Significance
Microbial communities contain cells of different shapes, and yet we know little about how these shapes affect community biology. We have developed a computational model to study the effects of microbial shape in communities. Our model predicts that shape will have strong effects on cells’ positioning, and, consequently, their survival and reproduction. Rod-shaped cells are better at colonizing the base of the community and its expanding edges, whereas round cells dominate the upper surface. We show that the same patterns occur in colonies of Escherichia coli, using strains with different shapes. Our work suggests that cell shape is a major determinant of patterning and evolutionary fitness within microbial communities.
Keywords: biofilms, cell morphology, biophysics, self-organization, synthetic biology
Abstract
The clearest phenotypic characteristic of microbial cells is their shape, but we do not understand how cell shape affects the dense communities, known as biofilms, where many microbes live. Here, we use individual-based modeling to systematically vary cell shape and study its impact in simulated communities. We compete cells with different cell morphologies under a range of conditions and ask how shape affects the patterning and evolutionary fitness of cells within a community. Our models predict that cell shape will strongly influence the fate of a cell lineage: we describe a mechanism through which coccal (round) cells rise to the upper surface of a community, leading to a strong spatial structuring that can be critical for fitness. We test our predictions experimentally using strains of Escherichia coli that grow at a similar rate but differ in cell shape due to single amino acid changes in the actin homolog MreB. As predicted by our model, cell types strongly sort by shape, with round cells at the top of the colony and rod cells dominating the basal surface and edges. Our work suggests that cell morphology has a strong impact within microbial communities and may offer new ways to engineer the structure of synthetic communities.
Single-celled microorganisms such as bacteria display significant morphological diversity, ranging from the simple to the complex and exotic (1–3). Phylogenetic studies indicate that particular morphologies have evolved independently multiple times, suggesting that the myriad shapes of modern bacteria may be adaptations to particular environments (4–6). Microbes can also actively change their morphology in response to environmental stimuli, such as changes to nutrient levels or predation (7, 8). However, understanding when and why particular cell shapes offer a competitive edge remains an unresolved question in microbiology.
Previous studies have characterized selective pressures favoring particular shapes (7, 9–11): for example, highly viscous environments may select for the helical cell morphologies observed in spirochete bacteria (12). Thus far, these studies have predominantly focused on selective pressures acting at the level of the individual cell. However, many species live in dense, surface-associated communities known as biofilms, which are fundamental to the biology of microbes and how they affect us—playing major roles in the human microbiome, chronic diseases, antibiotic resistance, biofouling, and waste-water treatment (13–17). As a result, there has been an intensive effort in recent years to understand how the biofilm mode of growth affects microbes and their evolution (18, 19), but we know very little of the importance of cell shape for biofilm biology.
In biofilms, microbial cells are often in close physical contact, making mechanical interactions between neighboring cells particularly significant. Recent studies have suggested that rod-shaped cells can drive collective behaviors in microbial groups because of their tendency to align their orientations with nearby cells and surfaces (20, 21). The resulting orientational order affects how cell groups expand in microfluidic channels and enables motile cells to swarm together in raft-like collectives (22, 23). Aligned cells are also subject to buckling interactions, which fold neighboring cell groups into one another to form fractal-like interdigitations (21, 24), and differences in cell sizes may drive depletion effects that lead to genetic demixing (25). These studies suggest that, by influencing biomechanical interactions between microbes, shape may have far-reaching consequences for the properties and prospects of a cell within a community.
Individual-based modeling has emerged as a powerful way to study biofilms. These models serve as a testing ground to study how phenotypes, including adhesion, antibiotics, and extracellular polymeric substances (EPSs), affect individual strains and biofilms as a whole (26–31). However, the majority of individual-based models do not allow cell shape to be altered (32). We have therefore developed a flexible simulation framework that allows us to incorporate cell shape alongside cell division, physical interactions, and metabolic interactions via nutrient consumption. Our analyses identify a mechanism by which different cell shapes can self-organize into layered structures, thereby providing particular genotypes with preferential access to favorable positions in the biofilm. We test our model predictions with experiments in which mutant Escherichia coli strains of different shapes are cultured together in colonies. Our work shows that differences in cell shape are central to both spatial architecture and fitness within microbial communities.
Results
To explore the consequences of bacterial cell shape within the biofilm environment, we used two approaches: computer simulations with an individual-based hybrid model (IbM) framework and experiments in which differently shaped bacteria are cultured together on agar plates.
Here, we introduce the model and its predictions, before going on to describe the experiments that we subsequently devised and performed to test it.
Individual-Based Modeling.
Our IbM framework considers microbial communities as collections of elongating cells on a hard surface (Fig. 1, times t1 and t2). Cells absorb diffusing nutrients from their surroundings to grow and divide, causing the community to expand in space (Fig. 1, “Dynamics”). All cells are represented as capsules of segment length and radius (Fig. 1, Top); bacillar and coccal cell shapes are modeled using short (S) or long (L) capsules, whose geometries approximate those of Staphylococcus aureus and E. coli, respectively (Fig. 1, “Cells”) (24, 33). Although simple, these morphologies are ubiquitous and frequently found together in bacterial communities (4, 7).
