Abstract
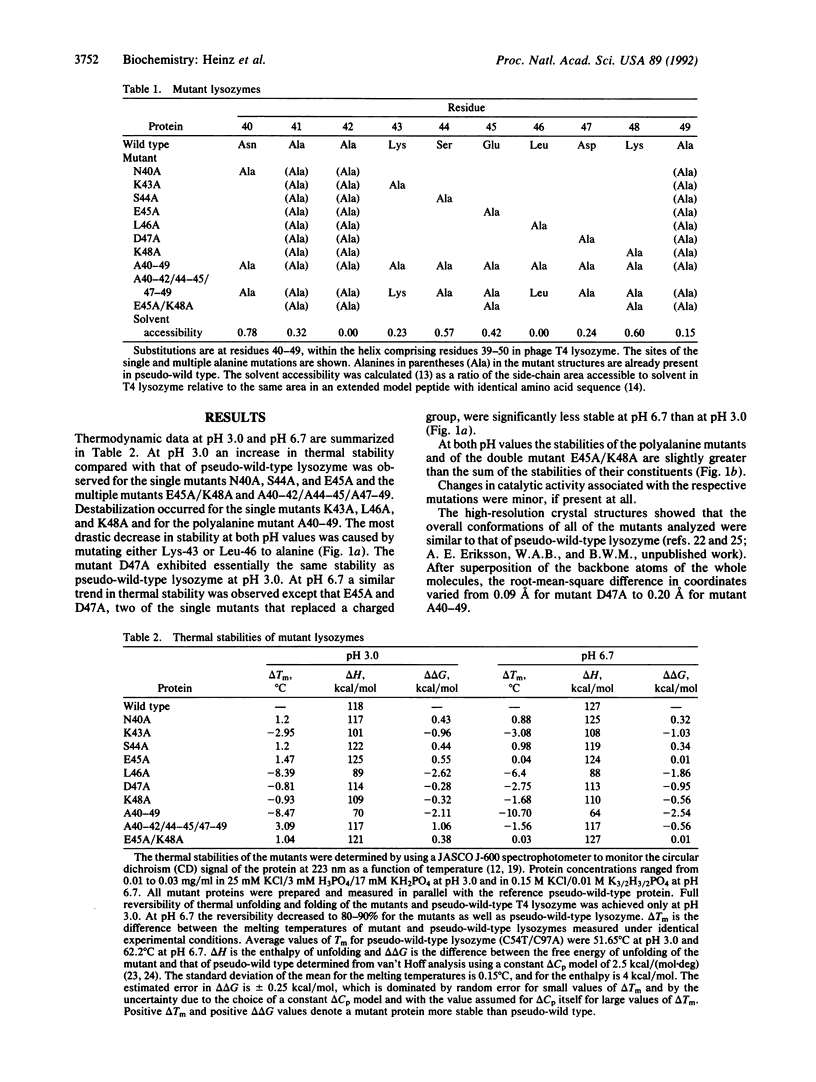
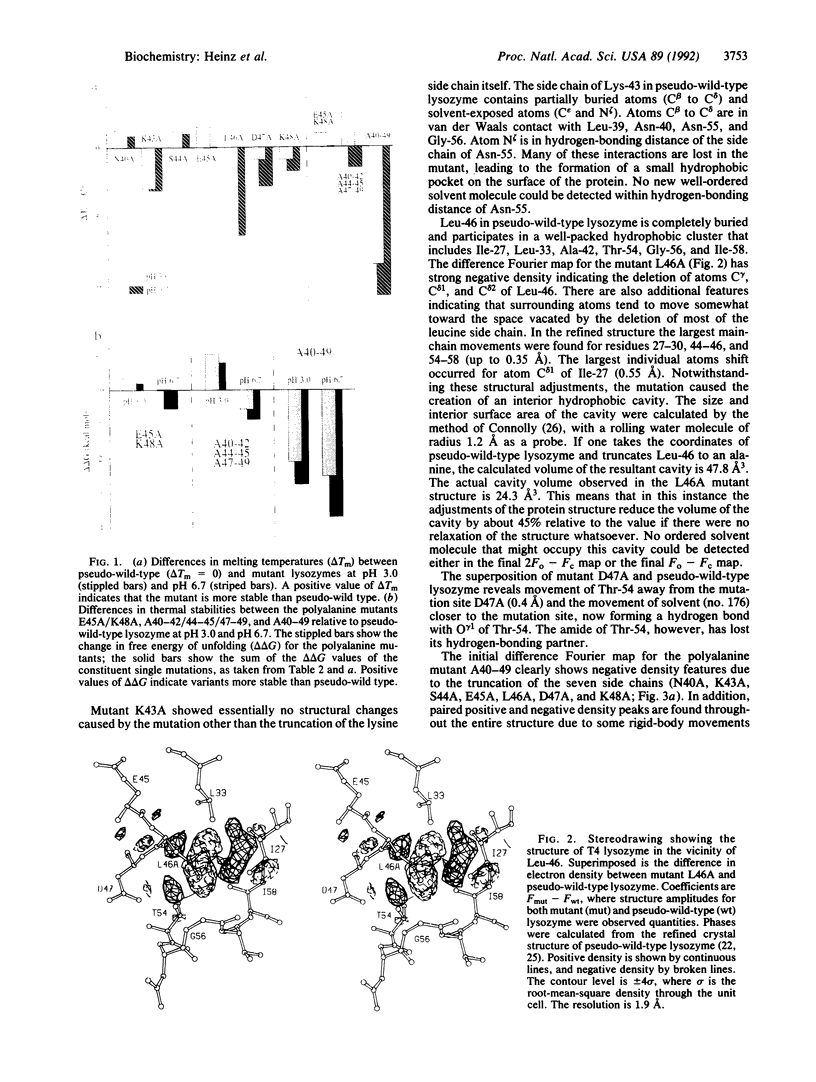
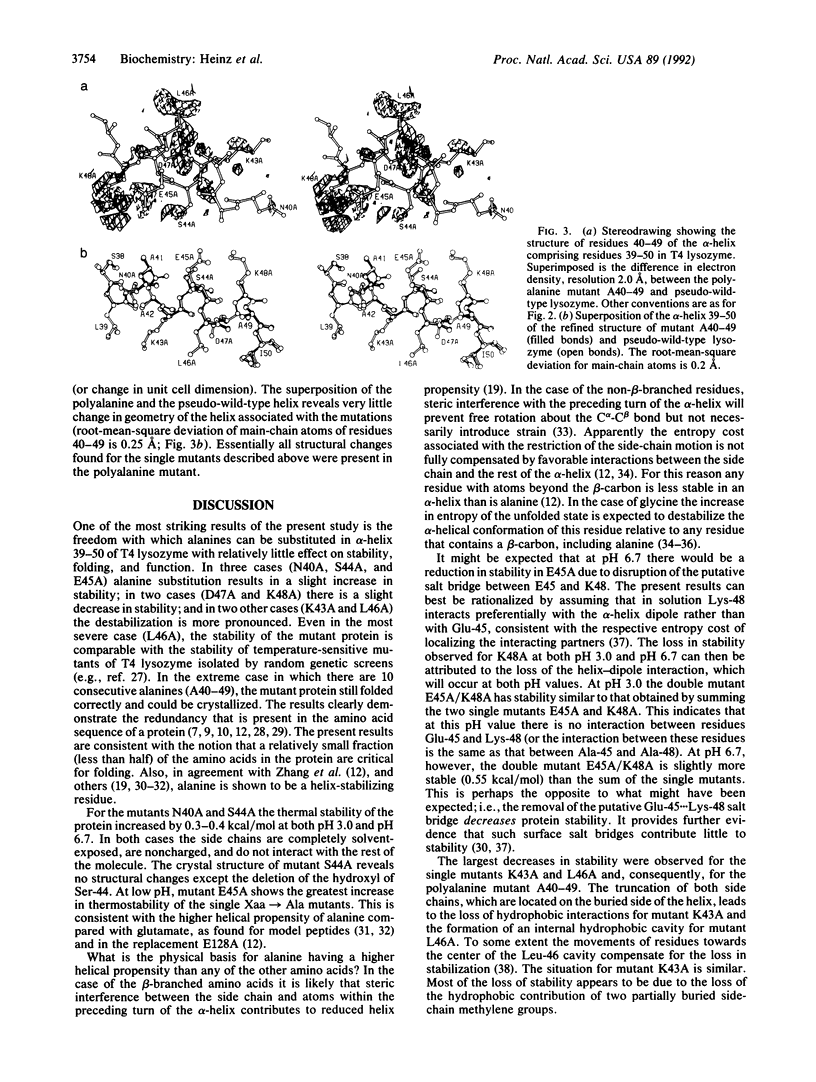
Single and multiple Xaa----Ala substitutions were constructed in the alpha-helix comprising residues 39-50 in bacteriophage T4 lysozyme. The variant with alanines at 10 consecutive positions (A40-49) folds normally and has activity essentially the same as wild type, although it is less stable. The crystal structure of this polyalanine mutant displays no significant change in the main-chain atoms of the helix when compared with the wild-type structure. The individual substitutions of the solvent-exposed residues Asn-40, Ser-44, and Glu-45 with alanine tend to increase the thermostability of the protein, whereas replacements of the buried or partially buried residues Lys-43 and Leu-46 are destabilizing. The melting temperature of the lysozyme in which Lys-43 and Leu-46 are retained and positions 40, 44, 45, 47, and 48 are substituted with alanine (i.e., A40-42/44-45/47-49) is increased by 3.1 degrees C relative to wild type at pH 3.0, but reduced by 1.6 degrees C at pH 6.7. In the case of the charged amino acids Glu-45 and Lys-48, the changes in melting temperature indicate that the putative salt bridge between these two residues contributes essentially nothing to the stability of the protein. The results clearly demonstrate that there is considerable redundancy in the sequence information in the polypeptide chain; not every amino acid is essential for folding. Also, further evidence is provided that the replacement of fully solvent-exposed residues within alpha-helices with alanines may be a general way to increase protein stability. The general approach may permit a simplification of the protein folding problem by retaining only amino acids proven to be essential for folding and replacing the remainder with alanine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Sun D. P., Nye J. A., Muchmore D. C., Matthews B. W. Temperature-sensitive mutations of bacteriophage T4 lysozyme occur at sites with low mobility and low solvent accessibility in the folded protein. Biochemistry. 1987 Jun 30;26(13):3754–3758. doi: 10.1021/bi00387a002. [DOI] [PubMed] [Google Scholar]
