapies. Our work emphasizes ecoevolutionary feedback in social evolution and demonstrates that social interactions may be considerably modified in natural, stressful environments.
Keywords: evolution, cooperation, antibiotics, social behavior, resistance
Abstract
Cheats are a pervasive threat to public goods production in natural and human communities, as they benefit from the commons without contributing to it. Although ecological antagonisms such as predation, parasitism, competition, and abiotic environmental stress play key roles in shaping population biology, it is unknown how such stresses generally affect the ability of cheats to undermine cooperation. We used theory and experiments to address this question in the pathogenic bacterium, Pseudomonas aeruginosa. Although public goods producers were selected against in all populations, our competition experiments showed that antibiotics significantly increased the advantage of nonproducers. Moreover, the dominance of nonproducers in mixed cultures was associated with higher resistance to antibiotics than in either monoculture. Mathematical modeling indicates that accentuated costs to producer phenotypes underlie the observed patterns. Mathematical analysis further shows how these patterns should generalize to other taxa with public goods behaviors. Our findings suggest that explaining the maintenance of cooperative public goods behaviors in certain natural systems will be more challenging than previously thought. Our results also have specific implications for the control of pathogenic bacteria using antibiotics and for understanding natural bacterial ecosystems, where subinhibitory concentrations of antimicrobials frequently occur.
Public goods production is a characteristic of a diverse range of taxa, from microbes to humans (1–3). Explaining the persistence of this costly behavior is challenging, because cheats can exploit the commons without contributing. Kin selection theory has proven to be a successful framework for addressing this question, with the central prediction that cooperation is favored by sufficient benefits to, and positive assortment between, cooperators (4–8). For example, recent study in experimental bacterial populations has elucidated mechanisms such as assortment emerging from limited or budding dispersal (9, 10) and kin discrimination (11, 12) that are consistent with kin selection fostering cooperative behaviors (e.g., refs. 8 and 13). However, despite this accumulating consensus, little is known about how social populations respond to differences and variation in abiotic and biotic components of their environment. In particular, it is unclear how ecological antagonisms affect the ability of cheats to invade cooperator communities.
Cooperation can be affected by stress directly through differential selection on cooperative phenotypes (14, 15), or by inducing specific plastic behaviors (16–22), especially when cooperation leads to increased stress resistance. However, in the absence of a direct benefit of the cooperative behavior against stress, the ecological and evolutionary outcomes of the interactions between nondefensive public goods and stress responses are less clear and are potentially complex. Cooperation may be influenced indirectly through impacts on population structure and dynamics (23), via epistasis or pleiotropy (24) or through the hitchhiking of cooperative genes with resistance mutations (25–27). In the case of hitchhiking, the fate of cooperators might in part be contingent on whether they represent the majority of the population when the environmental stress occurs. This is because the probability of the emergence of resistance or tolerance mutations should increase with population size (25, 28), and so these mutations are most likely to appear in the more numerous and/or fastest-growing subpopulation. On the other hand, we would more generally expect that, under sublethal stress, nonproducers limit the emergence and spread of resistant cooperators by cheating on public goods production. In addition, should stress responses (29–31) or the evolution of stress resistance (32–34) entail costs, such costs could potentially interact with social behaviors and accentuate selection for cheating. Evidence to support or refute such hypotheses is lacking. Addressing this gap crucially requires characterizing—both experimentally and in general theoretical models—how the fitness of cheats, relative to producers, depends on the level of ecological stress.
Microbial populations are an increasing focus for research on public goods dynamics (35–37). Microbes may exhibit rapid ecological and evolutionary responses and are amenable to controlled laboratory experimentation (36, 38). Bacteria, in particular, show a variety of behaviors consistent with basic social interactions. These frequently involve the coordinated secretion of metabolites that are potentially beneficial to others (i.e., public goods), leading to, for example, collective motility and/or resource acquisition (e.g., reviewed in refs. 35, 39, and 40). Bacteria are also confronted with a variety of antagonisms, including predation and parasitism (e.g., phages, metazoans, and plasmids), antimicrobials produced by other organisms (antibiotics, AMPs, and toxins), and abiotic environments (extreme temperatures, pH, and salinity) that can result in reduced fitness through decreases in survival and reproduction. In particular, subinhibitory concentrations of antimicrobials are pervasive in natural environments such as rivers, lakes, soils, and bacterial hosts (animals and plants). In human society, subinhibitory levels of anthropogenic antibiotics are important for their impacts on managed systems (e.g., animal husbandry), their effects as environmental pollutants, and their key role in the progressive increases in antibiotic resistance (41–43). Subinhibitory concentrations have been shown to affect cellular physiology and genetic variability and behaviors, yet the evolutionary implications for both social and asocial traits are largely unknown (41).
