Abstract
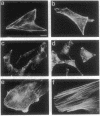
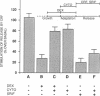
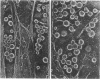
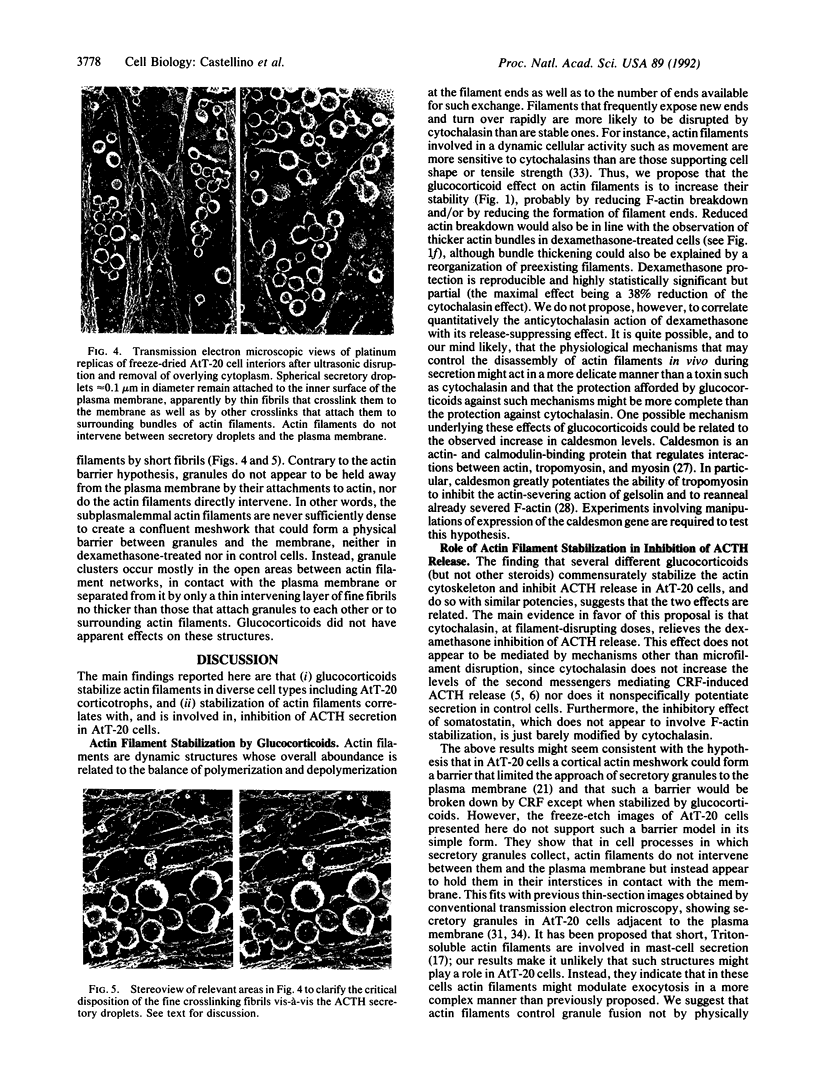
The mechanism by which glucocorticoids induce various cellular responses in different tissues is only partially understood. Here we demonstrate that glucocorticoids stabilize the actin cytoskeleton of several cell types, as revealed by increased resistance of actin filaments to the disrupting effect of cytochalasin and by visible thickening of actin filament bundles. These effects require several hours to develop, require protein synthesis, and are accompanied by increased expression of the actin-binding protein caldesmon. These data may help to explain why glucocorticoids inhibit corticotropin release from pituitary cells, if interpreted in terms of the current idea that an actin filament "barrier" modulates exocytotic secretion in various cell types. In support of this idea, we find that in "model" corticotrophs (AtT-20 cells), glucocorticoids stabilize actin filaments and inhibit corticotropin release with similar potencies. Furthermore, we show here that glucocorticoid inhibition is overcome by exposing AtT-20 cells to concentrations of cytochalasin B or D that disrupt their stabilized actin filaments. On the other hand, our freeze-etch electron microscopy of AtT-20 cells has shown that actin filaments do not, in fact, create a dense submembranous barrier that might prevent corticotropin secretory droplets from discharging; instead, they form open networks near the membrane that appear to hold secretory droplets in their interstices. We propose that the delicate physical crosslinks maintaining this actin-mediated membrane "docking" of secretory droplets may need to disconnect in order to permit corticotropin discharge and that these crosslinks may be stabilized along with the actin filaments in dexamethasone-treated cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aunis D., Bader M. F. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988 Sep;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Norman K. M. Identification of a secretory granule-binding protein as caldesmon. Nature. 1986 Jan 2;319(6048):68–70. doi: 10.1038/319068a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J., Barron J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc R Soc Lond B Biol Sci. 1982 Aug 23;216(1202):111–115. doi: 10.1098/rspb.1982.0064. [DOI] [PubMed] [Google Scholar]
- Cassimeris L., McNeill H., Zigmond S. H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol. 1990 Apr;110(4):1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Cyclic AMP inhibits both nicotine-induced actin disassembly and catecholamine secretion from bovine adrenal chromaffin cells. J Biol Chem. 1987 Aug 25;262(24):11663–11666. [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 1986 Oct 20;207(1):110–114. doi: 10.1016/0014-5793(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Coates T. D., Wolach B., Tzeng D. Y., Higgins C., Baehner R. L., Boxer L. A. The mechanism of action of the antiinflammatory agents dexamethasone and Auranofin in human polymorphonuclear leukocytes. Blood. 1983 Nov;62(5):1070–1077. [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M., Sterk A. R., Hirata F., Ishizaka T. Biochemical analysis of glucocorticoid-induced inhibition of IgE-mediated histamine release from mouse mast cells. J Immunol. 1982 Sep;129(3):1212–1218. [PubMed] [Google Scholar]
- Giguère V., Labrie F., Côté J., Coy D. H., Sueiras-Diaz J., Schally A. V. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3466–3469. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989 Feb;108(2):401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Sobue K., Kanda K., Harada A., Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989 Jan;108(1):111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R., Yamashiro S., Matsumura F. Annealing of gelsolin-severed actin fragments by tropomyosin in the presence of Ca2+. Potentiation of the annealing process by caldesmon. J Biol Chem. 1989 Oct 5;264(28):16764–16770. [PubMed] [Google Scholar]