- Becktel W. J., Schellman J. A. Protein stability curves. Biopolymers. 1987 Nov;26(11):1859–1877. doi: 10.1002/bip.360261104. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Reidhaar-Olson J. F., Lim W. A., Sauer R. T. Deciphering the message in protein sequences: tolerance to amino acid substitutions. Science. 1990 Mar 16;247(4948):1306–1310. doi: 10.1126/science.2315699. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826–4838. doi: 10.1021/ja00995a002. [DOI] [PubMed] [Google Scholar]
- Chakrabartty A., Schellman J. A., Baldwin R. L. Large differences in the helix propensities of alanine and glycine. Nature. 1991 Jun 13;351(6327):586–588. doi: 10.1038/351586a0. [DOI] [PubMed] [Google Scholar]
- Climie S., Ruiz-Perez L., Gonzalez-Pacanowska D., Prapunwattana P., Cho S. W., Stroud R., Santi D. V. Saturation site-directed mutagenesis of thymidylate synthase. J Biol Chem. 1990 Nov 5;265(31):18776–18779. [PubMed] [Google Scholar]
- Connolly M. L. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983 Aug 19;221(4612):709–713. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Dao-Pin S., Baase W. A., Matthews B. W. A mutant T4 lysozyme (Val 131----Ala) designed to increase thermostability by the reduction of strain within an alpha-helix. Proteins. 1990;7(2):198–204. doi: 10.1002/prot.340070208. [DOI] [PubMed] [Google Scholar]
- Daopin S., Alber T., Baase W. A., Wozniak J. A., Matthews B. W. Structural and thermodynamic analysis of the packing of two alpha-helices in bacteriophage T4 lysozyme. J Mol Biol. 1991 Sep 20;221(2):647–667. doi: 10.1016/0022-2836(91)80079-a. [DOI] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. Mutants generated by the insertion of random oligonucleotides into the active site of the beta-lactamase gene. Biochemistry. 1989 Jul 11;28(14):5703–5707. doi: 10.1021/bi00440a001. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Grutter M. G., Schellman J. Thermodynamic stability and point mutations of bacteriophage T4 lysozyme. J Mol Biol. 1984 May 15;175(2):195–212. doi: 10.1016/0022-2836(84)90474-1. [DOI] [PubMed] [Google Scholar]