We used a bacterial system to test the prediction that the direct cost of public goods production and indirect costs through exploitation by nonproducers accentuate both the ecological and evolutionary benefits of cheating when the population faces an environmental stress. Specifically, we examined how a public goods trait in the form of siderophore production interacts with resistance evolution to the antibiotic gentamicin in the pathogenic bacterium, Pseudomonas aeruginosa. We grew a strain of P. aeruginosa PAO1 producing the siderophore pyoverdin and/or a nonproducing strain under iron-limited conditions with different doses of the antibiotic. Siderophores are small secreted molecules that chelate poorly soluble iron in the environment, making the iron available to bacteria via specific outer-membrane receptors (44). Because any cell carrying these receptors can use chelated iron, siderophores are a public good in well-mixed environments. As such, costly siderophore production is vulnerable to “cheating” by cells that do not produce the molecule, but possess specialized receptors and reap the benefit of available iron (e.g., refs. 13 and 45). We used subinhibitory antibiotic concentrations, which the bacteria are most likely to encounter in natural settings and in host tissues (46–49). We assessed (i) the impact of antibiotic pressure on the interaction between the two production genotypes and (ii) the consequences for the population response to antibiotics, in particular the evolution of resistance. We found that antibiotic stress accelerates the decline of producers in mixed cultures, indicating that the environment can shape competitive interactions. Moreover, nonproducer resistance frequency was greater in mixed cultures compared with monocultures of either nonproducers or producers. A mathematical model shows that these observed qualitative patterns may be explained by the constitutive investment in pyoverdin production decreasing the capacity of producers to cope with antibiotic stress in the presence of nonproducers. Given the generality of the model, its predictions regarding the interplay between social and stress resistance traits should apply to many other biological systems. We discuss these findings in the contexts of social evolution and resistance to antagonisms, with a focus on bacterial evolution.
Experimental Results
Changes in Nonproducer Frequency.
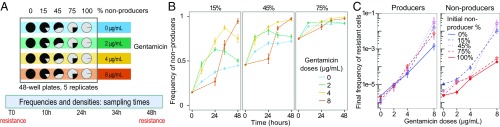
In all mixed populations, for all three initial frequencies and four antibiotic treatments (Fig. 1A), nonproducer frequency increased over the course of the experiment (Fig. 1B). Nonproducer frequencies were substantially higher in the presence of the antibiotic than in the antibiotic-free controls. At higher doses (4 and 8 µg/mL), nonproducers had often reached near fixation (>90%) after 48 h.
Fig. 1.
Experimental design and main experimental results. (A) Experimental design. (B) Change in nonproducer frequencies in experimental populations between beginning (T0) and end (T48) of the experiment. Panels correspond to different initial frequencies of nonproducers. Colors represent gentamicin doses. (C) Final frequency of resistant cells in producers (Left) and nonproducers (Right) in monocultures (solid lines) and in mixed cultures (dashed lines) for different doses of gentamicin. All bars are SEMs.
Antibiotic dose further affected the timing of changes in the relative frequencies of producers and nonproducers, as indicated by the significant Time × Gentamicin interaction [χ2(3) = 123.39, P < 0.0001]. Namely, at the two lower doses (2 and 4 µg/mL), nonproducer frequencies increased considerably during the first 24 h and then reached a peak (Fig. 1B). At the highest antibiotic dose (8 µg/mL), this increase was delayed by approximately 24 h.
These patterns were similar for all initial frequencies of nonproducers, and the significant three-way interaction [Time × Gentamicin × Initial Frequency, χ2(6) = 26.69, P < 0.001] likely reflects the lower absolute change in frequency for populations initiated with 75% of nonproducers.
Effects of the Antibiotic and Initial Nonproducer Frequency on Bacterial Antibiotic Resistance.
Experimental treatments affected resistance frequencies in three main ways. First, increasing the dose of gentamicin led to higher frequencies of resistant cells, with a difference of up to five orders of magnitude between the highest dose (8 µg/mL) and the control (Fig. 1C). Nonetheless, the frequency of resistance always remained below 10%.
Second, producer monocultures generally showed higher frequencies of resistance than nonproducer monocultures at all three gentamicin doses [producer vs. nonproducer: χ2(1) = 9.0, P < 0.005; Fig. 1C]. Third, resistance was more frequent in mixed culture than in monoculture, in particular at high antibiotic dose (Fig. 1C). This general pattern held for both producers [mono vs. mixed: χ2(1) = 43.7, P < 0.0001] and nonproducers [χ2(1) = 34.2, P < 0.0001], despite some deviations [significant Dose × Initial Nonproducer Frequency interactions for both producer types: χ2(9) > 16, P < 0.05 for both analyses]. Namely, for producers, the higher mixed-culture resistance was only consistently prominent in lines from the highest antibiotic dose treatment (Fig. 1C, Left). Nonproducers showed higher mixed-culture resistance over all dose treatments, but there was more variation among lines with different initial nonproducer frequencies (Fig. 1C, Right). A supplementary replicate experiment confirmed these main results (SI Appendix, Fig. S3 E–G).
The unexpected observation of higher frequencies of producer resistance in mixed culture led us to conduct a series of additional assays (SI Appendix) to investigate in more detail the quantitative levels of resistance (measured as the minimum inhibitory concentration) and associated fitness costs in mixed and monocultures. Our hypothesis was that producers in mixed culture might have acquired particular adaptations to selection pressures from both nonproducers and the antibiotic, resulting in highly resistant, fit producers that could coexist with nonproducers. Indeed, we found that, unlike nonproducers, producers tended to be more resistant (higher level of resistance) in mixed cultures compared with monocultures (P < 0.05, SI Appendix, Fig. S4 A–C). Moreover, this higher level of resistance did not come at an increased fitness cost: resistant producers showed an average reduction of growth of 40%, both in monocultures and mixed cultures, compared with nonresistant producers [ANOVA, F(1, 41) = 116.232, P < 0.001; SI Appendix, Fig. S4 D–F]. On the basis of the growth assays, the availability and production of pyoverdin were higher in resistant producers compared with nonresistant producers [availability: ANOVA, F(1, 9) = 21.603, P < 0.005; production: ANOVA, F(1, 9) = 69.362, P < 0.001]. However, we did not detect significant differences in pyoverdin availability [ANOVA, F(1, 9) = 0.069, P = 0.799; SI Appendix, Fig. S5A] or production per cell [ANOVA, F(1, 9) = 1.252, P = 0.292; SI Appendix, Fig. S5 B–D] between resistant colonies from mixed cultures and monocultures. This finding suggests that competition with nonproducers did not select for decreased pyoverdin production in resistant producers.