Fig. 1.
To simulate the growth of a mixed-species bacterial colony on a hard surface, we use a mixed-morphotype hybrid model. Bacterial cells are represented using rigid elastic capsules of variable length and fixed radius (Top Left); coccal and bacillar cells are approximated respectively using long (L) and short (S) capsules (“Cells”). Micrographs depict S. aureus (Top) and Escherichia coli (Bottom) bacteria. Biofilm growth is driven by three primary processes (“Dynamics”): cells absorb nutrients from their surroundings (process 1) to grow and divide (process 2), and repulsive interactions between growing cells lead to the expansion of the colony in three dimensions (process 3). By sequentially updating the cell configuration according to these rules, we simulate the development of colony structure from an initial inoculum of cells (time t1) to a mature biofilm (time t2). Micrographs provided by the Public Health Image Library (34) and Rocky Mountain Laboratories (35).
Overall, our model is similar in structure to previous models based on spherical cells (26–28), except that we can now also study communities containing nonspherical cell shapes. Although this extension requires the underlying mathematical formulation to be more detailed, our model is conceptually only incrementally more complex and, therefore, an appropriate minimum model for investigating the influences of cell shape in microbial communities. Further details on the framework are provided in SI Appendix.
Cell Shape Drives Spatial Patterning Within Colonies.
We first used our model framework to simulate the growth of vertical biofilm sections in two and three dimensions. In the 2D simulations, we used very thin domains so that cells were forced to grow as a monolayer; 3D simulations lift this constraint and allow full freedom of movement. Initial conditions and domain boundary treatments are described in Materials and Methods. As a first approximation, we allowed all cells to grow exponentially at a maximal growth rate , representing a scenario where nutrient perfusion in the biofilm is complete and spatially homogeneous. Later in this study, we remove this assumption to model competitive interactions between different cell shapes.
To investigate the impact of cell shape on biofilm growth, we grew colonies using three 1:1 combinations of S and L cell shapes, as shown at the top of Fig. 2 (SL, SS, and LL mixtures). We found that different spatial structures emerged, depending on the cell shapes that were present. SL cell mixtures spontaneously developed a layered structure, with groups of red S cells lying atop blue L cells. By contrast, SS mixtures tended to produce smooth, vertical boundaries between adjacent strain groups, with no layering. LL colonies produced a third type of spatial patterning, developing fractal-like interdigitations between adjacent cell groups, as reported in previous modeling studies (24, 32).
Fig. 2.
Cell morphology affects self-organization in simulations of exponential biofilm growth. (A) Sections of 2D biofilms grown from 1:1 mixtures of red- and blue-labeled strains form different spatial patterns depending on whether the strains have a coccal (short, S) or rod-like (long, L) morphology. (B) Volume-weighted histograms of cell coordinates () show that SL mixtures develop a layered structure in both 2D and 3D colonies, with S cells sitting above L cells. In SS and LL control simulations, this layering is absent. (C) Three-dimensional simulations, in which cell positions and orientations are no longer confined to the plane, produce spatial patterns similar to those shown in 2D. There were 20 simulations per case.
For these three cases, representative end-state snapshots of 2D colonies are shown in Fig. 2A, whereas Movie S1 shows these colonies growing side-by-side. In Fig. 2B, we plot histograms of cell coordinates for the two strains: here, corresponds to the biovolume distribution along the axis, normalized by total colony volume. This distribution quantifies the tendency for SL colonies to develop layered structures; by contrast, the vertical strain distributions seen in SS and LL control colonies are identical, confirming the absence of layering. This effect can be reproduced in 3D simulations, where cell centers and axes are not confined to the plane (Fig. 2 B and C).
In SI Appendix, we discuss further simulations carried out to interrogate the mechanism of the layering effect (SI Appendix, Figs. S1–S7 and Movie S2). We hypothesize that layering occurs because groups of rod-shaped cells form wedge-like structures, which, guided by the basal surface, collectively burrow beneath groups of coccal cells. The necessary group structure is, in turn, produced by steric interactions between neighboring cells and the basal surface, which produce nematic ordering in rod-shaped cells but not in coccal cells.
We also report tests that verify that the layering effect is robust with respect to changes in simulation boundary conditions (SI Appendix, Fig. S9), relative cell size (SI Appendix, Fig. S7A), simulation parameters such as the rate of division noise injection (SI Appendix, Fig. S7B), and changes in initial cell alignment (SI Appendix, Fig. S7B). However, we find that layering is completely dependent on the presence of the basal plane, confirming its importance in the layering mechanism (SI Appendix, Fig. S7B).
Overall, these observations suggest that individual cell shape is capable of driving significant structural changes in the wider biofilm environment—and, in particular, that combinations of differently shaped cells can drive the formation of layered structures, as reported previously in multispecies biofilms (36–38).
Shape-Driven Layering Alters Strain Fitness.
