Abstract
The Ca(2+)- and calmodulin-dependent protein phosphatase calcineurin is inhibited by the immunosuppressant drug cyclosporin A in the presence of cyclophilin A or B. Of the two isoforms, cyclophilin B is more potent by a factor of 2-5 when either the phosphoprotein [32P]casein or the [32P]phosphoserine [Ser(32P)] form of the 19-residue bovine cardiac cAMP-dependent protein kinase regulatory subunit peptide RII, [Ser(32P)15]RII, is used as substrate. With [Ser(32P15]RII as substrate, the concentrations of the cyclosporin A.cyclophilin A and cyclosporin A.cyclophilin B complexes, which cause 50% inhibition of calcineurin activity, are 120 and 50 nM, respectively. Lowering the concentration of calcineurin 80% with [32P]casein as substrate lowered the apparent inhibition constant for each complex even further; 50% inhibition of calcineurin was observed at 40 nM for cyclosporin A.cyclophilin A, whereas it was less than 10 nM for cyclosporin A.cyclophilin B. In all inhibition assays with [32P]casein or [Ser(32P)15]RII, the concentration of calcineurin required for measurable phosphatase activity is such that these complexes behave as tight-binding inhibitors of calcineurin, and steady-state kinetics cannot be used to assess inhibition patterns or Ki values. Limited trypsinization of calcineurin produces a fragment that is still inhibited, indicating that the interaction of cyclosporin.cyclophilin with calcineurin does not require either calmodulin or Ca2+.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Hexham J. M., Crumpton M. J. The association of type 1, type 2A and type 2B phosphatases with the human T lymphocyte plasma membrane. Biochem J. 1988 Dec 15;256(3):885–892. doi: 10.1042/bj2560885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Eder C., Gross M., Kersten H., Sylvester D., Appelbaum E., Cusimano D., Livi G. P., McLaughlin M. M., Kasyan K. The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms. J Biol Chem. 1991 Dec 5;266(34):23204–23214. [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D. K., Takio K., Hansen R. S., Krebs E. G. Dephosphorylation of cAMP-dependent protein kinase regulatory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J Biol Chem. 1986 Jun 25;261(18):8140–8145. [PubMed] [Google Scholar]
- Chernoff J., Sells M. A., Li H. C. Characterization of phosphotyrosyl-protein phosphatase activity associated with calcineurin. Biochem Biophys Res Commun. 1984 May 31;121(1):141–148. doi: 10.1016/0006-291x(84)90698-3. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Colombani P. M., Robb A., Hess A. D. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science. 1985 Apr 19;228(4697):337–339. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Kaetzel M. A. Calmodulin purification and fluorescent labeling. Methods Enzymol. 1983;102:1–8. doi: 10.1016/s0076-6879(83)02003-0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Strada S. J., Henderson P. J., Cohen P. A kinetic analysis of the effects of inhibitor-1 and inhibitor-2 on the activity of protein phosphatase-1. Eur J Biochem. 1983 May 2;132(2):309–313. doi: 10.1111/j.1432-1033.1983.tb07363.x. [DOI] [PubMed] [Google Scholar]