We then investigated genetic resistance to gentamicin in resistant and nonresistant individual colonies of producers and nonproducers from all treatments in the repeated 48-h experiment (SI Appendix). We sequenced the repressor gene and intergenic region of the efflux pump MexXY, described as the only identified pump mediating aminoglycoside resistance (50, 51). Although the observed selection for resistant phenotypes suggests an underlying genetic component, we did not detect any evidence of gene modification in these markers (SI Appendix). We further tested for the presence of nine genes coding for gentamicin-degrading enzymes and did not detect any of these genes.
Theoretical Framework
Our experimental results showed that (i) antibiotics increased the frequency of nonproducers in mixed cultures in a dose-dependent manner and (ii) the frequency of resistant cells was higher in mixed cultures than in either monoculture. We hypothesized that the cost of public goods production reduced the capacity of producers to cope with antibiotic stress, perhaps by depleting metabolic resources that would otherwise be expended on countering effects of the antibiotic. This would be especially pronounced in the presence of nonproducers because the latter constitute an indirect cost by removing iron from the environment. We developed and analyzed a mathematical model to examine this hypothesis and to investigate more generally how an ecological antagonism can influence competition between public goods producers and nonproducers.
Effects of an Ecological Antagonism on Interactions Between Producers and Nonproducers.
In analyzing the effects of an ecological antagonism, we are primarily interested in how rapidly the frequency of nonproducers increases during the exponential growth phase, when population size and public goods concentration are both relatively small. We therefore assume that the latter two factors have relatively little effect on the frequency dynamics and can be neglected. We further assume that the cost of public goods production is approximately constant. For an antagonism that slows growth or reproduction, the dynamical equations are then
where NPS and NQS are the numbers of producers and nonproducers (respectively), b is the baseline birth rate, c is the cost of public goods production, and εPS ≤ 1 and εQS ≤ 1 are the effects of the antagonism. The equations for a mortality-inducing antagonism are similar (SI Appendix). We assume the antagonism dose–response curve can be approximated by a sigmoidal Hill function kAh/(Ah + Mh), where A is the level of the antagonism, and k, h, and M are constant parameters. The Hill function form accords, for example, with the pharmacodynamics of antibiotics including gentamicin (52–54).
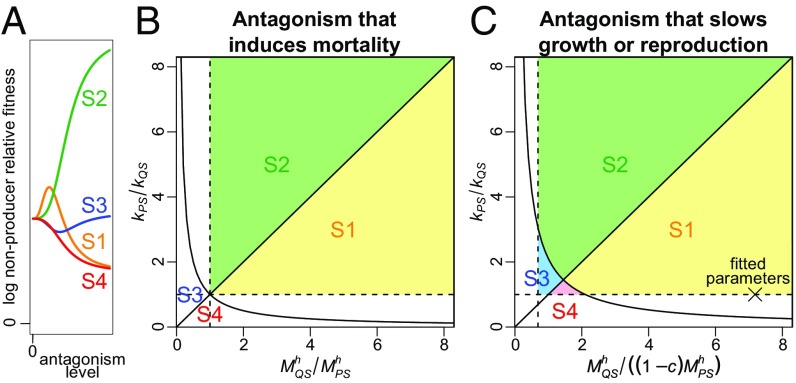
Analysis of our mathematical model reveals that, in general, the relative fitness of nonproducers can vary with the antagonism level in four qualitatively different ways. The type of relationship (monotonically increasing, monotonically decreasing, peaked, or valley shaped) depends on the relative sizes of the antagonism effects and the cost of public goods production (Fig. 2 and SI Appendix).
Fig. 2.
Results of a general mathematical model. (A) The level of an ecological antagonism and the relative fitness of public goods nonproducers can be related in four qualitatively different ways: S1, S2, S3, or S4. (B) For an antagonism that induces mortality, the form of the relationship depends on kPS/kQS (the ratio of the effects of the antagonism on producers and nonproducers, respectively, as the antagonism level approaches infinity) and MQS/MPS (the ratio of the antagonism levels at which the antagonism effect is half its maximum for nonproducers and producers, respectively) raised to the power of h (a constant, typically between 1 and 10). (C) For an antagonism that slows growth or reproduction, the class of the relationship also depends on the cost of public goods production, c. Boundaries defining the parameter regions of S1–S4 are shown as solid lines. Assuming the antagonism affects producers at least as much as nonproducers excludes the unshaded regions of the parameter space below and to the left of the dashed lines in each panel. Thus, for an antagonism that induces mortality, only relationships S1 and S2 are possible, whereas for an antagonism that slows growth or reproduction when c > 0, all four classes of relationship are possible (in the figure we set c = 0.31, which is the estimate obtained from our data). Our experimental system lies within the S1 region, as indicated by the cross labeled “fitted parameters.”