Next, we examined the consequences of layering phenomena on strain competition within the biofilm environment. Because biofilms are typically characterized as highly heterogeneous environments, systematic differences in the positioning of bacterial strains could lead to significant differences in their fitness, offering a competitive advantage to particular cell morphotypes (39–41).
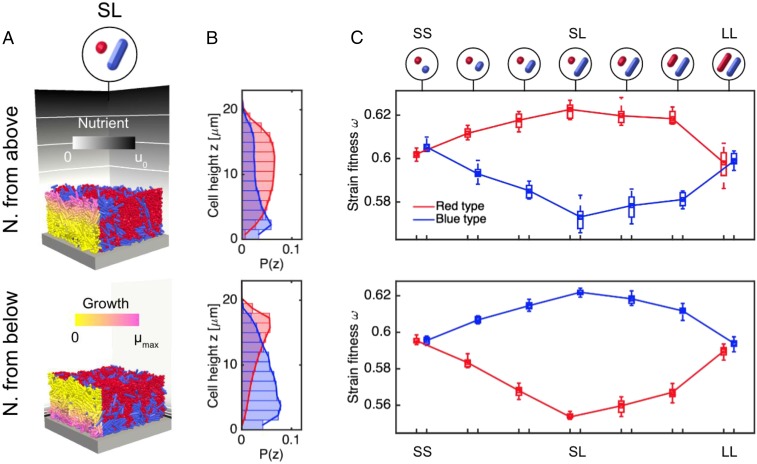
To examine this hypothesis, we repeated our 2D and 3D biofilm growth simulations under nutrient-limited conditions. Instead of allowing all cells to grow at the same rate as before, we coupled each cell’s growth rate to the local concentration of a rate-limiting nutrient field, evaluated at that cell’s centroid . As in previous studies (26, 28, 42, 43), we model this coupling using the Monod equation , where and are the maximum specific cell growth rate and the uptake saturation constant, respectively. By imposing different boundary conditions on the nutrient reaction-diffusion equation, we created opposing perfusion scenarios: one in which nutrient was supplied from above the colony and another in which supply came from below. In each simulation, we measured the fitness of each strain, , by computing the number of division events per unit time, . Here, refers to the total cell volume of a strain at the endpoint of the simulation . The results of these simulations are shown in Fig. 3. Nutrient field parameters are listed in SI Appendix, Table S3; these are chosen so as to create thin growth layers of similar thicknesses in the two perfusion scenarios. In both cases, the Damköhler number (the ratio of nutrient uptake rate to diffusion rate; Materials and Methods) was set to 0.01.
Fig. 3.
Cell morphology influences strain fitness within colonies. (A) Competition between different cell shapes is simulated by growing biofilm sections under nutrient-limited conditions. Nutrients are supplied either from above the colony (first row) or from below (second row). Cells on the left side of the colony are colored by their growth rate, showing that rapid cellular growth is limited to a thin layer at the top or at the base of the colony, in the two scenarios, respectively. Contours on the nutrient field (background) correspond to and . (B) Volume-weighted histograms () of cell heights in nutrient-limited SL colonies show similar layering effects to those created under homogeneous growth conditions, with S cells (red) growing atop L cells (blue). (C) Bar plots of strain fitness show that coccal morphologies outcompete rod-like morphologies in scenarios where nutrients are delivered from above the colony, but the reverse is true when nutrients are delivered from below. There were 10 simulations per case.
Fig. 3A provides example snapshots of both simulation scenarios, showing colonies grown from 1:1 SL cell mixtures after 12 h of growth. Cells in the bulk are colored by type as before, but cells on the left face of the colony are colored by their growth rate, indicating that rapid cellular growth is limited to a thin layer at the top or at the base of the colony, in the two scenarios respectively. In Fig. 3B, we plot histograms of cell heights in these colonies, showing that S and L cell strains still adopt a layered structure when grown together under nutrient-limited conditions. In SI Appendix, Fig. S8, we provide corresponding images and histograms for SS and LL mixtures, prepared in the same way.
In Fig. 3C, we explore the relationship between cell shape and strain fitness in the two nutrient competition scenarios. Here, we grow colonies not only of SS, SL, and LL cell strain mixtures but also using mixtures of intermediate cell aspect ratios. The top row of Fig. 3B provides a pictorial representation of the cell shapes used. Between the SS and SL cases, the birth aspect ratio of the blue strain is increased through the values 1.1, 1.5, 2.0, and 3.0. Correspondingly, the fitness of S cells increases at the expense of L cells, up to a maximum at the SL case. Next, between the SL and LL cases, the aspect ratio of the red strain is increased in the same way, and the fitness differential is reduced to ∼0. When the nutrient conditions are reversed, so too is the strain fitness, this time in favor of L cells.
Importantly, our simulations confirm the hypothesis that strain layering effects can, under idealized conditions, translate into differences in strain fitness. When nutrients are delivered from above the colony, S cells receive preferential nutrient access at the expense of L cells below. S cells are correspondingly afforded an increased growth rate and gain a fitness boost relative to the SS and LL cases where no layering exists. As a result, the number of S cells present in the upper layer of the colony is enhanced, as shown by the cell height histograms in Fig. 3B. In cases where nutrients are delivered from below the colony, the fates of S and L cells in SL mixtures are reversed.