- Kamel F., Kubajak C. L. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology. 1987 Aug;121(2):561–568. doi: 10.1210/endo-121-2-561. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M. E., Dallman M. F. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984 Winter;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Buckley K. M., Burgess T. L., Carlson S. S., Caroni P., Hooper J. E., Katzen A., Moore H. P., Pfeffer S. R., Schroer T. A. Membrane traffic in neurons and peptide-secreting cells. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):697–705. doi: 10.1101/sqb.1983.048.01.073. [DOI] [PubMed] [Google Scholar]
- Koffer A., Tatham P. E., Gomperts B. D. Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J Cell Biol. 1990 Sep;111(3):919–927. doi: 10.1083/jcb.111.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis D. M., Hall A. K., Weinstein L. A., Reese T. S. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988 May;1(3):201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Lelkes P. I., Friedman J. E., Rosenheck K., Oplatka A. Destabilization of actin filaments as a requirement for the secretion of catecholamines from permeabilized chromaffin cells. FEBS Lett. 1986 Nov 24;208(2):357–363. doi: 10.1016/0014-5793(86)81049-3. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A. G., Axelrod J. Inhibitors of the cytochrome P-450 enzymes block the secretagogue-induced release of corticotropin in mouse pituitary tumor cells. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1012–1014. doi: 10.1073/pnas.82.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Corda D., Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Schofield G., Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986 Nov;6(11):3128–3132. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L., Buckley K. M., Lowe A. W., Kelly R. B. Targeting of secretory vesicles to cytoplasmic domains in AtT-20 and PC-12 cells. J Cell Biol. 1988 Feb;106(2):239–251. doi: 10.1083/jcb.106.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E. H., Braun K., Johansen T. Reorganization of the subplasmalemmal cytoskeleton in association with exocytosis in rat mast cells. Histol Histopathol. 1989 Oct;4(4):473–477. [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Phillips M., Tashjian A. H., Jr Characterization of an early inhibitory effect of glucocorticoids on stimulated adrenocorticotropin and endorphin release from a clonal strain of mouse pituitary cells. Endocrinology. 1982 Mar;110(3):892–900. doi: 10.1210/endo-110-3-892. [DOI] [PubMed] [Google Scholar]
- Reisine T., Rougon G., Barbet J. Liposome delivery of cyclic AMP-dependent protein kinase inhibitor into intact cells: specific blockade of cyclic AMP-mediated adrenocorticotropin release from mouse anterior pituitary tumor cells. J Cell Biol. 1986 May;102(5):1630–1637. doi: 10.1083/jcb.102.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M. Steroid hormone regulation of gene expression. Annu Rev Pharmacol Toxicol. 1985;25:529–566. doi: 10.1146/annurev.pa.25.040185.002525. [DOI] [PubMed] [Google Scholar]
- Rodriguez Del Castillo A., Lemaire S., Tchakarov L., Jeyapragasan M., Doucet J. P., Vitale M. L., Trifaró J. M. Chromaffin cell scinderin, a novel calcium-dependent actin filament-severing protein. EMBO J. 1990 Jan;9(1):43–52. doi: 10.1002/j.1460-2075.1990.tb08078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Jacques A., Shin H. S., Lichtenstein L. M., Plaut M. Inhibition of T cell-mediated cytotoxicity by anti-inflammatory steroids. J Immunol. 1984 Jan;132(1):266–271. [PubMed] [Google Scholar]
- Sontag J. M., Aunis D., Bader M. F. Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur J Cell Biol. 1988 Jun;46(2):316–326. [PubMed] [Google Scholar]
- Tooze J., Burke B. Accumulation of adrenocorticotropin secretory granules in the midbody of telophase AtT20 cells: evidence that secretory granules move anterogradely along microtubules. J Cell Biol. 1987 Apr;104(4):1047–1057. doi: 10.1083/jcb.104.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Vitale M. L., Rodríguez Del Castillo A., Tchakarov L., Trifaró J. M. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol. 1991 Jun;113(5):1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Ishikawa R., Matsumura F. Mitosis-specific phosphorylation causes 83K non-muscle caldesmon to dissociate from microfilaments. Nature. 1990 Apr 12;344(6267):675–678. doi: 10.1038/344675a0. [DOI] [PubMed] [Google Scholar]