- Hecht M. H., Nelson H. C., Sauer R. T. Mutations in lambda repressor's amino-terminal domain: implications for protein stability and DNA binding. Proc Natl Acad Sci U S A. 1983 May;80(9):2676–2680. doi: 10.1073/pnas.80.9.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H., Sturtevant J. M., Sauer R. T. Stabilization of lambda repressor against thermal denaturation by site-directed Gly----Ala changes in alpha-helix 3. Proteins. 1986 Sep;1(1):43–46. doi: 10.1002/prot.340010108. [DOI] [PubMed] [Google Scholar]
- Kleina L. G., Miller J. H. Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol. 1990 Mar 20;212(2):295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Sauer R. T. Alternative packing arrangements in the hydrophobic core of lambda repressor. Nature. 1989 May 4;339(6219):31–36. doi: 10.1038/339031a0. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd Complete mutagenesis of the HIV-1 protease. Nature. 1989 Aug 3;340(6232):397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- Lyu P. C., Liff M. I., Marky L. A., Kallenbach N. R. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990 Nov 2;250(4981):669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Robbins V. H., Baldwin R. L. Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Matthews B. W. Control of enzyme activity by an engineered disulfide bond. Science. 1989 Feb 10;243(4892):792–794. doi: 10.1126/science.2916125. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Genetic and structural analysis of the protein stability problem. Biochemistry. 1987 Nov 3;26(22):6885–6888. doi: 10.1021/bi00396a001. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Nicholson H., Becktel W. J. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6663–6667. doi: 10.1073/pnas.84.19.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore D. C., McIntosh L. P., Russell C. B., Anderson D. E., Dahlquist F. W. Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol. 1989;177:44–73. doi: 10.1016/0076-6879(89)77005-1. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990 Nov 2;250(4981):646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Young V. B., Sauer R. T. Bacteriophage lambda cro mutations: effects on activity and intracellular degradation. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8829–8833. doi: 10.1073/pnas.83.23.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piela L., Nemethy G., Scheraga H. A. Conformational constraints of amino acid side chains in alpha-helices. Biopolymers. 1987 Aug;26(8):1273–1286. doi: 10.1002/bip.360260805. [DOI] [PubMed] [Google Scholar]
