Abstract
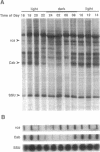
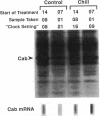
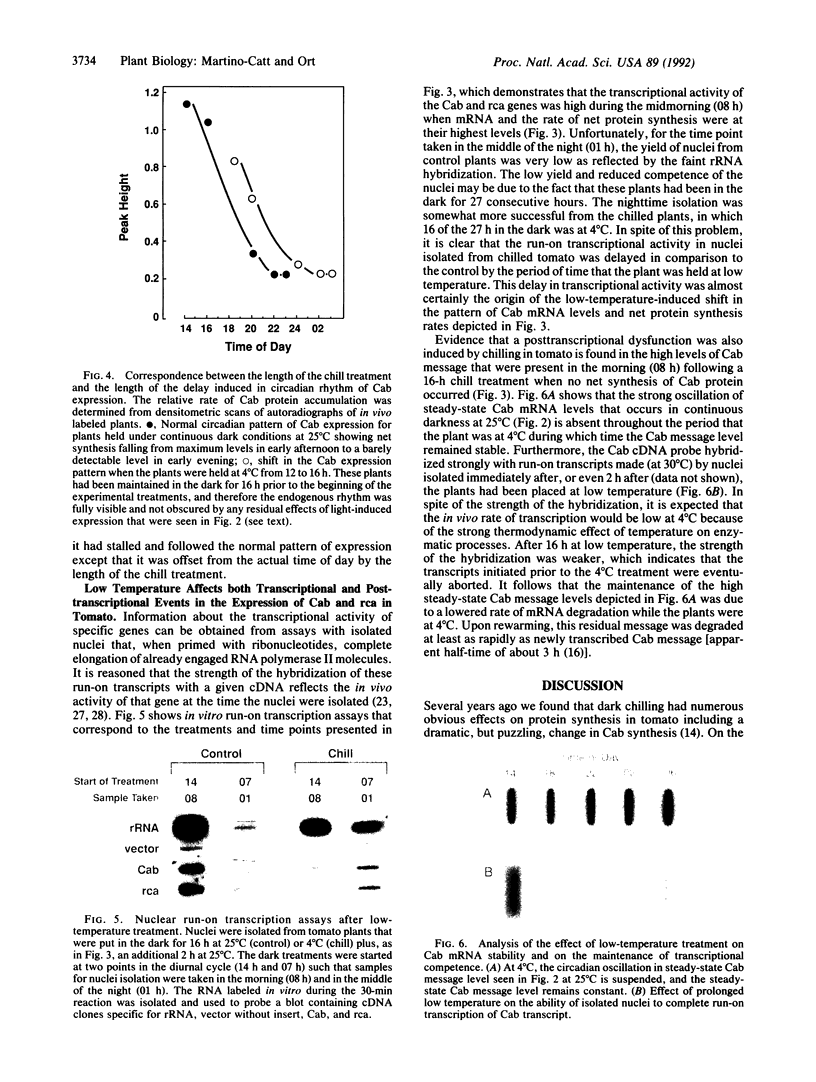
Impaired chloroplast function is responsible for nearly two-thirds of the inhibition to net photosynthesis caused by dark chilling in tomato (Lycopersicon esculentum Mill.), yet it has not been possible to localize the dysfunction to specific chloroplast reactions. We report here on an effect that low-temperature exposure has in tomato on the expression of certain nuclear-encoded chloroplast proteins, which may be directly related to the chilling sensitivity of photosynthesis. Transcriptional activity of genes for both the chlorophyll a/b binding protein of photosystem II (Cab) as well as for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase was found to be controlled by an endogenous rhythm. For Cab this rhythm was also visible at the level of newly synthesized protein, indicating that the circadian control of transcriptional activity normally ensures that this protein is synthesized only during daylight hours. However, low-temperature treatment suspended the timing of the rhythm in tomato so that, upon rewarming, the circadian control was reestablished but was displaced from the actual time of day by the length of the chilling exposure. In addition, we found that the normal turnover of Cab and Rubisco activase mRNA was suspended during the low-temperature treatment, but, upon rewarming, this stabilized message was not translated into protein. We believe that the low-temperature-induced mistiming of gene expression together with its effect on the translatability of existing transcripts may be an important clue in unraveling the basis for the chilling sensitivity of photosynthesis in tomato.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Opersteny D., Heusinkveld K. B. Prophylactic antiemetics for chemotherapy-associated nausea and vomiting. Cancer Nurs. 1983 Apr;6(2):117–123. [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busheva M., Garab G., Liker E., Tóth Z., Szèll M., Nagy F. Diurnal Fluctuations in the Content and Functional Properties of the Light Harvesting Chlorophyll a/b Complex in Thylakoid Membranes : Correlation with the Diurnal Rhythm of the mRNA Level. Plant Physiol. 1991 Apr;95(4):997–1003. doi: 10.1104/pp.95.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ort D. R. Changes in protein synthesis induced in tomato by chilling. Plant Physiol. 1988 Oct;88(2):454–461. doi: 10.1104/pp.88.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Hoffman N. E., Ko K., Scolnik P. A., Cashmore A. R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988 Dec 1;7(12):3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbrough P. B., Cullis C. A. Characterisation of the genes for ribosomal RNA in flax. Nucleic Acids Res. 1981 Mar 25;9(6):1301–1309. doi: 10.1093/nar/9.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Ma Y., Buchbinder B. U., Pyne J. W., Sun S. M., Bliss F. A. Messenger RNA for G1 protein of French bean seeds: Cell-free translation and product characterization. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Ort D. R., Boyer J. S. Impairment of photosynthesis by chilling-temperatures in tomato. Plant Physiol. 1981 Aug;68(2):329–334. doi: 10.1104/pp.68.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Paulsen H., Bogorad L. Diurnal and Circadian Rhythms in the Accumulation and Synthesis of mRNA for the Light-Harvesting Chlorophyll a/b-Binding Protein in Tobacco. Plant Physiol. 1988 Dec;88(4):1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler T. C., Naylor A. W. A Comparison of the Effects of Chilling on Thylakoid Electron Transfer in Pea (Pisum sativum L.) and Cucumber (Cucumis sativus L.). Plant Physiol. 1988 Jan;86(1):147–151. doi: 10.1104/pp.86.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesselmann S., Piechulla B. Effect of dark phases and temperature on the chlorophyll a/b binding protein mRNA level oscillations in tomato seedlings. Plant Mol Biol. 1990 Apr;14(4):605–616. doi: 10.1007/BF00027506. [DOI] [PubMed] [Google Scholar]
- Sassenrath G. F., Ort D. R., Portis A. R., Jr Impaired reductive activation of stromal bisphosphatases in tomato leaves following low-temperature exposure at high light. Arch Biochem Biophys. 1990 Nov 1;282(2):302–308. doi: 10.1016/0003-9861(90)90121-e. [DOI] [PubMed] [Google Scholar]
- Taylor W. C. Transcriptional regulation by a circadian rhythm. Plant Cell. 1989 Feb;1(2):259–264. doi: 10.1105/tpc.1.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Zielinski R. E., Ogren W. L. Structure and expression of spinach leaf cDNA encoding ribulosebisphosphate carboxylase/oxygenase activase. Proc Natl Acad Sci U S A. 1988 Feb;85(3):787–791. doi: 10.1073/pnas.85.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski R. E., Werneke J. M., Jenkins M. E. Coordinate Expression of Rubisco Activase and Rubisco during Barley Leaf Cell Development. Plant Physiol. 1989 Jun;90(2):516–521. doi: 10.1104/pp.90.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]