In both cases, we examined the effects of varying the nutrient availability in SI Appendix, Figs. S10 and S11, demonstrating that layering occurs irrespective of nutrient availability, but that this patterning translates into fitness differences only when the nutrient supply becomes limiting. Movie S3 shows an example of a 2D colony of S and L cells, growing with limited nutrient supply from above.
Experimental Tests of Shape-Driven Patterning.
Having shown that cell shape can affect community development and composition with our model, we then sought to test our predictions experimentally. Studying cell shape empirically is challenging, because differently shaped strains will typically be physiologically different as well, introducing confounding effects. However, the E. coli mutants recently identified and described by Monds et al. (44) provide a solution to this impasse. Strains in this library are genetically identical except for a point mutation in the gene coding for the cytoskeletal protein MreB. Critically, these mutations cause substantial and stable changes in cell aspect ratio, without significantly affecting exponential cell growth rates, which allows cells of different shapes to be cultured together in set proportions. [For example, the specific growth rate is for the ancestral (WT) strain and for the mutant REL606mreB A53T (44).]
To test the predictions of our model in vitro, we labeled the strains with fluorescent proteins and grew bacterial colonies using binary combinations of three different E. coli strains: the WT REL606 strain (referred to here as WT) and mutant strains REL606mreB A53S (AS) and REL606mreB A53K (AK). Cells of these three strains exhibit mean aspect ratios of 4.44, 3.55, and 2.50 respectively (44); Fig. 4, Inset shows individual cells of each these strains for visual comparison. Colonies were grown using each possible pairing of these three strains, labeled with red fluorescent protein (RFP) or GFP markers, before being imaged using epifluorescence and confocal laser scanning microscopy. The images shown are in pseudocolor, with the fluorescence signal from GFP-labeled cells shown in blue. Further details are provided in Materials and Methods.
Fig. 4.
Fluorescently labeled E. coli strains with different cell morphologies are grown together in solid culture, as an experimental test of morphotype-patterning relationships. The top row compares colonies composed of WT E. coli with those of actin homolog mutants mreBA53S (AS) and mreBA53K (AK), which differ in cell aspect ratio (Center Inset) but grow at the same rate. At higher magnification, representative confocal images of edge sections of each colony are shown alongside orthogonal projections (bottom row). These images corroborate the predictions of our simulations: WT colonies show complex, fractal-like mixing between red and blue cell groups, whereas AK colonies show smoother group boundaries extending vertically through the colony. AS colonies display intermediate behavior, showing patterning of lower fractal dimension than in WT colonies. Images were taken after 48 h of growth in each case. (Scale bars: top row, 1 mm; bottom row, 20 m.) Phase-contrast cell images (of width 3.5 m) were taken with permission from ref. 44.
Cell Morphology Drives Patterning in E. coli Colonies.
The E. coli colonies reproduced several key predictions of our model. Firstly, colonies composed of only one morphotype (AK-AK, AS-AS, WT-WT) showed that degree of interstrain (red-blue) mixing is strongly dependent on cell aspect ratio. Fig. 4 shows epifluorescent images of complete colonies (top row) and confocal images of colony edge sections (bottom row). Similarly to Fig. 2 (SS column), AK-AK colonies show smooth interstrain boundaries, whereas WT-WT mixtures reproduce the fractal-like interdigitations seen in the model (Fig. 2, LL column), again recapitulating the findings of previous studies (24). The intermediate AS cell shape (Fig. 4, center column) produces an intermediate level of mixing, generating fractal-like patterns as in the WT case, but with a lower fractal dimension. In SI Appendix, Fig. S12, we show additional colony images for other shape combinations, demonstrating that cell shape can affect the composition of the colony edge.
Our experiments also verify that mixing long and short cell strains together can result in the emergence of layered structures, with shorter cells lying on top of longer cells. In Fig. 5A, we show that WT cells (shown in blue) displace shorter AK cells (red) from the base of the colony. This effect is visible both from side projections of confocal z-stacks (Fig. 5A, top row) and from horizontal slices taken at increasing depths in the colony. Because nutrients are supplied from the agar below the colony, we also see an overgrowth of rod cells at the colony base, as predicted by the second modeling scenario shown in Fig. 3. Reversing the color labels produces the same result (Fig. 5B), demonstrating that layering is not an artifact produced by the fluorescent labeling scheme.
Fig. 5.