Nonproducer frequency is expected to increase fastest at intermediate antagonism levels (as in our experimental system) if the antagonism (i) affects producers more than nonproducers at low and intermediate levels, but (ii) impacts both populations similarly at very high levels. This pattern holds over a wide range of plausible parameter values, regardless of whether the antagonism affects growth, reproduction, or mortality, and is not sensitive to public goods dynamics (SI Appendix).
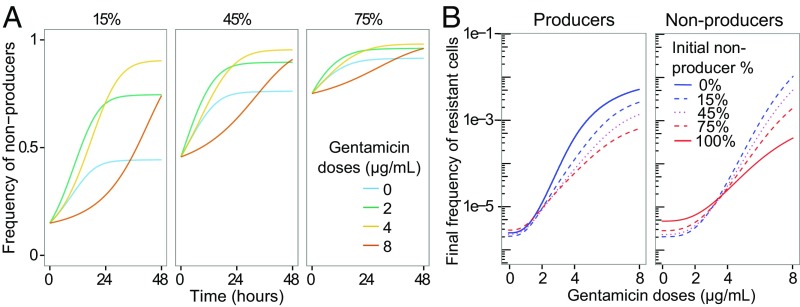
To further quantify antibiotic effects in our experimental system, we extended our mathematical model, such that the fitness of each subpopulation depended on the cost of public goods production, the population density, the beneficial effect of public goods, and effects of the antibiotic on bacterial growth. We then tailored this model to our particular biological system by specifying functional forms for each component (SI Appendix). When fitted to the bacterial population data using a Markov chain Monte Carlo (MCMC) method (SI Appendix), the dynamical model provides a good statistical fit to the experimental data (SI Appendix, Fig. S7), and shows frequency dynamics qualitatively consistent with experimental observations (cf. Figs. 1B and 3A). Initially, nonproducer frequency increases fastest at intermediate antibiotic concentrations and increases slowest at high antibiotic concentrations. However, under the reasonable assumption that the antibiotic effect decreases over time [which could occur, for example, due to drug degradation (55) or bacterial adaptation (56)], the model predicts that the rate of change will accelerate in the latter case, as we indeed observed in our experiments.
Fig. 3.
Results of the mathematical model fitted to the experimental data. (A) Change in nonproducer frequencies in a mathematical model between T0 and T48. Panels correspond to different initial frequencies of nonproducers. Colors represent gentamicin doses. (B) Resistance frequencies in a mathematical model at T48. Final frequency of resistant cells in producers (Left) and nonproducers (Right) in monocultures (solid lines) and in mixed cultures (dashed lines) for different doses of gentamicin. SI Appendix provides model definition and parameter values.
Effects of Nonproducer Frequency on Resistance to an Ecological Antagonism.
We next investigated the effects of the initial frequency of public goods nonproducers (and the antagonism level) on the frequency of resistance to an ecological antagonism, by extending the previously described mathematical model to include resistant and susceptible subpopulations of producers and nonproducers. To ensure generality, we analyzed various alternative ways in which the public goods concentration, the antagonism level, and the cost of resistance to the antagonism might affect the relative fitness of the resistant subpopulation (SI Appendix). This analysis reveals that, when the antagonism level is relatively high, a public good (such as pyoverdin) will increase the final frequency of resistance to the antagonism only if (i) the beneficial effect of the public good interacts with the effect of the antagonism or with the cost of resistance (or both); and (ii) the primary effect of the antagonism is to slow growth or reproduction. When these conditions are met, the beneficial effects of the public good accentuate the fitness difference resulting from the unequal effects of the antagonism on susceptible and resistant subpopulations.
According to the fitted model described previously (SI Appendix, Fig. S7), pyoverdin’s effect on the final frequency of resistance increased with antibiotic dose, within the range tested in our experiments (Fig. 3B). The model output resembles the data for the frequency of resistance in nonproducers, but is less accurate for the frequency of resistance in producers. An examination of the data (SI Appendix, Fig. S7) suggests that this discrepancy is a result of the rapid decrease in the susceptible producer population toward the end of the experiment, which may have been due to environmental deterioration (not included in the mathematical model).
Discussion
Social behaviors are widespread across the living world at all organizational levels (57). Whereas underlying intrapopulation interactions have been extensively studied, their interplay with environmental factors is only beginning to be understood. Importantly, very little is known about how ecological antagonisms affect the risk that cheating will undermine cooperation, particularly when cooperation is not directly involved in resistance or tolerance. Here, we focused on the interplay between antibiotic stress and siderophore cooperation in P. aeruginosa. We found that antibiotics constitute a cost to social behaviors, manifested by an increased benefit of cheating under stressful conditions. Mathematical analyses show the key driver to be the differential stress sensitivities (and fitness effects) of the two public goods strategies. Our experimental and theoretical results thus contribute to disentangling the complex ecological and evolutionary dynamics of public goods behaviors and their interactions with biological stressors such as antibiotics. Our mathematical model is sufficiently general for its testable predictions to apply to public goods cooperation across a wide array of biological systems. Below we discuss the importance of ecological antagonisms in the evolution of public goods behaviors and resistance.