- Pjura P. E., Matsumura M., Wozniak J. A., Matthews B. W. Structure of a thermostable disulfide-bridge mutant of phage T4 lysozyme shows that an engineered cross-link in a flexible region does not increase the rigidity of the folded protein. Biochemistry. 1990 Mar 13;29(10):2592–2598. doi: 10.1021/bi00462a023. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., Sun D. P., Nicholson H., Matthews B. W. Second-site revertants of an inactive T4 lysozyme mutant restore activity by restructuring the active site cleft. Biochemistry. 1991 Feb 5;30(5):1425–1432. doi: 10.1021/bi00219a037. [DOI] [PubMed] [Google Scholar]
- Reidhaar-Olson J. F., Sauer R. T. Combinatorial cassette mutagenesis as a probe of the informational content of protein sequences. Science. 1988 Jul 1;241(4861):53–57. doi: 10.1126/science.3388019. [DOI] [PubMed] [Google Scholar]
- Rennell D., Bouvier S. E., Hardy L. W., Poteete A. R. Systematic mutation of bacteriophage T4 lysozyme. J Mol Biol. 1991 Nov 5;222(1):67–88. doi: 10.1016/0022-2836(91)90738-r. [DOI] [PubMed] [Google Scholar]
- Shortle D., Stites W. E., Meeker A. K. Contributions of the large hydrophobic amino acids to the stability of staphylococcal nuclease. Biochemistry. 1990 Sep 4;29(35):8033–8041. doi: 10.1021/bi00487a007. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Sun D. P., Sauer U., Nicholson H., Matthews B. W. Contributions of engineered surface salt bridges to the stability of T4 lysozyme determined by directed mutagenesis. Biochemistry. 1991 Jul 23;30(29):7142–7153. doi: 10.1021/bi00243a015. [DOI] [PubMed] [Google Scholar]
- Weaver L. H., Matthews B. W. Structure of bacteriophage T4 lysozyme refined at 1.7 A resolution. J Mol Biol. 1987 Jan 5;193(1):189–199. doi: 10.1016/0022-2836(87)90636-x. [DOI] [PubMed] [Google Scholar]
- Wells J. A. Systematic mutational analyses of protein-protein interfaces. Methods Enzymol. 1991;202:390–411. doi: 10.1016/0076-6879(91)02020-a. [DOI] [PubMed] [Google Scholar]
- Zhang X. J., Baase W. A., Matthews B. W. Toward a simplification of the protein folding problem: a stabilizing polyalanine alpha-helix engineered in T4 lysozyme. Biochemistry. 1991 Feb 26;30(8):2012–2017. doi: 10.1021/bi00222a001. [DOI] [PubMed] [Google Scholar]