- Friedman J., Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991 Aug 23;66(4):799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait W. N., Harding M. W., Handschumacher R. E. Calmodulin, cyclophilin, and cyclosporin A. Science. 1986 Aug 29;233(4767):987–989. doi: 10.1126/science.3016900. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Hashimoto Y., King M. M., Soderling T. R. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7001–7005. doi: 10.1073/pnas.85.18.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansky S. J., Casey P. J., Stohs S. J. Cyclosporin A--a chemoprotectant against microcystin-LR toxicity. Toxicol Lett. 1990 Dec;54(2-3):279–285. doi: 10.1016/0378-4274(90)90195-r. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J., Spitzfaden C., Zurini M. G., Wider G., Widmer H., Wüthrich K., Walkinshaw M. D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991 Sep 19;353(6341):276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zydowsky L. D., Liu J., Walsh C. T. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
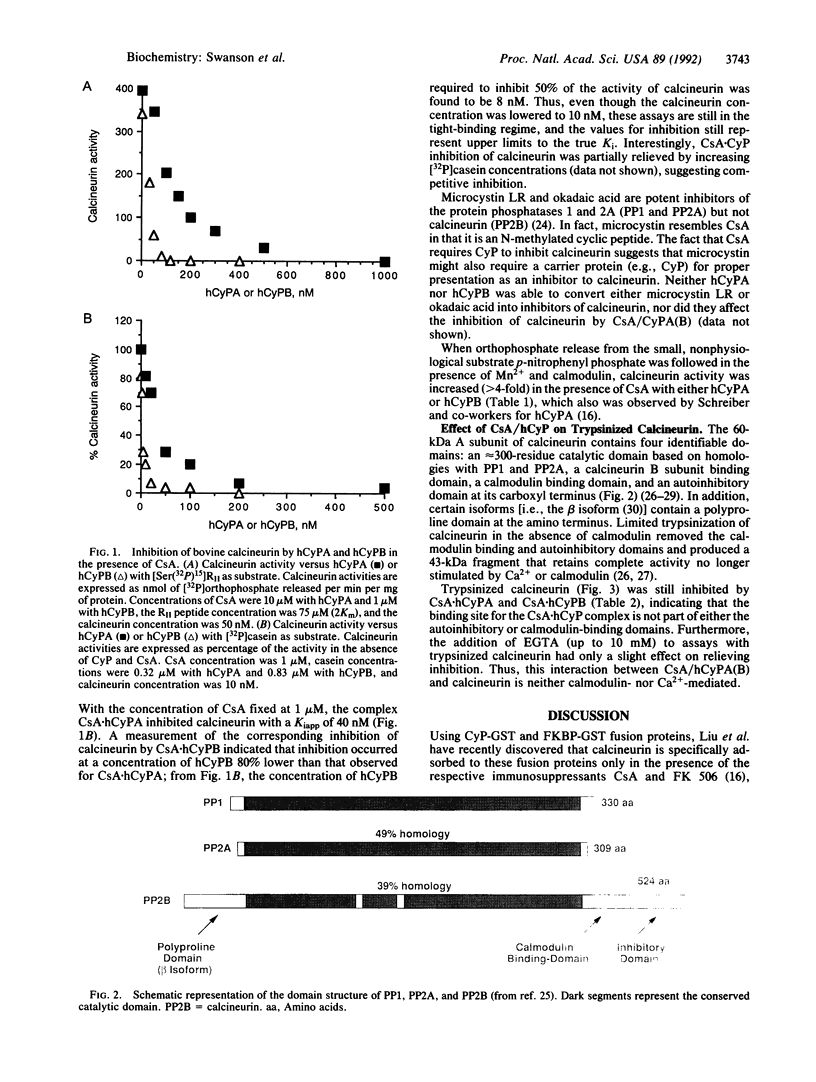
- Kincaid R. L., Nightingale M. S., Martin B. M. Characterization of a cDNA clone encoding the calmodulin-binding domain of mouse brain calcineurin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8983–8987. doi: 10.1073/pnas.85.23.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy T., Szafraniec L., Hunt D. F., Shabanowitz J., Yates J. R., 3rd, Hauer C. R., Carmichael W. W., Skulberg O., Codd G. A., Missler S. Structural characterization of toxic cyclic peptides from blue-green algae by tandem mass spectrometry. Proc Natl Acad Sci U S A. 1989 Feb;86(3):770–774. doi: 10.1073/pnas.86.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T., Takeda T., Hirai M., Ito A., Mukai H., Tanaka C. Evidence for a second isoform of the catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A). Biochem Biophys Res Commun. 1989 Dec 29;165(3):1352–1358. doi: 10.1016/0006-291x(89)92752-6. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Siekierka J. J., Sigal N. H. FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991 Apr 1;133(2):269–284. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Chen C. M., Schreiber S. L., Walsh C. T. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Activation of calcineurin by limited proteolysis. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Pallen C. J., Wang J. H., Graves D. J. Use of fluorinated tyrosine phosphates to probe the substrate specificity of the low molecular weight phosphatase activity of calcineurin. J Biol Chem. 1985 Dec 5;260(28):14932–14937. [PubMed] [Google Scholar]
- Metcalfe S. M., Richards F. M. Cyclosporine, FK506, and rapamycin. Some effects on early activation events in serum-free, mitogen-stimulated mouse spleen cells. Transplantation. 1990 Apr;49(4):798–802. [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Politino M., King M. M. Calcium- and calmodulin-sensitive interactions of calcineurin with phospholipids. J Biol Chem. 1987 Jul 25;262(21):10109–10113. [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F., Durette P., Siekierka J. J., Peterson L., Rich D. H., Dunlap B. E., Staruch M. J., Melino M. R., Koprak S. L. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J Exp Med. 1991 Mar 1;173(3):619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Activation of bovine brain calmodulin-dependent protein phosphatase by limited trypsinization. Biochemistry. 1984 Feb 28;23(5):973–979. doi: 10.1021/bi00300a027. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Calmodulin-dependent protein phosphatase: a developmental study. Biochemistry. 1983 Jul 19;22(15):3630–3635. doi: 10.1021/bi00284a014. [DOI] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Characterization of bovine brain calmodulin-dependent protein phosphatase. Arch Biochem Biophys. 1984 Jul;232(1):269–279. doi: 10.1016/0003-9861(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]