The essential element underlying all of our results is that, whereas public goods benefit every individual, only producers pay the associated fitness cost. This factor can explain why producer bacteria were more affected than nonproducers by antibiotic stress: the fitness cost of pyoverdin production (58) limited the capacity of producers to resist antibiotics (which is also associated with a metabolic fitness cost in antibiotic-free medium; SI Appendix, Fig. S4 D–F) (59) and to compete with nonproducers. In other words, in the absence of a “private benefit” to producers, it is growth in the absence of stress that, all else being equal, determines how well a strain can cope with an ecological antagonism. This is consistent with the findings of Mitri et al. (60) who used computer simulations in a spatial setting and showed that antagonism (in this case, ecological competition) is more detrimental to cooperators than to cheats when nutrients are limiting. The authors suggested that this effect occurs because the investment in cooperative secretions limits growth and thereby competitive ability. Alternatively, in some particular cases, antagonisms may directly increase the benefit to nonproducers by inducing the production of costly public goods (20, 61). In Staphylococcus aureus, for example, sublethal doses of ciprofloxacin, mupirocin, or rifampicin induce the expression of the costly effector molecule regulating the quorum-sensing system agr, thereby favoring agr-negative variants (61). Such findings add to the challenge of explaining how public goods cooperators and cheats coexist in nature. Our results specifically imply that cooperation may be even costlier in natural social bacterial systems than suggested by previous study (e.g., ref. 13). In many cases, a likely important factor enabling coexistence is spatial structure (62–64), whereby spatial segregation of nonproducers and producers limits the exploitative potential of the former.
In one of the few studies investigating interactions between antibiotics and social behaviors in bacteria, Diard et al. (14) assessed the in vivo impact of ciprofloxacin on competition between virulent cooperative Salmonella enterica serovar Typhimurium and avirulent defectors. In the absence of the antibiotic, defectors outcompeted cooperators in the gut lumen, whereas the antibiotic addition reversed the outcome, leading to selection for the virulent cooperators. Indeed, only the virulent cells were able to invade host tissues and escape antibiotic mortality in the lumen. The authors observed that, when antibiotic pressure decreased, the virulent strain reinvaded the gut lumen. Our results contrast with these findings of Diard et al., as we observed that antibiotics led to the selection of nonproducers over producers. We hypothesize that this finding is because of differences in spatial structure between the two studies: whereas the gut lumen is a highly structured environment, our in vitro studies were conducted under well-mixed conditions, where producers had no refuge from the antibiotic nor from exploitation by nonproducers.
Whereas public goods availability had relatively little effect on nonproducer frequency dynamics, we found it had a major role in the evolution of resistance to an ecological antagonism. Pure producer populations should have the highest public goods availability per individual, leading to the highest growth rates and population sizes, and therefore one might expect to see the highest final frequency of resistance in the absence of cheats. However, in our experiments we found that, under the highest antibiotic dose, the frequency of resistant cells was higher in mixed cultures. Our mathematical model shows that this pattern is predicted when a bacteriostatic antibiotic affects producers more than nonproducers, provided the beneficial effect of the public good interacts with the effects of the drug (SI Appendix). This model can explain why resistant nonproducers grew faster in mixed cultures, not only compared with resistant nonproducers in monoculture, but also compared with resistant producers in monoculture, thereby leading to more resistance in mixed populations. The optimal frequency for nonproducers appears to be low. When initially very frequent (75%), nonproducers did not evolve substantially higher frequencies of resistance compared with their populations in monoculture, possibly as uncommon producers yielded a low pyoverdin concentration, thereby contributing relatively little both to nonproducer growth (20) and to the generation of resistant variants. An additional experiment (SI Appendix) confirmed that differences in growth and antibiotic resistance between producers and nonproducers are mediated by pyoverdin availability and production: when populations grew under high iron availability conditions (i.e., where siderophores are not needed), the nonproducers did not invade the mixed populations (SI Appendix, Fig. S6A) and the frequency of resistance was similar for both strains in mixed cultures and in both monocultures (SI Appendix, Fig. S6 E–G). Our mathematical model also predicts that, when iron availability is limited, resistance among producers will be more frequent in monocultures than in mixed cultures, yet experimentally we observed the opposite pattern. This discrepancy between theory and experiments may be explained by the steep decline in sensitive producer densities near the end of the culture period, as they succumbed to the combined effects of antibiotics and exploitation by cheats.
Our results have implications for the control of cooperatively foraging or scavenging pathogenic bacteria using antibiotics in resource-limited infections as we have found that antibiotics can select for an overall higher prevalence of resistance when both producers and nonproducers are present. It has previously been shown that selection for nonproducers is expected to lower bacterial virulence (65, 66). We observed that highly resistant producers also arose in mixed cultures, but they were outcompeted by resistant nonproducers. This could have implications for predicting the direct and knockon effects of antibiotic dosing on treatment outcomes (e.g., see discussion in ref. 67).
Our findings also provide insights into competitive interactions in natural ecosystems. In particular, our study addresses two of the “outstanding questions” in a recent review by Ghoul and Mitri (68): How does the environment dictate the prevalence of competition? And: Is it possible to manipulate competition by altering environmental conditions? We have shown that the prevalence and outcomes of competition may be highly dependent on the environment, so that it is possible to manipulate the relative fitness of competitors by modifying their environment. Indeed, increased iron availability resulted in the higher relative fitness of producers. Moreover, the dose of antibiotics in the environment shapes the outcomes of competition between producers and nonproducers, with intermediate doses increasing the advantage to nonproducers.