Mixed-morphotype colonies reproduce the layering phenomena predicted by simulations. (A) Pseudocolor confocal images show that GFP-labeled WT E. coli (shown in blue) are able to burrow beneath shorter, wider AK mutants (red), leading to the WT cells’ enrichment at the base of the colony. (B) The same is true when fluorescent labels are reversed. Images correspond to vertical colony sections (A and B, top row) and horizontal slices taken at successive depths (A and B, bottom row). (C) We quantify layering effects by measuring the volume fraction of GFP-labeled cells as a function of depth in the colony, using automated image segmentation (Materials and Methods). (D) The gradients of these traces, corresponding to the percentage gain (or loss) of GFP signal per unit depth, are compared for various binary shape combinations. Cell micrographs in A, B, and D are taken with permission from ref. 44. (Scale bars: 20 m.) Confocal images and data taken after 48 h of growth.
In Fig. 5C, we use automated image analysis to quantify the layering effect. By counting the number of blue pixels in each layer of the stack (Materials and Methods), we estimate the volume fraction of AK (top row) or WT (bottom row) cells as a function of depth in the colony. These traces show an approximately linear reduction in the AK cell fraction (and corresponding increase in WT fraction) within the first 15 m below the colony surface, which is in agreement with the cell arrangements predicted in Figs. 2 and 3.
Finally, we repeated this analysis with images taken from colonies of other morphotype combinations, including the control colonies shown in Fig. 4. In each case, we quantified interstrain layering using the average slope of the GFP fraction traces, such as those shown in Fig. 5C, using linear fitting. Example image segmentations, along with the complete set of GFP fraction traces, are provided in SI Appendix, Fig. S13. The degree of layering for each type of mixture is shown in Fig. 5D. As predicted by the model, we observe a strong relationship between differences in cell shape and the degree of layering in the colony across these different genotypes. Our experiments, therefore, support multiple predictions of our model (Figs. 4 and 5). Moreover, this support came without needing to tune conditions; we performed the experimental tests after the modeling. This observation suggests that the effects we report in this study are robust to experimental conditions and may be common to many existing experimental systems that use mixtures of different cell shapes.
Discussion
We have used simulations and experiments to show that cell shape can be a determinant of both spatial patterning and composition within a microbial community. In particular, we find that mixtures of rod-shaped and coccal cells can produce layered colony structures, as observed previously in both biotic and abiotic environments (36, 45). This result extends our understanding of the functional value of cell shape, both as a competitive phenotype inthe biofilm context and as a means for bacteria to influence their environment through collective action.
From an evolutionary perspective, our work suggests that the biofilm environment may select for particular cell shapes in specific environments because of the ways in which they collectively influence biofilm architecture. Given the predominance of the biofilm environment for microbial life (18, 19), any selective effect produced might be expected to have a strong impact on the evolution of bacterial morphology. In natural biofilms, rapid cell growth is often limited to the upper regions of a biofilm (28, 41), which will select for coccal morphologies. Indeed, this selective pressure provides a rationale for morphological transitions associated with biofilm development (46–48) and may partly explain the ubiquity of the coccal morphology despite disadvantages, such as decreased nutrient absorption area (7).
In reality, microbial communities are far more intricate than the description used in our simulations. Like all models, our framework makes simplifying assumptions: we deliberately neglect the role of cell motility, detachment, and shear forces from the liquid surrounding the colony (42, 43, 49). Our representation of EPS secretions—a hallmark of microbial biofilms—is purely implicit, whereas the inclusion of explicit EPS particles has been shown to influence colony structure in previous studies (25, 41). Our model also neglects adhesive interactions, which could inhibit the layering effect if coccal cells were irreversibly attached to the basal surface.
However, these omissions allow a degree of realism to be traded for additional control and tractability. Rather than attempting to reproduce the exact dynamics of colony growth, our simulations instead predict the rich dynamics that can emerge when nonspherical cell shapes are introduced to existing modeling paradigms (28, 50). The fact that these predictions are corroborated by our experiments demonstrates the usefulness of this approach.
Our predictions highlight the need for further empirical and theoretical studies to examine mixed-shape colonies in more detail, treating cell shape as a physical variable instead of an incidental attribute. Bacterial strains such as those developed by Monds et al. (44) will be instrumental to this process, because they allow cellular shape to be varied in isolation of other confounding variables. Likewise, investigating how the predictions of previous theoretical studies are altered by the inclusion of morphological variability will help fully characterize the influences of cell shape in microbial communities. More generally, our work may also suggest new roles for shape and growth anisotropy in other biological systems—ultimately, shape may prove to be an important physical parameter not just for collectives of microbes but also for the morphogenesis of developing tissues and cancer tumors (51, 52).
Finally, although our study considers only antagonistic interactions between microbes, the patterning mechanisms we discuss could also influence more cooperative relationships. Metabolic exchanges between species are often associated with a layered structure in biofilms (36–38, 53–55), which suggests that shape-mediated self-organization could be used by experimenters to promote desirable colony structures. Furthermore, our results show that cell shape can affect the degree of strain mixing within the colony, which could in turn be used to control selective pressures for or against certain social strategies. For instance, because mixtures of coccal cells produce less genetic mixing than mixtures of rod-shaped cells, cooperative strategies such as enzyme secretion may become more evolutionarily stable (28). Our findings could therefore help to engineer more productive or stable synthetic microbial consortia, by selecting for community architectures that affect the interactions between strains and species (14).