Previous research has propounded the importance of ecology in social evolution and called for a deeper integration of ecological factors in social theory (69–73). Further to this claim, we suggest that ecological stressors could impact social evolution in microbes and in multicellular taxa more generally. Testing this broader hypothesis would require extensions of our mathematical model and experiments to assess the costs and payoffs of different social strategies in a wider range of environments with various types of spatial structure.
Materials and Methods
Experiment.
Experimental protocol.
We used P. aeruginosa PAO1 as the pyoverdin-producing wild type (“producers”) and an otherwise isogenic mutant PAO1ΔpvdD (74) unable to produce pyoverdin (“nonproducers”). We inoculated bacteria as either monocultures or mixed cultures of producers and nonproducers to a final density of approximately 107 bacteria per milliliter into fresh iron-limited medium with either a low (2 µg/mL), intermediate (4 µg/mL), or high (8 µg/mL) dose of gentamicin, or in antibiotic-free medium (Fig. 1A). Mixed populations were initiated with 15%, 45%, or 75% of nonproducers. Each treatment was replicated five times for a total of 100 populations (4 antibiotic conditions × 5 types of cultures × 5 replicates) that were arbitrarily distributed in 48-well plates. The experiment was run for 48 h at 37 °C under constant shaking (350 rpm, 8-mm stroke). We measured the densities and the relative frequencies of producers and nonproducers by plating samples of each population onto King’s B medium (KB) agar plates and subsequent counting of colony forming units (CFUs) at the beginning of the experiment (T0) and after 10 (T10), 24 (T24), 34 (T34), and 48 (T48) hours. In addition, we estimated the frequency of resistant cells at T0 and T48 by plating samples of each population onto antibiotic-free KB agar plates and onto KB agar with 10 µg/mL gentamicin, simultaneously. Experimental conditions are detailed in SI Appendix, section 1.
Additional assays.
Following the above-described experiment, we conducted a series of assays detailed in SI Appendix, sections 2 and 3. Briefly, we repeated the experiment for a subset of treatments and we isolated resistant and nonresistant clones from the ancestral and all of the evolved populations. We subsequently assayed pyoverdin production, the growth capacity and the level of gentamicin resistance of these clones. We explored the genetic basis of gentamicin resistance in the clones by target-sequencing and sequence amplification. Moreover, we controlled for the importance of pyoverdin cooperation in the observed dynamics by growing producers and nonproducers under iron-rich conditions (i.e., that did not require siderophore production).
Mathematical Analysis and Modeling.
We conducted a general mathematical analysis of the relative fitness of public goods producer and nonproducer subpopulations and of subpopulations resistant and susceptible to an ecological antagonism. The main assumptions are that public goods production and resistance to the antagonism incur fitness costs, and the antagonism may affect producers and nonproducers unequally. The full analysis can be found in SI Appendix, section 4.
We also developed a more specific mathematical model of our experimental system as a quantitative test of our assumptions. We fitted this model to the bacterial population data using a MCMC method (75, 76) and verified the fit with a different algorithm (77). Further details of the model are in SI Appendix, section 4.
Supplementary Material
Acknowledgments
We thank Pierre Cornelis and Melanie Ghoul for providing the PAO1 and PAO1ΔpvdD strains of P. aeruginosa; David Lunn for helping us acquire and set up software; and Guillaume Martin, Sylvain Gandon, Sonia Kéfi, Sébastien Lion, Sara Mitri, and Rolf Kümmerli for helpful advice. This work was supported by James S. McDonnell Foundation Studying Complex Systems Research Award 220020294 (to M.E.H.) and a doctoral grant from the French Ministry of Research (to M.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612522114/-/DCSupplemental.
References
- 1.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4(8):597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 2.Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol Evol. 2007;22(12):643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Cordero OX, Ventouras L-A, DeLong EF, Polz MF. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc Natl Acad Sci USA. 2012;109(49):20059–20064. doi: 10.1073/pnas.1213344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke AFG. The validity and value of inclusive fitness theory. Proc Biol Sci. 2011;278(1723):3313–3320. doi: 10.1098/rspb.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher JA, Doebeli M. A simple and general explanation for the evolution of altruism. Proc Biol Sci. 2009;276(1654):13–19. doi: 10.1098/rspb.2008.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7(1):1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7(1):17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.West SA, Pen I, Griffin AS. Cooperation and competition between relatives. Science. 2002;296(5565):72–75. doi: 10.1126/science.1065507. [DOI] [PubMed] [Google Scholar]
- 9.Gardner A, West SA. Demography, altruism, and the benefits of budding. J Evol Biol. 2006;19(5):1707–1716. doi: 10.1111/j.1420-9101.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- 10.Kümmerli R, Gardner A, West SA, Griffin AS. Limited dispersal, budding dispersal, and cooperation: An experimental study. Evolution. 2009;63(4):939–949. doi: 10.1111/j.1558-5646.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehdiabadi NJ, et al. Social evolution: Kin preference in a social microbe. Nature. 2006;442(7105):881–882. doi: 10.1038/442881a. [DOI] [PubMed] [Google Scholar]
- 12.Rendueles O, et al. Rapid and widespread de novo evolution of kin discrimination. Proc Natl Acad Sci USA. 2015;112(29):9076–9081. doi: 10.1073/pnas.1502251112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430(7003):1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 14.Diard M, et al. Antibiotic treatment selects for cooperative virulence of Salmonella typhimurium. Curr Biol. 2014;24(17):2000–2005. doi: 10.1016/j.cub.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Friman V-P, Diggle SP, Buckling A. Protist predation can favour cooperation within bacterial species. Biol Lett. 2013;9(5):20130548. doi: 10.1098/rsbl.2013.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleich R, Watrous JD, Dorrestein PC, Bowers AA, Shank EA. Thiopeptide antibiotics stimulate biofilm formation in Bacillus subtilis. Proc Natl Acad Sci USA. 2015;112(10):3086–3091. doi: 10.1073/pnas.1414272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelshtein A, Roth D, Ben Jacob E, Ingham CJ. Bacterial swarms recruit cargo bacteria to pave the way in toxic environments. MBio. 2015;6(3):e00074-15. doi: 10.1128/mBio.00074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman LR, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436(7054):1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan JB, et al. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3(4):e00198-12. doi: 10.1128/mBio.00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasse M, Torres-Barceló C, Hochberg ME. Phage selection for bacterial cheats leads to population decline. Proc R Soc B. 2015;282(1818):20152207. doi: 10.1098/rspb.2015.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira NM, et al. Biofilm formation as a response to ecological competition. PLoS Biol. 2015;13(7):e1002191. doi: 10.1371/journal.pbio.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle KE, Monaco H, van Ditmarsch D, Deforet M, Xavier JB. Integration of metabolic and quorum sensing signals governing the decision to cooperate in a bacterial social trait. PLOS Comput Biol. 2015;11(5):e1004279. doi: 10.1371/journal.pcbi.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Jacob E, et al. Bacterial cooperative organization under antibiotic stress. Phys Stat Mech Its Appl. 2000;282(1–2):247–282. [Google Scholar]
- 24.Yurtsev EA, Chao HX, Datta MS, Artemova T, Gore J. Bacterial cheating drives the population dynamics of cooperative antibiotic resistance plasmids. Mol Syst Biol. 2013;9(1):683. doi: 10.1038/msb.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan AD, Quigley BJZ, Brown SP, Buckling A. Selection on non-social traits limits the invasion of social cheats. Ecol Lett. 2012;15(8):841–846. doi: 10.1111/j.1461-0248.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley BJZ, López DG, Buckling A, McKane AJ, Brown SP. The mode of host–parasite interaction shapes coevolutionary dynamics and the fate of host cooperation. Proc R Soc B Biol Sci. 2012;279(1743):3742–3748. doi: 10.1098/rspb.2012.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waite AJ, Shou W. Adaptation to a new environment allows cooperators to purge cheaters stochastically. Proc Natl Acad Sci USA. 2012;109(47):19079–19086. doi: 10.1073/pnas.1210190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsayer J, Kaltz O, Hochberg ME. Evolutionary rescue in populations of Pseudomonas fluorescens across an antibiotic gradient. Evol Appl. 2013;6(4):608–616. doi: 10.1111/eva.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baharoglu Z, Mazel D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol Rev. 2014;38(6):1126–1145. doi: 10.1111/1574-6976.12077. [DOI] [PubMed] [Google Scholar]
- 30.Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012;67(9):2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 31.Schimel J, Balser TC, Wallenstein M. Microbial stress-response physiology and its implications for ecosystem function. Ecology. 2007;88(6):1386–1394. doi: 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- 32.Koskella B, Lin DM, Buckling A, Thompson JN. The costs of evolving resistance in heterogeneous parasite environments. Proc R Soc B Biol Sci. 2012;279(1735):1896–1903. doi: 10.1098/rspb.2011.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez JL, Rojo F. Metabolic regulation of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):768–789. doi: 10.1111/j.1574-6976.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Melnyk AH, Wong A, Kassen R. The fitness costs of antibiotic resistance mutations. Evol Appl. 2015;8(3):273–283. doi: 10.1111/eva.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16(4):178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 36.West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38(1):53–77. [Google Scholar]