Conclusions
Microorganisms of different shapes commonly grow together in their dense and genetically diverse communities, known as biofilms. We have used modeling and experiments to study the impact of cell morphology on biofilm architecture and competition. By building a simulation framework incorporating different cell shapes, we have predicted, described, and examined mechanisms of self-organization and spatial patterning driven by microbial morphology. We have also documented the same patterns emerging in real bacterial colonies. Our work suggests that cell shape is a major neglected determinant of patterning and evolutionary fitness within microbial communities.
Materials and Methods
IbM Simulations.
Simulations were performed using 2D and 3D domains. In both cases, domains were surrounded by hard walls to approximate repulsive mechanical forces from the basal surface and surrounding sections of the biofilm. In SI Appendix, Fig. S9, we show that the hard boundary approximation reproduces the effects of periodic boundary conditions, which are more realistic but computationally more demanding. In 2D simulations, the centroids and axes of 3D capsules were confined to a plane of base length m, whereas 3D simulations used a cuboidal domain with base dimensions m. Simulations were initialized using an inoculum of cells arranged randomly on the base of the domain, with cell axis vectors drawn randomly in the 3D case. Each inoculum consisted of a 1:1 mixture of two bacterial strains, marked with red and blue color labels for lineage tracking.
In total, 3,000 model simulations were performed on two NVIDIA Tesla C2075 6GB GDDR5 PCIe workstation graphics cards for simulations without nutrient fields and on two NVIDIA Quadro K5000 4GB GFX graphics card for simulations including them. To account for the stochastic noise terms in our model (SI Appendix), we increased simulation sample sizes until our measurements converged, using the convergence criterion
| [1] |
where denotes a mean vector of measurements for a sample size , and is a convergence tolerance set to ; represents the Euclidean norm operation.
Simulations were visualized and checked using Paraview 4.3.1, and the postprocessing of results was carried out using Matlab 2015a. Further details on the IbM framework, including model equations and assumptions, are provided in SI Appendix.
Bacterial Strains and Plasmids.
E. coli REL606 is the parent (WT) strain (56), referred to here as A53. A53K (REL606mreBA53K) and A53S (REL606mreBA53S) were previously constructed from REL606 with the respective nonsynonymous mutation at the 53rd amino acid residue of the MreB protein (44). A53K exhibits the shortest and widest cellular morphology, and A53S is intermediate, compared with A53. Importantly, all three strains share the same specific growth rate and differ only marginally in lag phase time (44).
We introduced plasmids pmaxRFP or pmaxGFP (Amaxa/Lonza) into all three strains for epifluorescence and confocal microscopy. For routine culturing in liquid, cells were grown shaking at 250 rpm in LB (Thermo-Fisher Scientific) at 37 °C. For plate cultures, cells were grown on Davis minimal (DM) medium (57), supplemented with 15 g L− 1 agar and either glucose (175 g mL− 1) or lactose (210 g mL− 1) as previously described (44) at 37 °C. All media were supplemented with kanamycin at 50 g mL− 1 to maintain plasmids.
Overnight cultures were serially diluted in PBS, mixed together in a 1:1 ratio, and then spotted in either 1- or 10-L volumes on the surface of DM plates. We observed no significant difference between glucose or lactose supplementation; data reported in this study are from glucose-supplemented plates spotted at 1-L volume. For each combination of cell strains and fluorescent labels, control experiments using reverse labeling were also carried out. Two days following inoculation, mixed-culture colonies were imaged by epifluorescence and confocal laser scanning microscopy as previously described (41, 57).
Confocal Image Analysis.
Following collection, confocal image stacks were enhanced in FIJI by setting pixel saturation to 1% and by normalizing signal to the full intensity range. Images were then segmented using the Matlab bioformats plugin, according to the following procedure. For each stack, incomplete or excessively dark images were excluded by removing any layer in which more than 1% of pixels had intensities less than 12% of the maximum, measured in composite grayscale images created by combining red and green channel data. These thresholds were chosen manually to optimize the segmentation accuracy. To ensure a sufficient number data points for curve fitting later on, confocal stacks with fewer than six images remaining were removed from the analysis outright. In each of the remaining images, the number of GFP-labeled cells was estimated by counting the number of pixels in the green channel data with intensities above a threshold, computed for each individual layer using Otsu’s method (58).
Supplementary Material
Acknowledgments
We thank Carolina Tropini for suggesting the use of mutant E. coli cells and Kerwyn Huang for providing the strains. We also thank NVIDIA donating the two Tesla C2075 graphics cards and Michel Quintard for providing access to the computing resources at Institut de Mécanique des Fluides de Toulouse. We thank Tim Rudge and Niall Murphy for their help with CellModeller and Mack Durham, Jonas Schluter, Dianne Newman, and two anonymous reviewers for helpful comments on the manuscript. W.P.J.S. is supported by an Engineering and Physical Sciences Research Council studentship (Grant EP/G03706X/1), and K.R.F. is supported by European Research Council Grant 242670. Both W.P.J.S. and K.R.F. received funding from the Calleva Research Centre for Evolution and Human Science (Magdalen College, Oxford).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613007114/-/DCSupplemental.