- 37.Xavier JB. Social interaction in synthetic and natural microbial communities. Mol Syst Biol. 2011;7(1):483. doi: 10.1038/msb.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strassmann JE, Queller DC. Evolution of cooperation and control of cheating in a social microbe. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10855–10862. doi: 10.1073/pnas.1102451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celiker H, Gore J. Cellular cooperation: Insights from microbes. Trends Cell Biol. 2013;23(1):9–15. doi: 10.1016/j.tcb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Velicer GJ. Social strife in the microbial world. Trends Microbiol. 2003;11(7):330–337. doi: 10.1016/s0966-842x(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 41.Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 42.Baquero F, Martínez JL, Cantón R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19(3):260–265. doi: 10.1016/j.copbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Gullberg E, et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7(7):e1002158. doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannauer M, et al. The PvdRT-OpmQ efflux pump controls the metal selectivity of the iron uptake pathway mediated by the siderophore pyoverdine in Pseudomonas aeruginosa. Environ Microbiol. 2012;14(7):1696–1708. doi: 10.1111/j.1462-2920.2011.02674.x. [DOI] [PubMed] [Google Scholar]
- 45.West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc Biol Sci. 2003;270(1510):37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaz Jauri P, Bakker MG, Salomon CE, Kinkel LL. Subinhibitory antibiotic concentrations mediate nutrient use and competition among soil streptomyces. PLoS One. 2013;8(12):e81064. doi: 10.1371/journal.pone.0081064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan GA, Berglund B, Khan KM, Lindgren P-E, Fick J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities: A study in Pakistan. PLoS One. 2013;8(6):e62712. doi: 10.1371/journal.pone.0062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice LB. The complex dynamics of antimicrobial activity in the human gastrointestinal tract. Trans Am Clin Climatol Assoc. 2013;124:123–132. [PMC free article] [PubMed] [Google Scholar]
- 49.Sukul P, Spiteller M. Fluoroquinolone antibiotics in the environment. Rev Environ Contam Toxicol. 2007;191:131–162. doi: 10.1007/978-0-387-69163-3_5. [DOI] [PubMed] [Google Scholar]
- 50.Poole K. Pseudomonas aeruginosa: Resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morita Y, Tomida J, Kawamura Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front Microbiol. 2012;3:408. doi: 10.3389/fmicb.2012.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(8):2626–2631. doi: 10.1128/AAC.01165-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regoes RR, et al. Pharmacodynamic functions: A multiparameter approach to the design of antibiotic treatment regimens. Antimicrob Agents Chemother. 2004;48(10):3670–3676. doi: 10.1128/AAC.48.10.3670-3676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR. Functional relationship between bacterial cell density and the efficacy of antibiotics. J Antimicrob Chemother. 2009;63(4):745–757. doi: 10.1093/jac/dkn554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13(6):151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garneau-Tsodikova S, Labby KJ. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Med Chem Comm. 2016;7(1):11–27. doi: 10.1039/C5MD00344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17(16):R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Dumas Z, Kümmerli R. Cost of cooperation rules selection for cheats in bacterial metapopulations. J Evol Biol. 2012;25(3):473–484. doi: 10.1111/j.1420-9101.2011.02437.x. [DOI] [PubMed] [Google Scholar]
- 59.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2(5):489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 60.Mitri S, Xavier JB, Foster KR. Social evolution in multispecies biofilms. Proc Natl Acad Sci USA. 2011;108(Supplement 2):10839–10846. doi: 10.1073/pnas.1100292108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulander W, et al. Antibiotic-mediated selection of quorum-sensing-negative Staphylococcus aureus. MBio. 2013;3(6):e00459-12. doi: 10.1128/mBio.00459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen B, Gore J, Nowak MA. Spatial dilemmas of diffusible public goods. eLife. 2013;2:e01169. doi: 10.7554/eLife.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kümmerli R, Schiessl KT, Waldvogel T, McNeill K, Ackermann M. Habitat structure and the evolution of diffusible siderophores in bacteria. Ecol Lett. 2014;17(12):1536–1544. doi: 10.1111/ele.12371. [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Fenoll C, Cavaliere M, Martinez-Garcia E, Poyatos JF. Exploitation by cheaters facilitates the preservation of essential public goods in microbial communities. bioRxiv. 2016 doi: 10.1101/040964. [DOI] [Google Scholar]
- 65.Buckling A, et al. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol Ecol. 2007;62(2):135–141. doi: 10.1111/j.1574-6941.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 66.Kirienko NV, Ausubel FM, Ruvkun G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112(6):1821–1826. doi: 10.1073/pnas.1424954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Day T, Read AF. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLOS Comput Biol. 2016;12(1):e1004689. doi: 10.1371/journal.pcbi.1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghoul M, Mitri S. The ecology and evolution of microbial competition. Trends Microbiol. 2016;24(10):833–845. doi: 10.1016/j.tim.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Brockhurst MA, Habets MGJL, Libberton B, Buckling A, Gardner A. Ecological drivers of the evolution of public-goods cooperation in bacteria. Ecology. 2010;91(2):334–340. doi: 10.1890/09-0293.1. [DOI] [PubMed] [Google Scholar]
- 70.Korb J, Foster KR. Ecological competition favours cooperation in termite societies. Ecol Lett. 2010;13(6):754–760. doi: 10.1111/j.1461-0248.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 71.Korb J, Heinze J. Ecology of Social Evolution. Springer; Berlin: 2008. [Google Scholar]
- 72.Rubenstein DR. Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10816–10822. doi: 10.1073/pnas.1100303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen S-F, et al. Unfavourable environment limits social conflict in Yuhina brunneiceps. Nat Commun. 2012;3:885. doi: 10.1038/ncomms1894. [DOI] [PubMed] [Google Scholar]
- 74.Ghysels B, et al. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology. 2004;150(Pt 6):1671–1680. doi: 10.1099/mic.0.27035-0. [DOI] [PubMed] [Google Scholar]
- 75.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS–a Bayesian modelling framework: Concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 76.Lunn DJ. WinBUGS Differential Interface—Worked Examples. Department of Epidemiology and Public Health, Imperial College School of Medicine; London: 2004. [Google Scholar]
- 77.Soetaert K, Petzoldt T. Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J Stat Softw. 2010;33(3):1–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.