References
- 1.Starr MP, Skerman VBD. Bacterial diversity: The natural history of selected morphologically unusual bacteria. Annu Rev Microbiol. 1965;19:407–454. doi: 10.1146/annurev.mi.19.100165.002203. [DOI] [PubMed] [Google Scholar]
- 2.Vos P, et al. Bergey’s Manual of Systematic Bacteriology: The Firmicutes. Vol 3 Springer Science & Business Media; The Netherlands: 2011. [Google Scholar]
- 3.Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3(8):601–610. doi: 10.1038/nrmicro1205. [DOI] [PubMed] [Google Scholar]
- 4.Siefert JL, Fox GE. Phylogenetic mapping of bacterial morphology. Microbiology. 1998;144(Pt 10):2803–2808. doi: 10.1099/00221287-144-10-2803. [DOI] [PubMed] [Google Scholar]
- 5.Tamames J, Gonzalez-Moreno M, Mingorance J, Valencia A, Vicente M. Bringing gene order into bacterial shape. Trends Genet. 2001;17(3):124–126. doi: 10.1016/s0168-9525(00)02212-5. [DOI] [PubMed] [Google Scholar]
- 6.Kysela DT, Randich AM, Caccamo PD, Brun YV. Diversity takes shape: Understanding the mechanistic and adaptive basis of bacterial morphology. PLoS Biol. 2016;14(10):e1002565. doi: 10.1371/journal.pbio.1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young KD. The selective value of bacterial shape. Microbiol Mol Biol Rev. 2006;70(3):660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice SS, Hunstad Da, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6(2):162–168. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 9.Young KD. Bacterial shape. Mol Microbiol. 2004;49(3):571–580. doi: 10.1046/j.1365-2958.2003.03607.x. [DOI] [PubMed] [Google Scholar]
- 10.Young KD. Bacterial morphology: Why have different shapes? Curr Opin Microbiol. 2007;10(6):596–600. doi: 10.1016/j.mib.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young KD. Bacterial shape: Two-dimensional questions and possibilities. Annu Rev Microbiol. 2010;64:223–240. doi: 10.1146/annurev.micro.112408.134102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura S, Adachi Y, Goto T, Magariyama Y. Improvement in motion efficiency of the spirochete Brachyspira pilosicoli in viscous environments. Biophys J. 2006;90(8):3019–3026. doi: 10.1529/biophysj.105.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AL, Foster KR. Host selection of microbiota via differential adhesion. Cell Host Microbe. 2016;19(4):550–559. doi: 10.1016/j.chom.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: Networks, competition, and stability. Science. 2015;350(6261):663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 15.Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA. 2008;299(22):2682–2684. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- 16.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 17.Klapper I, Dockery J. Mathematical description of microbial biofilms. SIAM Rev. 2010;52(2):221–265. [Google Scholar]
- 18.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 19.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 20.Volfson D, Cookson S, Hasty J, Tsimring LS. Biomechanical ordering of dense cell populations. Proc Natl Acad Sci USA. 2008;105(40):15346–15351. doi: 10.1073/pnas.0706805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer D, et al. Buckling instability in ordered bacterial colonies. Phys Biol. 2011;8(2):026008. doi: 10.1088/1478-3975/8/2/026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H, et al. Self-organization in high-density bacterial colonies: Efficient crowd control. PLoS Biol. 2007;5(11):e302. doi: 10.1371/journal.pbio.0050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copeland MF, Weibel DB. Bacterial swarming: A model system for studying dynamic self-assembly. Soft Matter. 2009;5(6):1174–1187. doi: 10.1039/B812146J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudge TJ, Federici F, Steiner PJ, Kan A, Haseloff J. Cell polarity-driven instability generates self-organized, fractal patterning of cell layers. ACS Synth Biol. 2013;2(12):705–714. doi: 10.1021/sb400030p. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh P, Mondal J, Ben-Jacob E, Levine H. Mechanically-driven phase separation in a growing bacterial colony. Proc Natl Acad Sci USA. 2015;112(17):E2166–E2173. doi: 10.1073/pnas.1504948112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picioreanu C, van Loosdrecht MCM, Heijnen JJ. Mathematical modeling of biofilm structure with a hybrid differential-discrete cellular automaton approach. Biotechnol Bioeng. 1998;58(1):101–116. doi: 10.1002/(sici)1097-0290(19980405)58:1<101::aid-bit11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Kreft J-U. Biofilms promote altruism. Microbiology. 2004;150(Pt 8):2751–2760. doi: 10.1099/mic.0.26829-0. [DOI] [PubMed] [Google Scholar]
- 28.Nadell CD, Foster KR, Xavier JB. Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol. 2010;6(3):e1000716. doi: 10.1371/journal.pcbi.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schluter J, Foster KR. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 2012;10(11):e1001424. doi: 10.1371/journal.pbio.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadell CD, et al. Cutting through the complexity of cell collectives. Proc Biol Sci. 2013;280(1755):20122770. doi: 10.1098/rspb.2012.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estrela S, Brown SP. Metabolic and demographic feedbacks shape the emergent spatial structure and function of microbial communities. PLoS Comput Biol. 2013;9(12):e1003398. doi: 10.1371/journal.pcbi.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storck T, Picioreanu C, Virdis B, Batstone DJ. Variable cell morphology approach for individual-based modeling of microbial communities. Biophys J. 2014;106(9):2037–2048. doi: 10.1016/j.bpj.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayama M, Shigemune N, Tsugukuni T, Tokuda H, Miyamoto T. Difference of EGCg adhesion on cell surface between Staphylococcus aureus and Escherichia coli visualized by electron microscopy after novel indirect staining with cerium chloride. J Microbiol Methods. 2011;86(1):97–103. doi: 10.1016/j.mimet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Matthew J, Arduino JC. 2007. Staphylococcus aureus SEM (image 6486). Accessed July 7, 2016.
- 35.Rocky Mountain Laboratories, NIAID,NIH 2006. Escherichia coli SEM. Accessed July 7, 2016.
- 36.Christensen BB, Haagensen JAJ, Heydorn AJ, Molin S. Metabolic commensalism and competition in a two-species microbial consortium. Appl Environ Microbiol. 2002;68(5):2495–2502. doi: 10.1128/AEM.68.5.2495-2502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen SK, Rainey PB, Haagensen JAJ, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445(7127):533–536. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- 38.Elias S, Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol Rev. 2012;36(5):990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 39.Wimpenny J, Manz W, Szewzyk U. Heterogeneity in biofilms. FEMS Microbiol Rev. 2000;24(5):661–671. doi: 10.1111/j.1574-6976.2000.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 40.Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009;33(1):206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Racimo F, Schluter J, Levy SB, Foster KR. Importance of positioning for microbial evolution. Proc Natl Acad Sci USA. 2014;111(16):E1639–E16347. doi: 10.1073/pnas.1323632111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picioreanu C, Van Loosdrecht MCM, Heijnen JJ. Effect of diffusive and convective substrate transport on biofilm structure formation: a two-dimensional modeling study. Biotechnol Bioeng. 2000;69(5):504–515. doi: 10.1002/1097-0290(20000905)69:5<504::aid-bit5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Kreft JU, Wimpenny JWT. Effect of EPS on biofilm structure and function as revealed by an individual-based model of biofilm growth. Water Sci Technol. 2001;43(6):135–141. [PubMed] [Google Scholar]
- 44.Monds RD, et al. Systematic perturbation of cytoskeletal function reveals a linear scaling relationship between cell geometry and fitness. Cell Rep. 2014;9(4):1528–1537. doi: 10.1016/j.celrep.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5(4):627–638. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serra DO, Richter AM, Klauck G, Mika F, Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio. 2013;4(2):e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergkessel M, Basta DW, Newman DK. The physiology of growth arrest: Uniting molecular and environmental microbiology. Nat Rev Microbiol. 2016;14(9):549–562. doi: 10.1038/nrmicro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendrickx L, Hausner M, Wuertz S. Natural genetic transformation in monoculture Acinetobacter sp. strain BD413 biofilms. Appl Environ Microbiol. 2003;69(3):1721–1727. doi: 10.1128/AEM.69.3.1721-1727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Picioreanu C, et al. Microbial motility involvement in biofilm structure formation - a 3D modelling study. Water Sci Technol. 2007;55(8-9):337–343. doi: 10.2166/wst.2007.275. [DOI] [PubMed] [Google Scholar]
- 50.Xavier JB, Foster KR. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci USA. 2007;104(3):876–881. doi: 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153(5):948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Basan M, Elgeti J, Hannezo E, Rappel W-J, Levine H. Alignment of cellular motility forces with tissue flow as a mechanism for efficient wound healing. Proc Natl Acad Sci USA. 2013;110(7):2452–2459. doi: 10.1073/pnas.1219937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol. 1999;65(3):1280–1288. doi: 10.1128/aem.65.3.1280-1288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilmes P, et al. Natural acidophilic biofilm communities reflect distinct organismal and functional organization. ISME J. 2009;3(2):266–270. doi: 10.1038/ismej.2008.90. [DOI] [PubMed] [Google Scholar]
- 55.Brenner K, Arnold FH. Self-organization, layered structure, and aggregation enhance persistence of a synthetic biofilm consortium. PLoS One. 2011;6(2):e16791. doi: 10.1371/journal.pone.0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenski RE, Rose MR, Simpson SC, Tadler SC. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat. 2016;138(6):1315–1341. [Google Scholar]
- 57.Kim W, Levy SB, Foster KR. Rapid radiation in bacteria leads to a division of labour. Nat Commun. 2016;7:10508. doi: 10.1038/ncomms10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otsu N. A threshold selection method from gray-level histograms. Automatica. 1975;11(285-296):23–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.